Abstract
Introduction
The impact of restricted diffusion on clinical outcome has not been well studied in childhood encephalitis. We hypothesized that the patients with lesions with restricted diffusion (LRD) would have worse clinical outcome.
Methods
We reviewed the MR studies of 83 children with encephalitis for LRD. An MRI scoring system (0–12) based on fluid-attenuated inversion recovery (FLAIR) imaging was created to evaluate the extent of imaging abnormality. Clinical outcome was graded by using Glasgow outcome scale (GOS) (1–5) in 1st and 12th month: 1 for death and five for full recovery. With respect to diffusion, the correlation between imaging score and GOS was assessed. Logistic regression analysis was used to explore the impact of diffusion and imaging score on clinical outcome. The patients were divided into three subgroups regarding imaging score: I, 0–4; II, 5–8; and III, 9–12.
Results
LRD was found in 28 patients. Negative significant correlation was found between imaging score and GOS in the group with LRD in both 1st month (R = −0.67, P < 0.001) and 12th month (R = −0.56, P = 0.001). Multivariate logistic regression showed that LRD (P < 0.001) and age (P = 0.026) were significant independent risk factors for unfavorable outcome in 1st month, and both LRD (P = 0.001) and imaging score (P = 0.043) were significant risk factors for unfavorable outcome in 12th month.
Conclusions
Patients with LRD have a worse clinical outcome than those without LRD. In patients with LRD, those with a greater extent of abnormality have a poorer outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Encephalitis in children is challenging to manage because of a rapidly progressive clinical course and a lack of standardized treatment protocol [1]. Although the mortality of patients with encephalitis has declined over the years, patients who have recovered from encephalitis often live with significant neurological sequelae. The extent of neuroimaging abnormalities has been correlated with patient outcome, revealing that the patients with a worse outcome had abnormal MR findings [2], diffuse lesions [3], deep white matter involvement [4], and brain stem involvement [5].
Diffusion-weighted MR imaging (DWI) has been reported to be more sensitive than conventional MRI in the early stage of encephalitis, showing more imaging abnormalities [6–9]. Some studies further revealed a correlation between the apparent diffusion coefficient (ADC) value of lesions and the duration of disease [8–10]. Lesions with restricted diffusion (LRD), consistent with cytotoxic edema, occur variably in different types of acute encephalitis [10, 11]. In a study comparing diffusion abnormality in patients with herpes simplex encephalitis (HSE) and those with Japanese encephalitis (JE) [10], LRD was more readily observed in HSE, which is considered as necrotizing encephalitis with extensive perivascular cuffing and inflammatory infiltrates [12, 13], while LRD was less frequently and less conspicuously observed in JE, which is a non-necrotizing encephalitis with microglial proliferation and sparse lymphocytic response [12, 13]. The pathologic changes in necrotizing encephalitis are more likely to be accompanied by necrosis, hemorrhage, and cell death [12] and thus may be associated with a poor prognosis. Moreover, in several small series, the presence of LRD has been reported to be associated with irreversible brain damage [14, 15] or poor clinical outcome [16, 17].
The purpose of this study was to determine the impact of the presence of LRD and the extent of imaging abnormality on the outcome of patients with acute and subacute encephalitis. We hypothesized that the patients with encephalitis showing LRD, after adjustment for the extent of brain involvement, would have worse outcome.
Materials and methods
Patients
MR studies of 83 patients with a final diagnosis of encephalitis, evaluated between 2005 and 2012, were retrospectively reviewed. Subjects were included in this study if they were diagnosed with acute or subacute encephalitis, were younger than 18 years of age, and admitted to our pediatric ward or pediatric intensive care unit. We excluded subjects with encephalopathy secondary to bacterial meningitis and associated intracranial complications, toxic or metabolic diseases, or vascular diseases and excluded those with preexisting neurological diseases. The recruited subjects consisted of 52 boys and 31 girls (1 to 16 years). Institutional review board approval was obtained for this study. The diagnosis of encephalitis was based on symptoms, signs, electroencephalographic findings, CSF analysis, serologic tests, and MRI findings. The identification of associated agents was based on neutralizing antibody titers in serum, CSF polymerase chain reaction, and virus culture of CSF, throat swab, urine, or stool. The time to imaging, defined as the interval between the first day of neurological symptoms and signs and the day of MR examination, was recorded.
