Abstract
Introduction
Flow diversion is being increasingly used to treat cerebral aneurysms. We present our experience using these stents to treat aneurysms distal to the circle of Willis with parent arteries smaller than 2.5 mm.
Methods
Aneurysms treated with a Pipeline™ Embolization Device in vessels less than 2.5 mm between June 2012 and August 2014 were included. We evaluated risk factors, family history of aneurysms, aneurysm characteristics, National Institute of Health Stroke Scale (NIHSS), and modified Rankin scale (mRS) on admission and angiography and clinical outcome at discharge, 6 months, and 1 year.
Results
We included seven patients with a mean age of 65 years. The parent vessel size ranged from 1.5 to 2.3 mm; mean 1.9 mm. Location of the aneurysms was as follows: two aneurysms centered along the pericallosal artery (one left, one right), one on the right angular artery, one aneurysm at the anterior communicating artery (ACom), one at the ACom-right A2 anterior cerebral artery (ACA), one at the lenticulostriate artery, and one at the A1-A2 ACA artery. Aneurysms ranged from 1 to 12 mm in diameter. All aneurysms were treated with a single Pipeline™ Embolization Device (PED). No peri- or post-procedural complications or mortality occurred. The patients were discharged with no change in NIHSS or mRS score. Angiographic follow-up was available in six patients. Angiography showed complete aneurysm occlusion in all. NIHSS and mRS remained unchanged at follow-up.
Conclusion
Our preliminary results show that flow diversion technology is an effective and safe therapy for aneurysms located on small cerebral arteries. Larger studies with long-term follow-up are needed to validate our promising results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Pipeline™ Embolization Device (PED; Ev3 Neurovascular, Irvine CA, USA) is currently approved in the United States for large or giant unruptured aneurysms in the internal carotid artery (ICA) from petrous to superior hypophyseal segments [1]. There are many case series and reports on the use of PED for off-label aneurysm sizes and locations [2–11]. However, its specific use in cerebral vessels smaller than 2.5 mm has not been fully investigated. We present seven cases of endovascular aneurysm treatment using a PED in the small cerebral vessels and discuss the feasibility, safety, and effectiveness of this treatment.
Materials and methods
Our hospital institutional review board approved this retrospective study.
Patients
We retrospectively reviewed all patients treated with PED at our institution from June 2012 to August 2014 and included aneurysms arising from parent arteries having a diameter <2.5 mm. Demographic data, vascular risk factors, and family history were also reviewed. Relevant clinical data included presentation symptoms, smoking, hypertension, past personal history of aneurysmal subarachnoid hemorrhage (SAH), and family history of vascular abnormalities. Baseline neurological status was assessed using the modified Rankin scale (mRS), and this was also captured at discharge and subsequent follow-up. Aneurysm size, location, and parent vessel size were assessed before intervention on working projection from digital subtraction angiography (DSA) and on 3D rotational angiography (RA) reconstructions. Vessel patency, aneurysm occlusion, intimal hyperplasia, and the incidence of thromboembolic events were reviewed after intervention and on follow-up imaging by an independent observer with an experience in Interventional Neuroradiology of 7 years. The imaging modality immediately after intervention and at subsequent follow-up included thin-section cone-beam computed tomography (CT) to evaluate wall apposition and the incidence of intimal hyperplasia. Before treatment, the patient and their family were presented with all therapeutic options regarding the aneurysm treatment, including but not limited to observation, surgical clipping, and various endovascular options. The risks and benefits for all options were discussed as well as the off-label indication of the PED.
Diagnostic angiography and intervention
All interventions were non-emergent; therefore, the patients were started on at least 81 mg of aspirin and 75 mg of clopidogrel for 5 days before the intervention. As per our policy, optimal platelet inhibition was tested using the VerifyNow P2Y12 assay showing at least 50 % inhibition or platelet reactive units (PRUs) less than 200. All patients continued taking clopidogrel for at least 6 months and will remain on lifelong aspirin.
