Abstract
Introduction
Brain herniations (BH) into arachnoid granulations (AG) in dural venous sinuses and calvarium have rarely been reported in the literature.
Methods
MRIs of 38 patients with BH into AG (BHAG) were retrospectively analyzed. Locations of BHAG, gyrus/lobe of the herniated brain, parenchymal abnormalities of the BH, and clinical and radiological conditions with raised intracranial pressure were recorded.
Results
Sixty-eight BHAG were found, by order of frequency, in the occipital squama (OS), transverse sinus (TS), lateral lacuna of the superior sagittal sinus (LLSSS), and straight sinus (SS), with cerebellar tissue being the most frequently involved in BHAG (94.5 % of OS, 55 % of TS, 100 % SS BHAG). Multiple BHAG were found in 58 % of the patients (up to five per patient). Parenchymal signal and structural changes (SSCG) were observed in 46 % of BHAG (100 % were cerebellar). Three patients had pseudotumor cerebri (PTCS); one patient had only MRI signs of PTCS. Twenty-one percent of patients had intracranial conditions susceptible of increasing cerebrospinal fluid (CSF) pressure other than PTCS.
Conclusions
BHAG occurred in the OS, TS, LLSSS, and the SS. SSCG of the herniated cerebellum were frequent and possibly result from tethering/strangulation in the AG. No symptoms could be clearly attributed to BHAG, though in three cases of PTCS, TS BHAG could have contributed to sustaining the raised CSF pressure. Various factors are probably involved in the development of BHAG including normal pia-arachnoid bridges between the brain surface and the AG, hydrodynamic constrains on the brain and AG, and, in some cases, increased intracranial pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachnoid granulations (AG), also known as Pacchionian granulations or Pacchionian bodies, develop from the arachnoid membrane [1, 2] and invaginate into the dura mater as they grow. Though Antonio Pacchioni is generally credited for describing AG for the first time in 1705, hence the eponymous designation, AG had already been reported by various investigators including Samuel Collins (1685), Alexis Littre (1684), and Thomas Willis (1664) and described and illustrated as far back as 1543 by Andreas Vesalius [1–3]. We owe Pacchioni a detailed description of AG and an attempt to explain their function. AG arise from arachnoid villi [2] that may be found on the visceral surface of the arachnoid and are filled with cerebrospinal fluid (CSF). CSF pulsations likely represent the principal mechanism of growth of arachnoid villi into AG. AG appear with age [2, 4], being virtually absent in early childhood and becoming a constant finding in adult life [1, 5]. As they grow, they invaginate into the dura forming a CSF-filled cavity and may erode into the calvarium [2, 3, 5]. Digitations of pia mater covering the brain surface extend into the AG, thus anchoring the brain to the dura matter, forming pia-arachnoid bridges [2, 3, 6, 7].
AG are found within dural venous sinuses or meningeal and diploic veins [2, 5, 8, 9], frequently located at points of cortical venous convergence into a dural sinus [1, 10]. AG may project into the lumen of the dural venous sinus and must be distinguished from thrombosis on imaging studies. Their most frequent location is within the lacunae laterales of the superior sagittal sinus (LLSSS) [2, 5]. They may also be found in the lumen of the superior sagittal sinus (SSS), the transverse sinuses (TS), the great vein of Galen/straight sinus junction, the straight sinus (SS), paracavernous sinuses, the middle meningeal veins in the middle cranial fossa, or in the tentorial sinuses [2, 5, 11]. AG in the TS most frequently arise from the infratentorial surface though they can also arise from the cerebral surface [5].
AG may also develop into the calvarium at a distance from a dural venous sinus and seemingly with no relation to venous structures, eroding the inner table, the diploe, and even the outer table. Although they may involve any part of the calvarium, they are frequently identified in the frontal bone and occipital squama, where they appear as well-marginated lytic lesions on plain films and CT and follow CSF signal on MRI [12–16]. The bony defect harboring these arachnoid granulations (arachnoid pits) may exceed 10 mm in diameter. AG in pneumatized regions of the base of the skull may provide a pathway for spontaneous CSF leaks and for meningocele/encephalomeningocele [17, 18].
The purpose of the present imaging study is to report cases of brain herniations (BH) into AG located in the dural venous sinuses and/or the calvarium that were encountered during routine diagnostic imaging investigations. BH into dural venous sinuses or the calvarial bony defects have rarely been reported in the literature. To the best of our knowledge, this is the largest series of BH into AG (BHAG). Physiopathological and clinical considerations and a review of the literature are provided.
Materials
This is a retrospective study based on MRIs performed at the Hospital of Sion. A keyword search using “brain herniation”, “meningo-encephalocele”, and “arachnoid granulation” of our RIS PACS system (Radoffice, WDS technologies SA, Geneva, Switzerland) was performed. All patients gave informed consent for MRI ± CT being performed during routine medical investigations. Informed consent of the patients for the study was waived by our IRB due to the retrospective nature of the study.
