Abstract
Introduction
The aim of the study is to assess the effect of shape, diameter, elongation and deviation criteria of basilar artery (BA), convergence angle and diameter variations of vertebral arteries, and concurrent chronic diseases on posterior circulation infarcts.
Methods
Between January 2010 and May 2013, 186 patients who underwent brain and diffusion magnetic resonance imaging (MRI) with suspected cerebrovascular accident and were diagnosed with posterior circulation infarct and 120 infarct negative control subjects were included in this case-control retrospective study. Vertebral artery (VA) and BA diameter, right (R) and left (L) VA angles at the level of bifurcation, and BA elongation-deviation, and shape of BA were assessed in a total of 306 subjects. Ischemic lesions in the posterior circulation were classified according to their anatomical location and vascular perfusion areas.
Results
No significant difference was noted between the control and patient groups with respect to BA diameter (p = 0.676). The most effective risk factors for posterior circulation infarcts were as follows: BA elongation of 2 or 3, BA transverse location of 2 or 3, increase in left VA angle, and history of hypertension, hypercholesterolemia, and diabetes mellitus.
Conclusion
Our results suggest that prominent elongation and deviation, C and J shape of BA, and increased L VA angle may be the predictors of at-risk patients in posterior circulation infarcts. Reporting marked morphological BA and VA variations detected at routine brain MRI will aid in selection of patients. Timely detection and treatment of at-risk patients may be life-saving.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term dolichoectasia is used for dilated, tortuous, and elongated arteries. Dilatation is the most striking feature and, therefore, it is also called dilatative arteriopathy. Dilatative arteriopathy is most commonly observed in intracranial vertebral and basilar arteries [1–3]. Some studies have observed that risk of ischemic symptom development is higher in cases with basilar artery ectasia, and those with tortuous and elongated basilar arteries may develop cranial nerve palsy [4–6].
The causes of posterior circulation infarcts are small vessel diseases in 15 %, cardiac embolism in 13 %, and multifactorial (arterial stenosis and/or occlusion, lacunar lesions, and cardiac embolism) in 13 % while a potential cause cannot be detected in 10 % [7]. The affected area of posterior circulation is brainstem in 60 %, cerebellum in 50 %; the infarcts are of basilar and/or vertebral artery origin in more than 50 % of patients [8].
Elongation and angulation of intracranial arteries cause reduced blood flow as a result of tension and distortion of arterial branches, especially penetrating branches of large arteries. Pontine infarcts at the territory of basilar artery (BA) are the best example [9, 10]. It has been reported that posterior and anterior circulation infarcts do not differ from each other with regard to risk factors but they have distinct symptomatologies as a result of involvement of different anatomical regions [11]. However, the most prominent difference is that arteries of the posterior circulation have a vertical course, while arteries of the anterior circulation have a horizontal course. This difference may explain why more than one infarct focus is observed in posterior circulation infarcts as compared to their anterior counterparts.
In clinically important pathologies of posterior circulation, timely detection and treatment of at-risk patients will be life-saving. This work will assess the effect of shape, diameter, elongation and deviation criteria of basilar artery, convergence angle and diameter variations of vertebral arteries, and concurrent chronic diseases in posterior circulation infarcts.
Materials and methods
Following approval by the Institutional Review Board of our institution, brain magnetic resonance images of 3120 patients who underwent brain and diffusion magnetic resonance imaging (MRI) with suspected cerebrovascular accident between January 2010 and May 2013 were accessed from our hospital’s picture archiving and communication system (PACS) and evaluated retrospectively. Four hundred eighteen of 3120 patients found to have a posterior circulation infarct were selected for analysis. Patients with a cardiac pathology with a possible causal relationship with infarct (e.g., atrial fibrillation, heart failure, aortic or mitral stenosis, etc.), and those with carotid, vertebral, or basilar artery stenosis or occlusion detected at digital subtraction angiography (DSA), computed tomography (CT) angiography, magnetic resonance angiography (MRA), or Doppler ultrasonography (DUSG), and with any infarct zone at the territory of internal carotid artery were excluded from the study. One hundred and eighty-six of 418 patients with an isolated posterior circulation infarct positive (PCIP) were included in this case-control retrospective study. One hundred twenty age and sex-matched having no similar posterior circulation infarct individuals (having no anterior and posterior circulation infarcts (PCIN)) were included as control group. Sixty of 120 PCIN control subjects were referred to MRI for headache, and 17 of them were evaluated for dizziness, also 43 of them had no particular health problem. Vertebral artery (VA) and BA diameter, right and left VA angles at the level of bifurcation, and elongation-deviation, and shape of BA were assessed in a total of 306 patients (120 infarct negative control group and 186 infarct positive patient group).