Diagnostic and interventional procedures were performed on a biplane digital angiography unit (Allura Biplane FD20/20, Philips Medical, Best, the Netherlands). All patients underwent treatment under general anesthesia under full heparin. An 8-Fr-long femoral sheath was placed in the right femoral artery. A 6-Fr shuttle sheath (Cook, Bloomington, IN, USA) was placed in the ipsilateral internal carotid artery. In all cases, a slow intra-arterial injection of 10 mg of verapamil was administered via the diagnostic catheter to prophylactically prevent vasospasm. Using a triaxial system, an intermediate catheter, Navien™ 058 guide catheter (Covidien, Plymouth, MN, USA) or Penumbra 5 MAX™ DDC guide catheter (Penumbra, Alameda, CA, USA), was navigated over an Excelsior® XT27 microcatheter (Boston Scientific, Natick, MA, USA) to the petrous or cavernous ICA segments. The Excelsior XT27 was navigated over 0.014-in. microwire in the target artery beyond the aneurysm. The 2.5-mm PED in varying lengths was used for all cases because the diameter of the parent artery was less than 2.5 mm. After the deployment, VasoCT was performed to identify PED wall apposition and Xper-CT was also done to assess for intracranial hemorrhage. After the treatment, an 8-Fr Angio-Seal™ closure device (Medtronic Inc., Minneapolis, MN, USA) was used at the arteriotomy puncture site for hemostasis.
Periprocedural technical complication (i.e., any additional maneuver due to misopening or occlusion of the PED) and procedure-related clinical complications (i.e., any new neurological deficit after the treatment related to ischemic stroke and/or intracranial hematoma or groin hematoma) were systematically assessed.
Control DSA was obtained immediately after placement of the device and at 6 months and 1 year. Additionally, a 3-month control angiogram was available in one patient as the intervention was recent. Contrast-enhanced (dilution 20 % Omnipaque 240) cone-beam CT was done after placement of the device and on follow-ups to ascertain the wall apposition and intimal hyperplasia. The aneurysm occlusion status, vessel patency, and luminal narrowing of the parent artery were assessed by a single neurointerventionalist not performing the treatment on immediate post-intervention and follow-up angiograms.
Results
Patient characteristics
A total of seven procedures in seven patients met our criteria (six females, 86 %). The mean age was 65 years (range from 51 to 71 years). Vascular risk factors included hypertension (86 %), smoking (14 %), and hyperlipidemia (42 %). In one patient (14 %), there were no vascular risk factors. In two patients (28 %), more than one cerebral aneurysm was present, and in three patients (42 %), there was a prior subarachnoid hemorrhage (SAH) from the aneurysm treated. (Table 1: Patient data and aneurysm characteristics).
Aneurysm characteristics
All aneurysms were located on the anterior circulation, with two pericallosal (one left and one right), one lenticulostriate artery, one at the anterior communicating artery, one at the angular branch of the right middle cerebral artery (MCA), one at the anterior communicating artery (ACom)-A2 junction, and one fusiform aneurysm at the right A1-A2 anterior cerebral artery (ACA). The parent vessel sizes varied from 1.5 to 2.3 mm (mean 1.9 mm). The aneurysm (or circulating portion in recanalized aneurysms) sizes varied from 1 to 12 mm (mean 3.9 mm). In four patients, the aneurysms of interest were identified as an incidental finding. In the remaining three patients, the target aneurysm had recanalized after a post-rupture endovascular coiling.
Procedure
All seven procedures were performed successfully. Deployment of PED in the parent vessels <2.5 mm had 86 % technical success on the first attempt. In one case (patient #6), the device was “corked” and removed due to migration of the distal aspect of the device. A second device was delivered without complication to the target location. Another periprocedural technical complication was recorded (patient #7), consisting in the occlusion of the contralateral ACA in an ACom aneurysm associated with occlusion of a cortical branch covered by the PED. This complication was satisfactorily managed by IV injection of Integrilin and verapamil, with complete reopening of the occluded vessels and no clinical consequence.
In our series, the PED was deployed using a modified technique with specific emphasis to control the distal placement of the delivery wire. In all cases, the strategy was to advance the delivery system and device at least 15 mm distal to the preferred landing site beyond the aneurysm neck. The PED was then only unsheathed from the microcatheter, without using the regular “pull and wag” technique, due to the risk of small branch perforation with the distal wire. Indeed, this maneuver kept the distal wire stationary, minimizing the risk of vascular injury. After complete deployment, the microcatheter was not advanced back into the implant in order to prevent any motion of the implant and also to prevent any motion of the distal recapture wire in the small vessel. The recapture coil was gently withdrawn back under rotatory motion under magnified, high-rate fluoroscopy, and special attention was paid to confirm the edge of the recapture coil did not get caught on the distal edge of the implant.