MRI were performed on a 1.5-T scan (Siemens Medical Systems, Erlangen, Germany). All MRI studies included non-contrast 3DT1 MPRAGE or post-gadolinium (0.1 mmol/kg) 3DT1 MPRAGE (Gd-3DT1) acquisitions, T2 Tirm dark fluid, TSE-T2 sequences, and susceptibility weighted imaging (SWI). In some cases, 3DT2 space or CISS acquisitions were also available. When CT studies (64 MDCT; GE, Fairfield, USA) were also available, they were included in the analysis. All cases of BHAG into a dural venous sinus or the calvarium were included. Patient medical history was analyzed. History and imaging signs of increased intracranial pressure or pseudotumor cerebri were sought for. The origin of the BH and the location of the BHAG were recorded. Signal or structural changes of the herniated brain were noted. In MRI studies with available Gd-3DT1 images, enhancement of the dura lining the AG in cases of OS BHAG was recorded. In the cases of BHAG into the calvarium where CT was available, erosions of the outer table were recorded.
Consensual analysis of the imaging and data was performed by a senior radiology resident and a senior board certified neuroradiologist (SM and DSM, respectively). All imaging sequences for each patient were analyzed at the same time.
A French and English literature review was performed with PubMed and Google. Cited references in pertinent published articles were also scrutinized in search for publications that could have been missed by the internet search.
Results
The keyword search of our RIS PACS system revealed 38 patients harboring a total of 68 BHAG between February 2013 and January 2016 (no cases found before February 2013). All patients were older than 18 years of age. Average age of patients was 63 ± 16.3 years and 66 % of patients were females.
BH were found by order of frequency in the OS, TS, LLSSS, and SS, all corresponding to typical locations of AG. BH were surrounded by a CSF rim or plainly located within a uni- or multiloculated CSF-filled defect in the dural venous sinus, the frontal/parietal bone, or OS (Figs. 1, 2, 3, 4, 5, and 6). Furthermore, when located in the dural venous sinuses, a zone of venous convergence was found at the point of the BH or in its vicinity. Though no statistical analyses in terms of frequency were performed, OS BHAG often contained inferior cerebellar or vermian veins and bore a close anatomical relation with OS diploic veins.
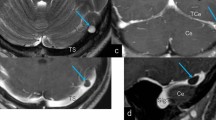
Thirty-three-year-old male with subarachnoid hemorrhage of unknown origin (patient 15). Herniation of the ITG and LOTG (large arrowhead) is seen into the left TS. This BHAG was suspected on initial PC-CT. ITG looks normal. It is surrounded by CSF that contained an AG (small arrowheads (d, e)). Coronal MPR reformat: CTA (a), 3DT1 MPRAGE (b), Gd-3DT1 MPRAGE (c), 3DT2 space (d); axial: TSE-T2 (e), 3DT1 MPRAGE (f), Gd-3DT1 MPRAGE (g)
Eighty-five-year-old woman with right frontal glioblastoma multiforme (patient 25). Bilateral SSL (arrows) herniation into AG in the TS (large arrowheads (b–d)). SSCG of the herniated SSL surrounded by gliosis (small arrowheads (a)). The herniated tissue appears tethered. Enhanced TS (small double arrows (d, e)) is visible around the BHAG. Coronal T2 tirm dark fluid (a); axial TSE-T2 (b, c); reformat Gd-3DT1 MPRAGE (d—axial oblique, e—coronal oblique)
Thirty-four-year-old woman with suspected meningitis and multiple BHAG (patient 24). There is a small herniation of normal looking ISL (arrow) into an AG of the OS (arrowhead) on the left side. Typical CSF filled and multilobulated appearance of the AG OS. There is substantial contrast enhancement of the wall of the AG OS (arrowhead (d)), which is much more conspicuous than normal looking dura mater (small double arrow). This enhancement could result from either tethering of the dura or inflammation. TSE-T2 (a), 3DT2 space (b), 3DT1 MPRAGE (c), and Gd-3DT1 MPRAGE (d)
Seventy-four-year-old male with transient global amnesia (patient 22). Herniation of the SSL (arrow) into an AG of the distal SS (arrowhead). The herniated SSL looks tethered and slight SSCG are observed. This BHAG was mistaken for a large AG of the SS on contrast-enhanced CT (d, e), though the herniated SSL was visible (e) and retrospectively identified as a BHAG after the MRI study. A second large AG (double arrow (d)) was also visible on CT at the great vein of Galen and SS junction but contained no brain tissue. Coronal oblique reformat 3DT1 MPRAGE (a) and 3DT2 space (b); sagittal reformat 3DT2 space (c), sagittal and coronal oblique reformat CTA (d, e)