The brain MRI was performed in a routine supine natural position using a 1.5-Tesla scanner (Intera, Gyroscan, Philips Medical Systems, The Netherlands). All images were taken according to a standard protocol using axial T2-weighted turbo spin echo (repetition time/echo time (TR/TE): 4466/100, slice thickness (ST) 5 mm, number of excitation (NEX) 3), coronal and sagittal T2-weighted turbo spin echo (TR/TE: 4800/100, ST: 4 mm, NEX: 3), axial fluid-attenuated inversion recovery (TR/TE: 6000/100, ST: 5 mm, NEX: 3), axial T1-weighted spin echo sequences (TR/TE: 462/11, ST: 5 mm, NEX: 3) covering the whole brain. Echo-planar images were obtained with diffusion gradients in the x, y, z planes at b values of 0, 500, 1000 s/mm2 (TR/TE: 3130/74, ST: 5 mm, NEX: 3). Apparent diffusion coefficient (ADC) maps were automatically created. Brain MRI was evaluated, and measurements were obtained by a radiologist (GÇ, with over 4 years of experience reading brain MRI). Image analysis was performed blinded to the grouping of the patients. On axial T2-weighted brain magnetic resonance images, the diameter of the intracranial part of the vertebral arteries, diameter of BA, basilar bifurcation height (elongation), transverse position of BA (deviation), and shape of BA were evaluated. The right (R) and left (L) VA angles were measured on the angiography and coronal T2-weighted images at the level of the bifurcation (Fig. 1). Shape of BA was classified as normal (N), C, S, and J. Basilar and vertebral artery diameters were measured as in millimeter (mm) at their widest point from axial T2-weighted images. Based on the study by Smoker et al. [12, 13], the elongation of the BA was divided into three groups as mild (1; within the suprasellar cistern), moderate (2; at level of third ventricle floor), and severe (3; indenting and elevating the floor of the third ventricle). Also, deviation criteria was classified as severe (3; in cerebellopontine angle cistern), moderate (2; lateral to lateral margin of clivus or dorsum sellae), and mild (1; medial to lateral margin of clivus or dorsum sellae).
Based on the work by Voetsch et al. [14], ischemic lesions at the posterior circulation in the patient group were classified according to proximal, middle, and distal infarct zone. Combined effects of all possible risk factors for infarct were assessed with multivariate logistic regression analysis.
Statistical analysis
Data analysis was performed with SPSS Windows 11.5 software package (SPSS ver. 11.5, SPSS Inc., Chicago, IL, USA). Normality of continuous variables was tested using Kolmogorov–Smirnov test. Descriptive statistics were presented as mean ± standard deviation (SD) and median (minimum-maximum) for continuous variables while categoric variables were presented as number of cases and (%).
The significance of the difference between the groups with respect to mean and median values was tested with Student’s t test and Mann Whitney U test, respectively. Categoric variables were analyzed with Pearson’s chi-square, Fisher’s exact chi-square, or likelihood ratio test.
A multivariate logistic regression analysis was used to determine the factor(s) most effective for distinguishing patient and control groups. Variables with a p < 0.25 at univariate test statistics were included in the regression model as candidate risk factors. In addition, odds ratio and 95 % confidence interval were calculated for each variable.
A p value less than 0.05 was considered statistically significant.
Results
A total of 186 PCIP patients having no occlusion or prominent stenosis in basilar and vertebral arteries (mean age 67.9 (SD ± 11) years, 78 females (41.9 %), 108 males (58.1 %)) were included. Posterior circulation infarct negative (PCIN) group included 120 cases with a mean age of 68.8 (SD ± 12.2) years, of whom 63 were female (52.5 %) and 57 were male (47.5 %). No significant difference was noted between both groups with respect to mean age (p = 0.749) and sex distribution (p = 0.085).
Posterior circulation infarct positive group had a significantly higher rate of chronic diseases including hypertension (HT), hypercholesterolemia, and diabetes mellitus (DM) (p < 0.05). The demographic data of PCIN and PCIP groups are given on Table 1.