All post-procedural cone-beam CTs showed good wall apposition. The parent artery and all side branches covered by the PED were well preserved on the post-procedural angiograms. Groin hematoma was seen in one patient and was managed conservatively.
Angiography results
Immediate post-interventional control angiographies after PED placement showed good flow in the parent vessel as well as in covered branches. No vasospasm was noted in the intracranial circulation related to the device placement. There was flow stasis inside the aneurysm in one patient, significantly reduced flow in one patient, mildly reduced flow in four patients, and no apparent change in flow in one patient.
Six-month and 1-year follow-up angiography was available in two patients (28 %), only 6-month follow-up angiography in two patients (28 %), only 1-year follow-up in one patient (14 %), 3-month follow-up in one patient (14 %), and no follow-up yet in one patient (14 %) (Table 2). The patient with no follow-up was not due for angiography at the time of the manuscript preparation. Follow-up angiography was thus available in six patients (86 %) and showed complete aneurysm occlusion in all patients. No significant intimal hyperplasia was seen. Two patients (28 %) showed mild intimal hyperplasia within the stent. One-year control angiography demonstrated stable occlusion of the aneurysms. The intimal hyperplasia remained stable on the 1-year angiogram in one patient. There was no evidence of in-stent thrombosis/stenosis or distal thromboembolic events (Table 2).
Aspirin and clopidogrel were continued for an additional 6 months in the one case where mild intimal hyperplasia was seen at the 6-month angiography time point. There was no evidence of parent vessel occlusion. There was mild asymptomatic stenosis of a fronto-polar branch of the ACA covered by the PED in one patient with pericallosal artery aneurysm.
Illustrative cases
Case 1
A 71-year-old female had a history of headaches, and workup showed a right posterior cerebral artery (PCA) aneurysm. She underwent stent-assisted coil embolization of this PCA aneurysm. She was also found to have a fusiform aneurysm in M2 inferior division (angular artery) of the right MCA (Fig. 1). The size of the aneurysm was 3.1 mm × 3.6 mm × 3.7 mm with a neck of 3.1 mm. The diameter of the parent artery distal and proximal to the aneurysm was 2.1 and 2.3 mm, respectively. A tiny branch originated from the neck. We successfully navigated and placed a 2.5 mm × 10 mm PED across the neck. There was no evidence of acute thrombus formation/distal emboli or vasospasm. Post-procedural angiogram and VasoCT showed mild flow stagnation within the aneurysmal sac, patency of the vasculature, and good wall apposition. The patient tolerated the procedure very well and was discharged with a mRS score of 0. Follow-up angiogram at 6 months showed no residual aneurysm and patency of angular artery and branches. Mild in-stent stenosis at the proximal portion due to intimal hyperplasia was revealed, and no evidence of ischemic events was recorded during the clinical follow-up. The patient was continued on dual anti-platelet medication for another 6 months. One-year follow-up angiography showed stable aneurysm occlusion and intimal hyperplasia. The patient continued to do well; mRS score remained 0.
Case 2
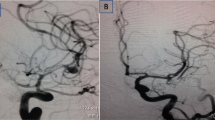
A 71-year-old female presented with a history of subarachnoid hemorrhage secondary to the rupture of an ACom-right A2 ACA junction fusiform aneurysm (11.5 mm × 5.5 mm). Due to the fusiform nature of the aneurysm and the presence of a normal branch arising close to distal aspect of the aneurysm, it was decided to pursue balloon-assisted sub-total coiling for the acute period. A two-month follow-up angiogram showed progressive recanalization of the aneurysm. The patient then underwent PED placement from the right A2 ACA to the right A1 ACA. The size of the residual aneurysm was 6 mm × 6 mm with no appreciable neck due to the fusiform nature of the aneurysm. The right A2 ACA artery distal to the aneurysm neck measured 1.9 mm and proximal 1.8 mm in diameter (Fig. 2). A vascular branch was present arising from the neck of the aneurysm along its distal aspect. We placed a 2.5-mm × 16-mm PED across the neck of the aneurysm successfully. The final angiogram showed flow stagnation within the aneurysm and good patency of the anterior cerebral artery and the branch arising from the aneurysm base. VasoCT also showed good wall apposition. The patient tolerated the procedure very well and was discharged with a mRS score of 0. Follow-up angiogram at 6 months showed no residual aneurysm and patency of the parent artery and branches. The patient continued to do well; mRS score remained 0.