Seventy-year-old female with squamous cell carcinoma of the base of the tongue, cerebral metastasis, and meningeal carcinosis (patient 14). There is a giant AG of the OS (AGOS—arrowhead) on the midline and a small one on the left (double arrowhead). The giant AGOS contains herniated, tethered ISL and SSL with mild SSCG from the right cerebellum. Note how the right cerebellar hemisphere is distorted from the attraction into the AG. In comparison with Fig. 4, there is faint contrast enhancement of the walls of the AGOS (b, c) identical to that of normal dura. Non-contrast CT (a); reformat Gd-3DT1 MPRAGE (b, c); reformat 3DT2 (d–f)
Seventy-eight-year-old male with left sigmoid sinus DAVF and normal pressure hydrocephalus harboring two LLSSS BHAG from the SFG (patient 32). The more posteriorly located of the two BHAG (large arrowhead) is seen to cross the entire thickness of the calvarium, eroding through the outer table (small arrowheads). The herniated SFG (arrow) reaches the diploic spaces, while the CSF component from the AG surrounding the BH extrudes slightly beyond the outer surface of the outer table. a Coronal TSE-T2, b coronal reformat Gd-3DT1 MPRAGE, c sagittal oblique reformat Gd-3DT1 MPRAGE
Sixty-three percent of the BHAG were in the OS (43/68), 29.5 % in the TS (20/68), 6 % in the LLSSS (4/68), and 1.5 % in the SS (1/68). Seventy-eight percent of all BHAG arose from the cerebellum. Ninety-five percent of OS BHAG (41/43) arose from the cerebellum and 5 % from the occipital lobe (2/43). In the latter, the OS AG was located supratentorially above the torcular herophilii. Eighty-eight percent of OS BHAG arose from the inferior semilunar lobule (ISL) (Fig. 3), 4.5 % from both the ISL and the superior semilunar lobule (SSL) (2/43) (Fig. 5), 4.5 % from the occipital lobe, and 2 % from the SSL (1/35). Herniated brain into TS AG arose from the inferior temporal gyrus (ITG) in 40 % of the cases (9/20)from the SSL in 55 % (11/20) (Fig. 2), and from the ITG and lateral occipito-temporal gyrus (LOTG) in 5 % of the cases (1/20) (Fig. 1). One hundred percent of SS BHAG arose from the SSL (1/1) (Fig. 4). One hundred percent of LLSSS BHAG arose from the superior frontal gyrus (Fig. 6), all eroding into the parietal bone except for one which eroded the frontal bone, immediately in front of the coronal suture.
Multiple BHAG were noted in 58 % of the patients (22/38). Eighty-two percent of the patients with multiple BHAG had two BHAG, 9 % had three BHAG, 4.5 % had four, and 4.5 % had five.
Signal and structural changes (SSCG) of the BH within the AG or the stem of the BH (Figs. 2 and 5) were noted in 46 % of the BHAG (31/68). These changes included focal brain atrophy (widening of sulci or folia) and/or hyper-T2 and T2-tirm signal consistent with gliosis (no restricted diffusion, no enhancement, no blood residues on SWI). SSCG were found in 53 % of OS BHAG (23/43), 35 % of TS BHAG (7/20), 100 % of SS BHAG (1/1), and 0 % of LLSSS BHAG. One hundred percent of SSCG involved herniated cerebellar tissue.
Enhancement of the dura mater lining the OS AG was observed in 19 of the 28 OS BHAG (68 %) where Gd-3DT1 sequences were available.
Non-contrast or gadolinium-enhanced volumetric T1 images and volumetric T2 images were respectively obtained in 100 % and 48 % of the studies. All BHAG were detectable on 3DT1 (with or without gadolinium enhancement) and 3DT2. Gd-3DT1 images were not superior to non-enhanced 3DT1 images for the detection of BHAG (100 % were visible on non-enhanced 3DT1 images), but they could better delineate the structures surrounding the BH (CSF rim, boundaries of the dural venous sinus lumen), demonstrate enhancement of the dura lining OS AG, and show pial/diploic veins in the vicinity of the BHAG.
Eighty-four percent of the patients had a non-contrast CT study of which 63 % included contrast-enhanced CT (either CTA, CTV, or delayed venous phase CT). BHAG were identifiable retrospectively in 87 % of CT studies and suspected on CT prior to MRI in 58 % (initial imaging workup). Ninety percent of TS BHAG were identifiable retrospectively and 45 % were suspected on the initial CT (all were post-contrast CT) against 84 and 65 %, respectively, for OS BHAG. One hundred percent LLSSS BHAG were suspected on the initial study in the patient with LLSSS BHAG that had pre-MRI CT. The SS BHAG was not suspected on initial non-contrast and post-contrast CT but was identifiable retrospectively.
Erosions measuring up to 4 mm of the outer table of the calvarium were found in 13 % of BHAG (9/68), seven of which corresponded to OS BHAG and two to LLSSS BHAG (Fig. 6). No brain tissue was seen to herniate beyond the outer table. In one case of LLSSS BHAG (Fig. 6) and one case of OS BHAG, CSF from the BHAG was seen to extrude beyond the outer table defect on MRI (patients 34 and 37).