MR image acquisition
The examinations were performed on a 1.5 T (Intera; Philips Medical Systems, Best, The Netherlands) or a 3 T (Discovery MR750, GE Healthcare, Milwaukee, WI) scanner. MR pulse sequences included axial T1WI, axial and coronal fluid-attenuated inversion recovery (FLAIR) imaging, sagittal T2WI, axial DWI with ADC map, and axial and coronal postcontrast T1WI. DWI was performed with a single-shot spin echo EPI sequence by using the following parameters: TR/TE = 64/4,000 ms on 1.5 T scanner and 80/6,000 ms on 3 T scanner; on both scanners: image matrix = 128 × 128 (interpolated to 256 × 256), section thickness = 4 mm, FOV = 220 mm, and number of averages = 2.
MRI analysis
Two neuroradiologists, both having over 10 years of experience in practicing neuroradiology which included interpretation of MRI with DWI, independently reviewed all MR images and were blinded to the results of outcome assessment. One of them is a pediatric neuroradiologist having 1 year of fellowship training in pediatric neuroradiology and over 10 years of experience in practicing pediatric neuroradiology. Different interpretations were solved by consensus. An MRI scoring system using FLAIR imaging was devised to facilitate appraisal of the imaging abnormalities according to a point system (0–12 points) based on the extent of imaging abnormalities (Figs. 1 and 2). The scoring system was as follows: one or two lobes of a cerebral hemispheres = 1 point; three or four lobes of a cerebral hemisphere = 2 points; any one of caudate nucleus, lentiform nucleus, or thalamus on one side = 1 point; any two of these deep gray nuclei on one side = 2 points; one cerebellar hemisphere = 1 point; any part of the brain stem = 2 points. The LRD was defined as a lesion showing hyperintensity on DWI and hypointensity on the ADC map, consistent with cytotoxic edema [18].
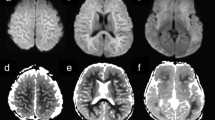
A 5-year-old boy with encephalitis 4 days after disease onset. a, b Axial FLAIR images show diffuse hyperintensites in the cerebral cortex, with a higher intensities in the frontal (arrowheads) and parietal (arrows) cortical regions (a) caudate heads (arrowheads), lentiform nuclei (double arrowheads), and thalami (triple arrowheads) (b). Relatively normal signal intensity is noted in the occipital regions. c, d Axial DWI shows hyperintensities indicating restricted diffusion in most of the lesions visible on the FLAIR images
Clinical outcome assessment
A pediatric neurologist, with 9 years of experience in pediatric critical care, who was blinded to the imaging findings, assessed the outcome of the patients based on data from medical records, outpatient visits, and telephone interviews. The clinical outcome was graded by using the Glasgow outcome scale [19–21] at the end of the 1st and 12th month after the onset of disease: one for death, two for neurovegetative state, three for severe disability incompatible with independent living, four for moderate disability but compatible with independence, and five for full recovery.
Statistical analysis
Linear regression analysis was used to evaluate the correlation between imaging score (independent variable) and GOS (dependent variable) at the end of the 1st and 12th month for all patients. Logistic regression was conducted to predict clinical outcome based on explanatory variables consisting of age, sex, LRD, and imaging score, at the end of the 1st and 12th month. The clinical outcome was dichotomized according to GOS: unfavorable, GOS = 1–3, and favorable, GOS = 4–5. Univariate logistic regression was performed to evaluate the association of age, sex, imaging score, and LRD with unfavorable outcome. Variables with a P value of less than 0.1 in univariate analysis were included and evaluated by multivariate analysis with the forward selection method. To facilitate statistical calculation, the patients were divided into three subgroups according to their imaging score: subgroup I, 0–4; subgroup II, 5–8; subgroup III, 9–12. Shapiro-Wilk test was performed to test normality of continuous variables. t test or Mann-Whitney U test was used to compare the age and the time to imaging between the LRD (+) and LRD (−) groups. A P value less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 17.0.