There were no significant differences between the groups with respect to median R VA diameter, L VA diameter, and R VA angle (p > 0.05). Compared to the control group, the patient group had a significantly higher median L VA angle (p = 0.018). Vascular morphological characteristics of PCIN and PCIP groups are given in detail on Table 2.
Median basilar artery diameter was 2.75 mm in the PCIN group and 2.8 mm in the PCIP group. No significant difference was present between both groups with regard to median BA diameter (p = 0.158).
The ratio of subjects with an N-shaped BA shape was significantly lower (p < 0.001) whereas the ratio of those with a J and C-shaped BA was significantly higher (p < 0.05) in the PCIP group. The two groups were not different with respect to the ratio of subjects with an S-shaped BA (p > 0.05).
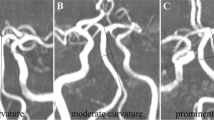
Classification according to elongation criteria in the PCIP group revealed that the ratio of subjects with a BA elongation of 1 was significantly lower (p < 0.001), whereas the ratio of subjects with a BA elongation of 2 or 3 was significantly higher (p < 0.001 vs p = 0.002) (Fig. 2). Classification based on the deviation criteria in the PCIP group showed that the ratio of subjects with a BA transverse location of 1 was significantly lower (p < 0.001), while the ratio of subjects with a transverse location of 2 or 3 was significantly higher (p < 0.001) (Fig. 2). Representative images of patients with posterior circulation infarcts are shown in Figs. 3 and 4.
a–d shows MR images in a 57-year-old female with acute pons infarct. On axial b-1000 (a), ADC maps (b), and FLAIR (c) images, there is focal acute pons infarct. On 3-D reformatted image of the posterior circulation (d), right VA (thin white arrow) shows prominent tortuosity compared with left side (highlighted white arrow), there is C-shaped BA and BA showing prominent deviation and elongation (thick white arrow)
Combined effects of all possible risk factors for infarct were assessed with multivariate logistic regression analysis. After correction for other risk factors, factors most effective on infarct development were a BA elongation of 2 or 3 followed by a BA transverse location of 2 or 3, an increased L VA angle, and history of HT, DM, and hypercholesterolemia. Odds ratios and 95 % confidence intervals of all possible risk factors for infarct are given on Table 3.
In our study, the distribution of subjects according to location of infarct zone at the posterior circulation was as follows: middle in 108 (58.1 %) patients, proximal in 70 (37.6 %) patients, and distal 54 (29 %) in patients. Also, the distribution according to topographical localization was in pons in 56 (30.1 %), in cerebellum in 111 (59.7 %), in occipital lobe in 36 (19.3 %), in medulla oblongata in 6 (3.2 %), in mesencephalon in 6 (3.2 %) of 186 patients.
No significant difference existed between the subjects with a proximal infarct location and those with a middle or distal infarct location with respect to chronic disease, BA diameter, shape, elongation, and transverse location, R VA diameter, L VA diameter, R VA angle, and L VA angle (p > 0.05).
Evaluation of segments of basilar artery in 186 patients with posterior circulation infarct did not yield any signs of occlusion or significant stenosis in the entire patient group.
Discussion
Our study demonstrated that factors effective on posterior circulation infarct development was, in descending order, a BA height (elongation) of 2 or 3, a BA transverse location (deviation) of 2 or 3, and an increase in left VA angle. However, there were no significant differences between the groups with respect to diameter measurements that have been suggested to be the most important criteria for dolichoectasia as mentioned in previous studies [15–17]. Previous studies demonstrated that chronic diseases such as HT, hypercholesterolemia, and DM are the well-known risk factors for posterior circulation infarcts [15, 18]; we found similar findings in our study (Tables 1 and 3).
One patient who has a fenestration in the proximal basilar trunk of the BA is excluded from the study. There has also been speculation about associations between vertebrobasilar artery fenestrations and brainstem ischemia, or infarctions, although their relationships are controversial [16].
The term basilar artery dolichoectasia defines elongation, tortuosity, and dilatation of basilar arteries. Thus, it is also called dilatative arteriopathy. A basilar arterial diameter more than 4.5 mm is accepted as ectasia. Smoker et al. defined dolichoectasia criteria with semi-quantitative assessment with CT [12, 13]. No standard and widely accepted dolichoectasia criteria are present with MRI and MRA examinations, and some difficulties exist with routine MRI reporting. A cohort study by Ubogu et al. showed that a vertebrobasilar artery diameter more than 4.5 mm, a deviation more than 10 mm, and a length more than 29.5 mm were independent risk factors for transient or permanent posterior circulation deficits [17].