Four patients had imaging signs of pseudotumor cerebri syndrome (PTCS). Imaging signs of PTCS were considered in the presence of enlarged sella, “empty or partially empty sella”, dilated optic nerve sheaths, tortuous optic nerves, and dilated CSF spaces of Meckel’s cave or around the oculomotor nerves and/or abducens nerves [19]. Three patients (patients 1, 8, and 38) had clinical symptoms of PTCS that were managed conservatively with acetazolamide treatment. Patients 1 and 8 had TS BHAG, arising from the ITG bilaterally for patient 1 and from the SSL bilaterally for patient 8. BHAG were responsible for severe bilateral TS stenosis in patient 8. In patient 1, there was severe stenosis of the right TS caused by the BHAG and AGs and a hypoplastic left TS with severe stenosis from AG and extrinsic compression from increased CSF pressure (left TS BHAG did not cause significant stenosis). As the patients evolved favorably under conservative medical treatment, no TS stenting was contemplated and no TS pressure measurements were performed. The third patient was recently diagnosed with PTCS (patient 38) secondary to venous outflow obstruction from a torcular herophili meningioma invading the origin of the right TS that was severely stenosed, and from a left TS severe stenosis secondary to AG containing herniated SSL. The patient is waiting for follow-up consult. One patient had imaging signs of PTCS (patient 7) without symptoms of PTCS and could have corresponded to a case of “burnt out” PTCS. In this patient, BHAG were located in the right TS and in the right OS.
Twenty-one percent of patients (8/38) had or had had intracranial conditions potentially causing chronically raised intracranial pressure (Table 1).
Discussion
Sixty-eight BHAG were found in 38 patients in this study based on MRI findings. Sixty-three percent occurred in the OS, 29.5 % in the TS, 6 % in the LLSSS, and 1.5 % in the SS. Average age was 63 years, suggesting that BHAG occur more frequently with advancing age. Two thirds of patients were females.
That BH occurred into AG is supported by several morphological features in the present study: BH were found in typical locations for AG (OS, TS, LLSSS, and SS); they were surrounded by a CSF rim or plainly located within a uni- or multiloculated CSF-filled defect in the dural venous sinus or calvarium; when located in the dural venous sinuses, a zone of venous convergence was generally found at the point of the BH or in its vicinity; and when located in the OS, the osseous defect harboring the BH often contained inferior cerebellar or vermian veins and/or bore a close anatomical relation with OS diploic veins. There is also evidence in the literature to support that the BH reported in our series occur in AG. In a paper on venous pseudotumor cerebri [20], it is mentioned that “small hernias of brain tissue are frequently seen in the base of arachnoid granulations at craniotomy” (M Besser; personal communication). No follow-up publication to this “personal communication” could be found to provide further details. More importantly, Wolbach [7] presented nine autopsy cases of “hernias of the cerebrum and cerebellum” due to increased intracranial pressure, six of which had BH into dural venous sinuses or into the occipital squama (Table 2). In five of these cases, the author specified that BH entered arachnoid villi and he provided microscopic correlation of one case where distorted cerebellar tissue herniated into large arachnoid villi in the right lateral sinus. Beneke, quoted by Wolbach [7], described an autopsy case of intracranial hypertension secondary to a choroid plexus papilloma in which he found numerous BH into arachnoid villi along the superior sagittal sinus. These findings were confirmed histologically as quoted by Wolbach: “The hernias consisted of brain tissue in various stages of disorganization, some containing vessels of considerable size, which were carried along from their original positions… The entrance of the hernias into the dura takes place through endothelial lined fissures, which Beneke states normally contain processes of arachnoid tissue. These arachnoid processes, he goes on to say, are the smallest “Anlagen” of the Pacchionian granulations”.
Descriptions of BHAG in the radiological literature are scant (summarized in Table 2). Kollar et al. [21] reported a case of TS dura arteriovenous fistula (DAVF) containing a round filling defect in the fistulous portion of the TS on digital subtraction angiography (DSA). The DAVF was embolized and subsequently resected surgically. Herniated brain tissue was found within the resected TS that corresponded to the filling defect observed on DSA. Other than its ectopic location, the herniated brain was histologically normal. Liang et al. [22] mention that two out of 433 filling defects in the dural venous sinuses on MRI were due to invaginated brain into the dural venous sinus. Their figure 5 illustrates an occipital lobe BH into the right TS, though no other information concerning these two cases was provided. Recently, five individual case reports and a series of five cases [23–28] reported a total of ten patients harboring 13 BH. A study including 20 patients and 21 BH [29] was published while the present manuscript was being finalized. In this study, 62 % of BH occurred in the TS, 19 % in the torcular, 14 % into the OS, and 5 % into the sigmoid sinus. Four cases involved pediatric patients of 8, 9, and 11 (two patients) years of age [24–26, 29], the rest of BHAG being encountered in adult patients as in the present series. Some authors have suggested that these BH occurred into AG [24, 27]; others do not take a clear position [23, 25] or suggest that BH could also invaginate into dural venous sinuses at points of dural weakness or where cortical veins enter the venous sinus without clearly ruling out AG [26, 29].