a Angiogram (3D DSA) at the time of acute SAH showed a bilobed ACom-right A2-ACA junction aneurysm with a branch arising from the distal part of the aneurysm (arrow). b Angiogram at the time of the flow diverter treatment showed the residual aneurysm and parent vessel sizes. c Cone-beam CT demonstrating the implant placement. d Follow-up angiography demonstrated no aneurysm filling with vascular remodeling
Case 3
A 69-year-old female had an incidental discovery of a fusiform right A1-A2 ACA aneurysm during workup for vertigo. The aneurysm measured 12 mm × 10 mm × 10 mm with no appreciable neck (Fig. 3). The aneurysm incorporated the distal right A1 and the proximal A2 ACA. In addition, the aneurysm also had a pseudolobule in its proximal aspect and the recurrent artery of Heubner arising from the distal base of the aneurysm. The parent vessel distal to the aneurysm measured, 1.9 mm, and the vessel at the planned proximal landing zone was 2.0 mm. The distal vessel was accessed using a “buddy wire” technique. During deployment of the PED, the distal aspect of the PED retracted more than anticipated, the device was corked, and a new device was then used. We placed a 2.5-mm × 20-mm PED spanning from the right A2-ACA to the right A1-ACA, covering the neck of the aneurysm. The final angiogram showed complete flow stagnation within the aneurysm and good patency of the ACA and the branch arising from the aneurysm base. This flow stagnation was followed over 20 min with angiograms to rule out any hyperacute thrombosis within the aneurysm. The patient tolerated the procedure very well and was discharged with a mRS score of 0. Follow-up angiogram at 3 months showed no residual aneurysm and patency of the parent artery and branches. Minimal intimal hyperplasia was seen within the device. The patient continued to do well; mRS score remained 0. Case of a pericallosal artery aneurysm treated with PED is illustrated in Fig. 4.
a Frontal angiogram showed a large fusiform right A1-A2 ACA aneurysm. The recurrent artery of Heubner was seen close to the aneurysm (black arrows). b 3D DSA demonstrated the artery of Heubner arising from the distal aspect of the aneurysm sac (arrow). Note the presence of a pseudolobule along the proximal portion of the aneurysm sac (double arrows). c Left ICA DSA showing no filing of the aneurysms from the left ICA (ACom non-patent). d Post-implant deployment cone-beam CT showed the aneurysm coverage. Follow-up angiogram (e) and cone-beam CT (f) demonstrated complete aneurysm occlusion with minimal intimal hyperplasia (white arrow). Note the visualization of the recurrent artery of Heubner on control DSA (e, black arrows), which remained patent
Discussion
The Food and Drug Administration has only approved using PED for large aneurysms on the internal carotid artery, so the feasibility, safety, and clinical results of PED for smaller vessels are not known. Herein, we present preliminary evidence in a small patient population of consecutive PED treatments of aneurysms arising from small parent arteries.
Conventional coil embolization is the standard of care in most cerebral aneurysms. Coil embolization can be relatively difficult for distal aneurysms located on a small parent artery. Both balloon- and stent-assisted coil embolizations (SACE) are viable techniques for wide-neck aneurysms; however, both techniques require placing multiple catheters in the parent artery, which may have a higher risk of thromboembolic events in small vessels.
The safety, technical success, and effectiveness of SACE with self-expanding stents in vessels ≤2 mm have been demonstrated [12]. However, SACE requires placing two microcatheters in small distal vessels for the jailing and semi-jailing techniques. Accessing the aneurysm after placing the stent can be difficult in small vessels and small aneurysms after placement of the stent. Additionally, in cases where branches arise from the aneurysm neck/ base, the aneurysm is deliberately under-coiled, which may lead to higher risks of recurrence.