In our study, the median basilar artery diameter was 2.8 mm in the PCIP group and it was not different from the control group. This size is similar to that found in the study by Keyik et al. [19] but smaller than those found by many other studies [17, 20].
Comparison of the groups with respect to shape of basilar artery revealed that the ratio of patients in the PCIP group with an N-shaped BA was significantly lower (p < 0.001), while the ratio of those with a J and C-shaped BA was significantly higher (p < 0.05). Evaluation of data about elongation, deviation, and shape all together suggest that dolichoectasia criteria other than diameter had an undeniable effect on infarcts originating from the middle segment of basilar artery. Elongation and angulation of intracranial arteries cause reduced blood flow as a result of tension and distortion of arterial branches, especially penetrating branches of large arteries. Pontine infarcts at the territory of basilar artery are the best example [9, 10]. Blood flow in dilated arteries in a to-and-fro fashion causes reduced antegrade blood flow [21, 22]. Reduced antegrade blood flow leads to blood stagnation in the dilated arterial segment and thrombus formation. Luminal thrombosis occludes arterial branches and causes distal embolism [23, 24]. These events culminate in persistent or transient brain ischemia.
In the PCIP group, a BA elongation or deviation grade of 2 or 3 had the strongest effect on infarct development. They were followed by an increased L VA angle. In the PCIP group, 154 (82.8 %) patients were diagnosed to have a codominance in VA, 5 (2.7 %) had R VA dominance, and 27 (14.5 %) had L VA dominance. These results may explain the role of the increased left VA angle in infarct development in the posterior circulation territory.
Voetsch et al. [14] found that the infarct was located in middle perfusion area in 75 %, in proximal perfusion area in 30 %, and in distal perfusion area in 50 % of patients. In our study, the distribution according to infarct zone by posterior circulation territory was 108 (58.1 %) middle, 70 (37.6 %) proximal, and 54 (29 %) distal perfusion areas. This variation was attributed to exclusion of patients with stenosis/occlusion of BA or VA and cardiac pathology.
Devuyst et al. [8] found that the affected area of posterior circulation according to topographical localization was brainstem in 60 %, cerebellum in 50 %; the infarcts are of basilar and/or vertebral artery origin in more than 50 % of patients. In our study, the distribution according to topographical localization was in pons in 30.1 %, in cerebellum in 59.7 %, in occipital lobe in 19.3 %, in medulla oblongata in 3.2 %, in mesencephalon in 3.2 % of 186 patients.
It has been suggested that dolichoectasia develops as a result of atherosclerotic degeneration in arterial wall, and arterial hypertension is a pathogenetic risk factor acting alone or in combination. Hegedus [25] indicated that it is a congenital anomaly with findings of smooth muscle atrophy and defects in internal elastic membrane on histological sections. Its association with dilatation of other cerebral vessels and aortic aneurysm supports the theory of diffuse arterial defect. Hypertension and atheromatous process play an important role for ischemia development in dolichoectatic patients. The relationship between vertebrobasilar artery dolichoectasia and ischemic symptoms is not completely understood. Literature data mostly comes from case reports. Some studies have tried to explain its etiology and mechanism. Infarcts at distal sites have been linked to artery-to-artery embolism, while brain stem and some cerebellar infarcts to atherothrombotic occlusion of basilar artery [26, 27].
Acute posterior circulation infarcts are diagnosed more rapidly and earlier with diffusion-weighted imaging (DWI). The examination time is on the order of seconds. It is substantially effective in ischemic stroke despite artefacts and limitations in posterior fossa [28]. Routine MRI can detect acute infarcts at 24–48 h at the earliest, while the corresponding figure associated with DWI ranges between half an hour and 6 h [29]. A retrospective analysis of our study showed that the posterior circulation infarct was detected at chronic period in 116 (62.4 %) patients, at acute period in 63 (33.8 %) patients, and at subacute period in 7 (3.8 %) patients.
Tortuous and elongated arteries particularly cause medullary and pontine compression and distortion. Resta et al. [4] demonstrated the relationship between arterial shift of the vertebrobasilar system and neurological findings in the posterior fossa in 77.3 % of angiographic-clinic correlations and concluded that the greater degree of arterial shift means greater number of positive clinical cases. In our study, 10 patients had vertigo, 2 had tinnitus, and 2 had facial paralysis secondary to vascular compression as additional symptoms.