Several authors have referred to BHAG into dural venous sinuses as encephaloceles [23, 27, 28]. Battal et al. [26, 29] argue, however, that BH into dural venous sinuses and calvarium do not represent encephaloceles as “encephaloceles are composed of meninges and brain extruding outside the skull”. In our study, no brain tissue was seen to herniate outside the outer table of the calvarium. Erosions of the outer table were, however, observed in 13 % of BHAG, and in two of those cases, CSF from the BHAG extruded outside the outer table on MRI (Fig. 6). Beneke, cited by Wolbach [7], described an autopsy case of “papilloma of ependymal origin arising from the choroid plexus. There were symptoms [of increased intracranial pressure] for about 1 year before death…There was marked internal hydrocephalus. The [brain] hernias were particularly numerous in the regions of the arachnoid villi along the superior longitudinal sinus. A number had completely perforated the bone”. An analogy can be drawn between BHAG of the skull vault and dural venous sinuses and temporal and sphenoidal acquired meningo-encephaloceles where AG located in the cranial fossae seem to be implicated in the development of the meningo-encephalocele [18]. Finally, the definition of “cele” (from the greek word “Kele”) is that of “a swelling or hernia in a specified part” (Oxford, British and World English Dictionary, Online). BHAG, therefore, correspond to a form of acquired meningo-encephocele.
Though rare, BHAG may be encountered during routine brain imaging. An incidence of 0.32 % has recently been reported [29]. They must be distinguished from venous sinus thrombosis, dural-based tumors, or other normal filling defects of dural venous sinuses, such as septa, AG, fat, etc.[22, 30, 31]. MRI readily demonstrated BHAG, particularly if high-resolution volumetric T1 and T2 sequences are obtained, in keeping with findings from Battal et al. [29]. Indeed, conventional brain sequences have proved to be inadequate for the detection of small BH due to thick slice thickness [29]. In our study, CT was also effective in demonstrating BHAG, which were retrospectively identified in 87 % of CT studies and suspected on the initial CT prior to MRI in 58 % of the cases. Though OS BHAG are readily detectable on non-contrast CT (NC-CT), post-contrast CT (PC-CT) was generally necessary to identify TS BHAG. PC-CT shows a filling defect in the dural venous sinus that can draw attention to an otherwise unsuspected BH due to the herniated brain appearing isodense to the dural venous sinus on NC-CT.
All OS and SS BHAG arose from the cerebellar hemispheres except for two OS BHAG in one patient that were located supratentorially, arising from the occipital lobe. Interestingly, 55 % of TS BHAG arose infratentorially from the SSL, while 45 % arose from the inferior surface of the temporal lobe. Herniation of the cerebellum into the undersurface of the TS is possible because AG principally develop on the cerebellar surface of the TS as reported by Le Gros Clark [5]. BHAG locations differed from previous reports, with the most frequent BHAG location being the OS followed by the TS, as opposed to the TS being the most frequent location in the radiological literature [21–27, 29]. Cerebellar herniation into TS AG was previously reported in the radiological literature by Battal et al. [29] in one case and by Chan et al.[24], though close inspection of their figures 1–3 suggests that this could have in fact been a case of ISL herniation into an OS AG. Wolbach [7] reported cerebellar herniations into AG of the lateral sinuses (transverse and sigmoid sinuses) in four out of the six autopsy cases described above. BHAG into LL SSS have been described by Wolbach [7] (three cases) and by Beneke, cited by Wolbach [7] (one case), but no reference was found in the radiological literature. Unlike other authors, we did not encounter BH into the sigmoid sinus or the torcular herophilii [29], or occipital lobe BH into the TS [22, 29].
Multiple BHAG were a frequent finding in the present study, being identified in 58 % of the patients with a maximum of five BHAG in one case (patient 36). This is a higher rate than reported by Battal et al.[29] in the largest series up to day with 1 patient out of 20 (5 %) harboring multiple BHAGs. A case of three BHAG was also reported by Battal et al. [26] in their series of five patients. Wolbach [7], however, reports multiple BHAG in 100 % of his patients, with multiple BHAG being found both in the same location and in different locations at the same time. In all but one patient, patients died with signs of or from longstanding increased intracranial pressure that could account for the higher number of BHAG encountered by Wolbach.
Physiopathological considerations
The mechanism by which the brain may herniate into an AG likely responds to a combination of anatomical and hydrodynamic (mechanical) factors. As mentioned earlier, AGs originate in the arachnoid membrane as arachnoid villosities. As AG grow due to the continuous effect of CSF pulsations, they invaginate through points of dural weakness, dura mater defects, and/or points of venous convergence, drawing the adjacent pia mater from the surface of the brain into the AG [2, 7, 32]. A pia-arachnoid bridge is thus formed, constituting a potential scaffold through which the brain can be pulled into the AG. The same pulsatile forces that drive the growth and progressive invagination of AG into the dura and calvarium may act on the brain resulting in BHAG. In the case of TS BHAG arising from the temporal lobe BHAG, the temporal lobe lies in intimate contact with the dural venous sinus, likely favoring the herniation. This is not the case in TS BHAG arising from the cerebellum or the SS BHAG (Figs. 2 and 3). Pre-existing pia-arachnoid bridges between the AG and the brain could explain how the cerebellum may herniate cranially into the TS and SS AG, seemingly against gravity and CSF pulsations. In the same line of thought, Wolbach [7] states, “The more compact layers of the pia and arachnoid extend upwards into the villus, around the periphery of its base. This arrangement practically makes a break in the pia arachnoid covering of the brain and probably is of mechanical importance in the formation of hernias”.