Since the Pipeline™ Embolization Device for Uncoilable or Failed Aneurysms (PUFS) trial [1], many studies have been published regarding the use of flow diverters to treat large and small aneurysms [13–16]. Meta-analysis of flow diversion in more than 1500 aneurysms confirmed the aneurysm occlusion efficacy rates as high as 76 %, with treatment-related mortality and morbidity of 4 and 5 %, respectively [17]. Even if recent studies have focused on the safety and effectiveness of flow diversion on mid-size (<10 mm) [10] and small (<7 mm) [4] intracranial aneurysms, only limited data are available on the feasibility, safety, and effectiveness of flow diversion in aneurysms arising from the small parent artery (<2.5 mm).
Martin et al. have published a case series on the use of small flow diverters (≤3 mm) in 12 patients showing a complete occlusion rate of 75 % at a median follow-up of 18 months and a procedural complication rate of 8 % from an embolic infarct [11]. In this case series from three Canadian centers, the 2.5-mm device was used in five patients. However, the size of the target vessels is not described in the article. In another multi-institutional series of 25 patients, flow diverters were used to treat aneurysms located at the level of the circle of Willis and beyond, with a 64 % occlusion rate at 6 months and an in-stent stenosis rate of 27 % [3]. The authors published a morbidity of 12 % with no mortality. The 2.5-mm implant was used in only three cases in this case series, again without mention of the target vessel sizes. Pistocchi et al. demonstrated the use of flow diverters at or beyond the circle of Willis with an aneurysm occlusion rate of 82 % and a neurological complication rate of 11.1 % [8]. In-stent stenosis was seen in 21.7 % cases, but patients were asymptomatic in all cases. Similarly, Yavuz et al. treated 25 MCA aneurysms in 21 patients with flow diversion. An aneurysm occlusion rate of 84 % was reported in the available follow-ups at the time of publication [2]. Complications were seen in five patients, with no mortality. More recently, few case series have been published on FDS treatment in ACA and MCA aneurysms [3, 18–21] also located on small parent artery. In these series, the occlusion rate varied from 62 to 97.3 % at follow-up; the complication rate was acceptable. The results of our series are very comparable to previously published use of flow diverters in off-label situations. Follow-up was available in six patients (86 %); all showed complete aneurysm occlusion. No mortality or neurological complications were seen. There was one case of groin hematoma, which was managed conservatively. There were two cases of in-stent mild intimal hyperplasia on follow-up (28 %) without any flow limitation. There was one case of asymptomatic stenosis at the origin of a fronto-polar branch after flow diverter placement in the pericallosal artery.
The minimum size of the PED is 2.5 mm in diameter; therefore, when a PED is deployed in a small vessel less than 2 mm, it may be elongated and the stent pores may become larger. This increase in porosity may impair the flow diversion effect and lower the chances of intra-aneurysmal thrombosis. However, in all the patients who underwent control DSA in our study, complete aneurysm occlusion was observed.
The main limitations to our series are the retrospective nature and limited follow-up.
Additionally, control MRI was not systematically performed since this imaging modality is not used in our protocol for embolized aneurysms’ follow-up. Brain MRI is only performed if a symptomatic complication is depicted at neurological examination. Thus, some silent ischemic strokes due to occlusion of perforating branches may have been missed in our series.
Conclusion
Our limited case series shows promising results regarding the use of flow diverters in small intracranial vessels as a feasible, safe, and efficacious mode of aneurysm treatment.
References
Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G et al (2013) Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 267(3):858–68
Yavuz K, Geyik S, Saatci I, Cekirge HS (2014) Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the pipeline embolization device. AJNR Am J Neuroradiol 35(3):529–35
Martinez-Galdamez M, Romance A, Vega P, Vega A, Caniego JL, Paul L, et al (2014) Pipeline endovascular device for the treatment of intracranial aneurysms at the level of the circle of Willis and beyond: multicenter experience. J Neurointervent Surg.