Our study indicates the role of the morphological assessment of the both basilar and vertebral artery for predicting the patients at risk in posterior circulation infarcts; the results should be interpreted in light of the limitations of the study. One of the limitations was that we did not evaluate the severity of the chronic diseases; all the patients included in the PCIP group were under medications due to chronic diseases (HT, hypercholesterolemia, and DM). Also, time length for an exposure to a certain risk factor would significantly influence the incidence of bad outcome; in this study, we did not perform a correlative analysis about time effect, and further studies are needed.
To our knowledge, our study would be the first study that evaluated the posterior circulation infarcts in the direction of the morphological assessment of the both basilar and vertebral arteries. Our results suggest that prominent elongation and deviation, C and J shape of BA, and increased L VA angle may be the predictors of at-risk patients in posterior circulation infarcts. Reporting marked morphological BA and VA variations detected at routine brain MRI will aid in selection of patients for primary stroke prevention. In considering stroke prevention, one should be aware of the mechanisms of disease underlying the clinical stroke syndrome, and therefore, preventive measures should be tailored to the disease mechanism [18]. Risk factors for atherosclerosis and ischemic stroke include smoking, HT, DM, and elevated cholesterol. Statin therapy reduces LDL cholesterol level, with each 10 % reduction in LDL cholesterol estimated to decrease the risk for stroke by 15 % [30]. The optimal offensiveness for glycemic control remains controversial. Some proofs suggest tight glycemic control in patients with ischemic cardiovascular disease actually worsens outcomes; inversely, others favor moderately aggressive glycemic control [31]. Also, the benefit of statins for stroke prevention in patients with DM is clear [32]. The relationship between HT and lacunar stroke is particularly strong and may occur in individuals with no other stroke risk factors. It is clear that screening for HT and treatment of HT are important and effective in stroke prevention [18]. Also, for lowering stroke risk, the 5 factors are body mass index >25 kg/m2, 30 min/day of moderate activity, not smoking, modest alcohol intake, and scoring in the top 40 % on a healthy diet score. The prevention of recurrent strokes has focused on the use of antiplatelet therapy and blood pressure control [18]. If a patient has lacunar or small size infarct in posterior circulation territory, that means this patient must be undertaken on anticoagulant and antiplatelet therapy to decrease recurrent stroke risk. Timely detection and treatment of at-risk patients may be life-saving.
References
Pico F, Labreuche J, Touboul PJ, Leys D, Amarenco P (2005) Intracranial arterial dolichoectasia and small vessel disease in stroke patients. Ann Neurol 57:472–479
Pico F, Labreuche J, Cohen A, Touboul PJ, Amarenco P, GENIC investigators (2004) Intracranial arterial dolichoectasia is associated with enlarged descending thoracic aorta. Neurology 63:2016–2021
Pico F, Labreuche J, Touboul PJ, Amarenco P, GENIC Investigators (2003) Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology 61:1736–1742
Resta M, Gentile MA, Di Cuonzo F, Vinjau E, Brindicci D, Carella A (1984) Clinical-angiographic correlations in 132 patients with megadolichovertebrobasilar anomaly. Neuroradiology 26:213–216
Castelnovo G, Jomir L, Le Bayon A, Bouly S, Thiebaut C, Labauge P (2003) Lingual atrophy and dolichoectatic artery. Neurology 61:1121
Deeb ZL, Jannetta PJ, Rosenbaum AE, Kerber CW, Drayer BP (1979) Tortuous vertebrobasilar arteries causing cranial nerve syndromes: screening by computed tomography. J Comput Assist Tomogr 3:774–778
Bogousslavsky J, Regli F, Maeder P, Meuli R, Nader J (1993) The etiology of posterior circulation infarcts: a prospective study using magnetic resonance imaging and magnetic resonance angiography. Neurology 43:1528–1533
Devuyst G, Bogousslavsky J, Meuli R, Moncayo J, de Freitas G, van Melle G (2002) Stroke or transient ischemic attacks with basilar artery stenosis or occlusion: clinical patterns and outcome. Arch Neurol 59:567–573