Signal and structural changes (SSCG) of the HB were frequently observed (46 % of BHAG). In all cases, SSCG involved cerebellar tissue, with no cases of supratentorial SSCG being found. Progressive thinning of the herniated cerebellum towards the AG and variable degrees of distortion of the cerebellum around the stem of the BH were observed (Figs. 2 and 5). We found no mention of SSCG of BHAG in the radiological literature. Distorted cerebellar tissue herniating into the lateral sinus (transverse and sigmoid sinuses) was, however, observed by Wolbach [7], and Beneke, quoted by Wolbach [7], found that “the hernias consisted of brain tissue in various stages of disorganization”. These autopsy findings could correspond to the SSCG encountered in cerebellar BHAG in our study.
Tethering of the herniated cerebellum by pia-arachnoid bridges or strangulation of the BH at the neck of AG or a combination of both could account for SSCG and distortion of the cerebellum. Tethering of the cerebellum is likely favored by it being suspended from the dura, rather than resting on it, as is the case for the undersurface of the temporal lobe. In addition, continuous downward forces pulling the cerebellum away from its dural attachments could further enhance tethering. Indeed, no SSCG were observed in TS BHAG arising from the inferior surface of the temporal lobe, where, because the temporal lobe lies against the dural venous sinuses, tethering is unlikely. Finally, inflammatory processes leading to fibrous tissue within the AG contributing to strangulation and tethering cannot be discarded, in keeping with the progressive fibrosis observed in mature AG [2]. Enhancement of the dural lining of the AG was found in up to 68 % of the cases where volumetric Gd-enhanced T1 studies were available. Although in some cases the dural enhancement around BHAG was linear and as intense as in normal dura, in other cases, enhancement was very conspicuous (Fig. 4) suggesting inflammatory changes. Alternatively, this conspicuous dural enhancement could represent focal pachymeningitis secondary to the dura being pulled by the pia-arachnoid bridges and BH attaching it to the brain surface.
BHAG as a cause of neurological symptoms
A cause to effect relation between BHAG and neurological symptoms is difficult to ascertain in both the present study and previous reported cases, with the majority of presenting conditions being unrelated to BHAG (see Table 1).
In the three patients with PTCS of our series, venous outflow obstruction from stenosis caused by the BHAG could have contributed to raising CSF pressure. Two of these patients evolved favorably under conservative medical treatment; therefore, no TS stenting was contemplated and no TS pressure measurements were performed. The hemodynamic significance of the stenosis was, therefore, not quantified. The third case of PTCS was recently diagnosed and has not yet returned for follow-up. Whether the BHAG were the cause of the PTCS or the result of raised CSF pressure is impossible to determine in the absence of imaging studies prior to the development of PTCS. BHAG into the TS could, however, contribute to the PTCS by causing a venous outflow obstruction in both situations. BHAG into the TS from CSF hypertension could accentuate the stenosis and further raise the venous pressure, thus perpetuating and worsening a pre-existing PTCS. The question of whether BHAG into a dural venous sinus is a contraindication to stenting in cases of refractory PTCS with pressure gradient across the stenosis must be raised. Compression of viable HB by the stent could be of concern in cases where the neck of the AG is narrow.
In the published radiological literature, there is no specific mention of patients with PTCS and venous outflow obstruction caused by BHAG. Battal et al.[26] describe a patient with a medical history of spontaneous CSF leak presumably from a ethmoidal encephalocele, who carried two TS temporal lobe BHAG, one sigmoid sinus left cerebellar hemisphere BHAG and bilaterally hypoplastic transverse sinuses; though not specifically mentioned, PTCS would be a reasonable assumption in this case.
A relation with headaches is suggested by Battal et al. [29] as patients with headaches in their series of BHAG were higher than the general population, though this difference did not reach statistical significance. Coban et al.[27] describe a case of a 31-year-old patient with a 5-day history of dizziness, imbalance, and pressure sensation in the left ear and suggested that symptoms were caused by a left TS BHAG from the inferior temporal lobe. There is no mention of how the patient evolved clinically. A cause to effect relation is therefore difficult to establish for this case.
Finally, SSCG were frequently observed in the present study, but none involved frontal, temporal, or occipital lobe BH, all SSCG being exclusively found in the cerebellum. Supratentorial BH with SSCG could theoretically cause seizures. Indeed, temporal lobe encephaloceles have been shown to present with seizures as reported by Saavalainen et al. [33] in a series of 23 patients of which 12 underwent surgery. Histopathological examination of the resected herniated brain showed gliosis in all cases and mild cortical laminar disorganization in 42 %. These changes could, when imaged, correspond to SSCG observed on MRI in our study. One could posit that SSCG are necessary for BH to cause seizures, with the reserve that strangulated herniated brain in an AG could be sufficient to induce seizures and that BH without SSCG on imaging findings does not exclude minor gliotic changes and mild cortical laminar disorganization.