Chalouhi N, Zanaty M, Whiting A, Yang S, Tjoumakaris S, Hasan D, et al (2015) Safety and efficacy of the Pipeline Embolization Device in 100 small intracranial aneurysms. J Neurosurg. 1–5
Mitchell B, Momin E, Jou LD, Shaltoni H, Morsi H, Mawad M (2014) Extensive bilateral vertebral artery remodeling following treatment of dissection using pipeline embolic device. J Vasc Interv Neurol 7(5):5–8
Cinar C, Oran I, Bozkaya H, Ozgiray E (2013) Endovascular treatment of ruptured blister-like aneurysms with special reference to the flow-diverting strategy. Neuroradiology 55(4):441–7
Chalouhi N, Zanaty M, Tjoumakaris S, Gonzalez LF, Hasan D, Kung D et al (2014) Treatment of blister-like aneurysms with the pipeline embolization device. Neurosurgery 74(5):527–32, discussion 32
Pistocchi S, Blanc R, Bartolini B, Piotin M (2012) Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke 43(4):1032–8
Amenta PS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH et al (2012) Successful treatment of a traumatic carotid pseudoaneurysm with the Pipeline stent: case report and review of the literature. Surg Neurol Int 3:160
Lin LM, Colby GP, Kim JE, Huang J, Tamargo RJ, Coon AL (2013) Immediate and follow-up results for 44 consecutive cases of small (<10 mm) internal carotid artery aneurysms treated with the pipeline embolization device. Surg Neurol Int 4:114
Martin AR, Cruz JP, O’Kelly C, Kelly M, Spears J, Marotta TR (2014) Small pipes: preliminary experience with 3-mm or smaller pipeline flow-diverting stents for aneurysm repair prior to regulatory approval. AJNR Am J Neuroradiol.
Turk AS, Niemann DB, Ahmed A, Aagaard-Kienitz B (2007) Use of self-expanding stents in distal small cerebral vessels. AJNR Am J Neuroradiol 28(3):533–6
Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J et al (2012) Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 33(6):1150–5
Chalouhi N, Zanaty M, Whiting A, Yang S, Tjoumakaris S, Hasan D et al (2015) Safety and efficacy of the Pipeline Embolization Device in 100 small intracranial aneurysms. J Neurosurg 122(6):1498–502
Chalouhi N, Starke RM, Yang S, Bovenzi CD, Tjoumakaris S, Hasan D et al (2014) Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke 45(1):54–8
Wakhloo AK, Lylyk P, de Vries J, Taschner C, Lundquist J, Biondi A et al (2015) Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol 36(1):98–107
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF (2013) Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44(2):442–7
Clarencon F, Di Maria F, Gabrieli J, Shotar E, Zeghal C, Nouet A, et al (2015) Flow diverter stents for the treatment of anterior cerebral artery aneurysms: safety and effectiveness. Clin Neuroradiol.
Gawlitza M, Januel AC, Tall P, Bonneville F, Cognard C (2015) Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: a single-center series with special emphasis on covered cortical branches and perforating arteries. J Neurointervent Surg.
Saleme S, Iosif C, Ponomarjova S, Mendes G, Camilleri Y, Caire F et al (2014) Flow-diverting stents for intracranial bifurcation aneurysm treatment. Neurosurgery 75(6):623–31, quiz 31
Caroff J, Neki H, Mihalea C, D’Argento F, Abdel Khalek H, Ikka L, et al (2015) Flow-diverter stents for the treatment of saccular middle cerebral artery bifurcation aneurysms. AJNR Am J Neuroradiol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the Institutional Review Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
ASP consults for Codman Neurovascular and Covidien and has research grants from Stryker Neurovascular and Covidien. AKW consults for Stryker Neurovascular, has a research grant from Philips Healthcare and the Wyss Institute and speaks for the Harvard Postgraduate Course and Miami Cardiovascular Institute. MJG consults for Codman Neurovascular and Stryker Neurovascular and has research grants from the NIH, eV3/Covidien Neurovascular, Codman Neurovascular, Fraunhofer Institute, the Wyss Institute, Philips Healthcare, Stryker Neurovascular, Silk Road and Lazarus-Effect. PK consults for Stryker Neurovascular, Covidien and MicroVention. FC consults for Covidien and Codman Neurovascular.
Rights and permissions
About this article
Cite this article
Puri, A.S., Massari, F., Asai, T. et al. Safety, efficacy, and short-term follow-up of the use of Pipeline™ Embolization Device in small (<2.5mm) cerebral vessels for aneurysm treatment: single institution experience. Neuroradiology 58, 267–275 (2016). https://doi.org/10.1007/s00234-015-1630-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1630-5