Pessin MS, Chimowitz MI, Levine SR, Kwan ES, Adelman LS, Earnest MP, Clark DM, Chason J, Ausman JI, Caplan LR (1989) Stroke in patients with fusiform vertebrobasilar aneurysms. Neurology 39:16–21
Passero S, Fillosomi G (1998) Posterior circulation infarcts in patient with vertebrobasilar dolichoectasia. Stroke 29:653–659
Libman RB, Kwiatkowski TG, Hansen MD, Clarke WR, Woolson RF, Adams HP (2001) Differences between anterior and posterior circulation stroke in TOAST. Cerebrovasc Dis 11:311–316
Smoker WRK, Price MJ, Keyes WD, Corbett JJ, Gentry LR (1986) High-resolution computed tomography of the basilar artery: 1. Normal size and position. AJNR Am J Neuroradiol 7:55–60
Smoker WRK, Corbett JJ, Gentry LR, Keyes WD, Price MJ, McKusker S (1986) High-resolution computed tomography of the basilar artery: 2. Vertebrobasilar dolichoectasia: clinical-pathologic correlation and review. AJNR Am J Neuroradiol 7:61–72
Voetsch B, DeWitt D, Pessin MS, Caplan LR (2004) Basilar artery occlusive disease in the New England Medical Center posterior circulation registry. Arch Neurol 61:496–504
Caplan LR (1989) Intracranial atheromatous disease: a neglected understudied and underused concept. Neurology 39:1246–1250
Dodevski A, Lazareska M, Tosovska-Lazarova D, Zhivadinovik J, Stojkoski A (2011) Basilar artery fenestration. Folia Morphol 70:80–83
Ubogu EE, Zaidat OO (2004) Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry 75:22–26
Marsh JD, Keyrouz SG (2010) Stroke prevention and treatment. J Am Coll Cardiol 56:683–691
Keyik B, Coban GS, Yanik B, Hekimoglu B (2010) Evaluation of the dolichoectasia of the basilar artery in patients with posterior circulation infarcts. New Med J 27:101–105
Kumral E, Kisabay A, Ataç Ç, Kaya Ç, Çalli C (2005) The mechanism of ischemic stroke in patients with dolichoectatic basilar artery. Eur J Neurol 12:437–444
Hennerici M, Ratenberg W, Schwartz A (1987) Transcranial Doppler ultrasound for assessment of intracranial arterial flow velocity. II. Evaluation of intracranial arterial disease. Surg Neurol 27:523–532
Schwartz A, Ratenberg W, Hennerici M (1993) Dolichoectatic intracranial arteries: review of selected aspects. Cerebrovasc Dis 3:273–279
De Georgia M, Belden J, Pao L, Pessin M, Kwan E, Caplan L (1999) Thrombus in vertebrobasilar dolichoectatic artery treated with intravenous urokinase. Cerebrovasc Dis 9:29–33
Shokunbi MT, Vinters HV, Kaufmann JC (1988) Fusiform intracranial aneurysms: clinicopathologic features. Surg Neurol 20:263–270
Hegedus K (1985) Ectasia of the basilar artery with special reference to possible pathogenesis. Surg Neurol 24:463–469
Freund W, Kassubek J, Aschoff A, Huber R (2008) MRI-based separation of congenital and acquired vertebrobasilar artery anomalies in ischemic stroke of the posterior circulation. Stroke 39:2382–2384
Caplan LR (2005) Dilatative arteriopathy (dolichoectasia): what is known and not known. Ann Neurol 57:469–471
Oppenheim C, Stanescu R, Dormont D, Crozier S, Marro B, Samson Y, Rancurel G, Marsault C (2000) False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol 21:1434–1440
Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR (1995) Acute human stroke studied by whole brain echo planar diffusion weighted magnetic resonance imaging. Ann Neurol 37:231–241
Amarenco P, Labreuche J, Lavallee P, Touboul PJ (2004) Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke 35:2902–2909
The Action to Control Cardiovascular Risk in Diabetes Study Group (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Colhoun HM, Betteridge DJ, Durrington PN et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Institutional Review Board of our institution and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript was accepted by European Congress of Radiology and presented as a poster on March 2014 in Vienna.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 46 kb)
Rights and permissions
About this article
Cite this article
Çoban, G., Çifçi, E., Yildirim, E. et al. Predisposing factors in posterior circulation infarcts: a vascular morphological assessment. Neuroradiology 57, 483–489 (2015). https://doi.org/10.1007/s00234-015-1490-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1490-z