Clinical conditions leading to the formation of BHAG
In the present series, four patients had imaging findings of PTCS (three with clinical signs of PTCS, one without) corresponding to 10.5 % of the cases. A 10.5 % incidence of PTCS/“burnt out PTCS” in the present series is significantly higher (P value <0.0001) than the 0.9/100,000 annual incidence of PTCS in the general population [34]. Eight additional patients (21 %) had or had had intracranial conditions potentially causing chronically raised intracranial pressure (Table 1). Put together, 32 % patients (8/38) had or may have had increased intracranial pressure. In the series by Battal et al. [29], 20 % of the patients presented with intracranial mass lesions that could have led to increased intracranial pressure. In the autopsy cases reported by Wolbach and Beneke, all patients had died with signs of or from increased intracranial pressure from various conditions, and presented with multiple BHAG each. Overall, these findings suggest that increased intracranial pressure may bring about the formation of BHAG, though it is not a prerequisite. Indeed, given that the majority of the patients with BHAG do not have increased intracranial pressure, the chronic effect of CSF pulsations and pia-arachnoid bridges described above may generally suffice for BHAG to develop.
Limitations of the present study
The present study carries limitations inherent to all retrospective studies. Discrepancies exist between our study and previously reported cases, mainly concerning the location of BHAG, the origin of the BH, and the presence of SSCG that have not previously been reported. BHAG locations not reported in the study may have been missed given the setup of the study (keyword search).
Conclusion
BHAG represent a form of acquired meningo-encephaloceles. They occur, by order of frequency, in the OS, the TS, the LLSSS, and the SS, though other locations have been described in the literature. When located in a dural venous sinus, BHAG must be distinguished from thrombosis or other meningeal base processes such as meningioma. SSCG of the herniated cerebellum (but not of the temporal lobes) were frequent findings and possibly result from tethering and/or strangulation of the cerebellar tissue in the AG. No clinical symptoms could be clearly attributed to BHAG, though in our cases of venous PTCS, TS BHAG could have contributed to sustaining the raised CSF pressure. Various factors are likely involved in the development of BHAG, including normal pia-arachnoid bridges between the brain surface and the AG and hydrodynamic constrains on the brain and AG. Raised intracranial pressure may favor the development of BHAG. Scrutiny for imaging and clinical signs and conditions leading to raised intracranial/CSF pressure is, therefore, recommended when BHAG is discovered.
Abbreviations
- AG:
-
Arachnoid granulation
- BH:
-
Brain herniation
- BHAG:
-
Brain herniation into an AG
- ITG:
-
Inferior temporal gyrus
- ISL:
-
Inferior semilunar lobule
- L:
-
Left
- LLSSS:
-
Lateral lacunae of the SSS
- LOTG:
-
Lateral occipito-temporal gyrus
- NC-CT:
-
Non-contrast CT
- OS:
-
Occipital squama
- PC-CT:
-
Post-contrast CT
- PTCS:
-
Pseudotumor cerebri syndrome
- R:
-
Right
- SiS:
-
Sigmoid sinus
- SS:
-
Straight sinus
- SSCG:
-
Signal and structural changes
- SSL:
-
Superior semilunar lobule
- SSS:
-
Superior sagittal sinus
- TS:
-
Transverse sinus
References
Faivre EJ (1853) Les granulations méningiennes. Ecole de Médecine de Paris, Paris
Trolard P (1892) Les granulations de Pacchioni. Les lacunes veineuses de la dure-mère. J de l’anatomie et de la physiologie normales et pathologiques de l’homme et des animaux 28:28–57/172–210
Turner L (1961) The structure of arachnoid granulations with observations on their physiological and pathological significance. Ann R Coll Surg Engl 29:237–264
Basmajian JV (1952) The depressions for the arachnoid granulations as a criterion of age. Anat Rec 112(4):843–846
Le Gros Clark WE (1920) On the pacchionian bodies. J Anat 55:40–48
Trolard P (1890) Les veines méningées moyennes. Rev Sci Biol 485–499
Wolbach SB (1908) Multiple hernias of the cerebrum and cerebellum, due to intracranial pressure. J Med Res 19(1):153–174 157
Cooper ER (1958) Arachnoid granulations in man. Acta Anat (Basel) 34(3):187–200
Cooper ER (1960) Further studies of arachnoid granulations in man. Acta Anat (Basel) 42:88–104
Gailloud P, Muster M, Khaw N, Martin JB, Murphy KJ, Fasel JH, Rufenacht DA (2001) Anatomic relationship between arachnoid granulations in the transverse sinus and the termination of the vein of Labbe: an angiographic study. Neuroradiology 43(2):139–143
Grzybowski DM, Herderick EE, Kapoor KG, Holman DW, Katz SE (2007) Human arachnoid granulations part I: a technique for quantifying area and distribution on the superior surface of the cerebral cortex. Cerebrospinal Fluid Res 4:6. doi:10.1186/1743-8454-4-6
Grossman CB, Potts DG (1974) Arachnoid granulations: radiology and anatomy. Radiology 113(1):95–100. doi:10.1148/113.1.95
Branan R, Wilson CB (1976) Arachnoid granulations simulating osteolytic lesions of the calvarium. AJR Am J Roentgenol 127(3):523–525. doi:10.2214/ajr.127.3.523
Rosenberg AE, O’Connell JX, Ojemann RG, Plata MJ, Palmer WE (1993) Giant cystic arachnoid granulations: a rare cause of lytic skull lesions. Hum Pathol 24(4):438–441
Esposito G, Della Pepa GM, Sturiale CL, Gaudino S, Anile C, Pompucci A (2011) Hypertrophic arachnoid granulation of the occipital bone: neuroradiological differential diagnosis. Clin Neuroradiol 21(4):239–243. doi:10.1007/s00062-011-0059-4
Tural Emon S, Orakdogen M, Akpinar E, Hakan T, Zafer Berkman M (2012) Arachnoid granulations: a rare cause of lytic occipital bone lesion. Neurol Neurochir Pol 46(6):603–606
Alonso RC, de la Pena MJ, Caicoya AG, Rodriguez MR, Moreno EA, de Vega Fernandez VM (2013) Spontaneous skull base meningoencephaloceles and cerebrospinal fluid fistulas. Radiographics 33(2):553–570. doi:10.1148/rg.332125028
Connor SE (2010) Imaging of skull-base cephalocoeles and cerebrospinal fluid leaks. Clin Radiol 65(10):832–841. doi:10.1016/j.crad.2010.05.002
San Millan D, Kohler R (2014) Enlarged CSF spaces in pseudotumor cerebri. AJR Am J Roentgenol 203(4):W457–W458. doi:10.2214/AJR.14.12787
Owler BK, Parker G, Halmagyi GM, Johnston IH, Besser M, Pickard JD, Higgins JN (2005) Cranial venous outflow obstruction and pseudotumor cerebri syndrome. Adv Tech Stand Neurosurg 30:107–174
Kollar C, Johnston I, Parker G, Harper C (1998) Dural arteriovenous fistula in association with heterotopic brain nodule in the transverse sinus. AJNR Am J Neuroradiol 19(6):1126–1128
Liang L, Korogi Y, Sugahara T, Ikushima I, Shigematsu Y, Takahashi M, Provenzale JM (2002) Normal structures in the intracranial dural sinuses: delineation with 3D contrast-enhanced magnetization prepared rapid acquisition gradient-echo imaging sequence. AJNR Am J Neuroradiol 23(10):1739–1746
Karatag O, Cosar M, Kizildag B, Sen HM (2013) Dural sinus filling defect: intrasigmoid encephalocele. BMJ Case Rep. doi:10.1136/bcr-2013-201616
Chan WC, Lai V, Wong YC, Poon WL (2011) Focal brain herniation into giant arachnoid granulation: a rare occurrence. Eur Radiol Radiol Extra 78:e111–e113
Kocyigit A, Herek D, Balci YI (2015) Focal herniation of cerebral parenchyma into transverse sinus. J Neuroradiol 42(2):126–127. doi:10.1016/j.neurad.2014.05.009
Battal B, Castillo M (2014) Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J 27(1):55–62
Coban G, Yildirim E, Horasanli B, Cifci BE, Agildere M (2013) Unusual cause of dizziness: occult temporal lobe encephalocele into transverse sinus. Clin Neurol Neurosurg 115(9):1911–1913. doi:10.1016/j.clineuro.2013.05.032
Asadi H, Morokoff A, Gaillard F (2015) Occult temporal lobe encephalocoele into the transverse sinus. J Clin Neurosci 22(7):1202–1204. doi:10.1016/j.jocn.2015.01.020
Battal B, Hamcan S, Akgun V, Sari S, Oz O, Tasar M, Castillo M (2015) Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. doi:10.1007/s00330-015-3959-x
Tokiguchi S, Kurashima A, Ito J, Takahashi H, Shimbo Y (1988) Fat in the dural sinus—CT and anatomical correlations. Neuroradiology 30(1):78–80
Browder J, Browder A, Kaplan HA (1972) Benign tumors of the cerebral dural sinuses. J Neurosurg 37(5):576–579. doi:10.3171/jns.1972.37.5.0576
Trolard P (1890) De quelques particularités de la dure mère. Journal de l’anatomie et de la physiologie normales et pathologiques de l’homme et des animaux 26:407–418
Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A (2015) Temporal anteroinferior encephalocele: an underrecognized etiology of temporal lobe epilepsy? Neurology 85(17):1467–1474. doi:10.1212/WNL.0000000000002062
Durcan FJ, Corbett JJ, Wall M (1988) The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol 45(8):875–877
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that while all patients gave informed consent for imaging studies, due to the retrospective nature of this work, Institutional Review Board approval was not required.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Malekzadehlashkariani, S., Wanke, I., Rüfenacht, D.A. et al. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology 58, 443–457 (2016). https://doi.org/10.1007/s00234-016-1662-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1662-5