Results
Associated agents were enterovirus in four patients, cytomegalovirus in three, rotavirus in three, herpes simplex virus in five, influenza virus in two, norovirus in two, parainfluenza virus in two, adenovirus in three, enterovirus and rotavirus in one, enterovirus and influenza virus in one, enterovirus and cytomegalovirus in one, enterovirus and parainfluenza virus in one, rotavirus and influenza virus in one, norovirus and respiratory syncytial virus in one, and rotavirus and cytomegalovirus in one. No causative agent was identified in 49 (59 %) patients. The distribution of patients with LRD according to associated agents is shown on Fig. 3.
Twenty-eight patients (median 2.75 years, interquartile range 7.30 years) had LRD, but 55 patients (median 5.00 years, interquartile range 4.50 years) did not. There were no significant differences between the age of the LRD (+) and LRD (−) groups (Mann-Whitney U test, P = 0.23). The GOS results are summarized in Table 1, according to LRD and the three subgroups with different imaging scores. In the LRD (+) group, the proportion of lesions visible on FLAIR showing restricted diffusion ranged from 30 to 100 %.
In the LRD (−) group, the median interval between the onset of disease and the initial MR study was 7.00 days (interquartile range 8.00 days), whereas in the LRD (+) group, the median interval was 7.50 days (interquartile range 6.00 days). No significant difference of interval was found between these two groups (Mann-Whitney U test, P = 0.41).
Clinical outcome at the end of the first month
Negative significant correlation was found between imaging score and GOS (R = −0.67, P < 0.001) in the LRD (+) group (Fig. 4a). No significant correlation was found between imaging score and GOS in the LRD (−) group.
Multivariate logistic regression analysis showed that LRD (odds ratio [OR], 9.82; 95 % confidence interval [CI] 2.97, 32.49; P < 0.001) and age (OR, 0.84; 95 % CI 0.72, 0.98; P = 0.026) were significantly associated with unfavorable outcome (Table 2).
Clinical outcome at the end of the 12th month
Negative significant correlation was found between imaging score and GOS (R = −0.56, P = 0.001) in the LRD (+) group (Fig. 4b). No significant correlation was found between imaging score and GOS in the LRD (−) group.
Multivariate logistic regression analysis showed that LRD (OR, 5.77; 95 % CI 1.98, 16.80; P = 0.001) and imaging score (P = 0.043) were significantly associated with unfavorable outcome. For imaging score, when subgroup I was used as a reference, the relative risk of unfavorable outcome rose from 1.0 in subgroup I to 4.70 in subgroup III (95 % CI 1.28, 17.31; P = 0.020) (Table 2).
Discussion
In children with encephalitis, the extent of neuroimaging abnormality has been correlated with patient outcome, revealing that the patients with a worse outcome had a greater extent of abnormality or more diffuse lesions. Our results showed LRD to be a strong predictor of unfavorable outcome both at 1-month (OR, 9.82; P < 0.001) and 12-month (OR, 5.77; P < 0.001) follow-up. We did not directly compare the outcome of the LRD (+) group with that of the LRD (−) group, because a potential interaction may exist between diffusion of the lesions and the extent of the lesions, which was represented by the imaging score in our study. The extent of abnormality is generally believed to be related to the outcome of patients in encephalitis [2–5]. In order to delineate such potential interaction, we used logistic regression analysis to evaluate the association of LRD with the outcome of these two groups of patients. Also, separately in the LRD (−) group and LRD (+) group, we performed linear regression to assess the correlation between patient outcome and imaging score.
The histopathologic basis of the decrease in ADC in encephalitis is unclear because of a lack of correlation between DWI images and pathologic slices of brain lesions. A decrease in extracellular spaces may be a reasonable explanation in areas of LRD [11]. The causes of LRD in encephalitis have been attributed to the swelling of neuroglial cells or myelin resulting in cytotoxic edema [15, 22]. In encephalitis, LRDs were more readily observed in necrotizing encephalitis with neuropathologic findings of perivascular cuffing and inflammatory infiltrates [10, 12, 13]. In a series of neonatal herpes type 2 encephalitis, many of the LRDs progressed to irreversible changes on follow-up imaging [14]. Our purpose was to study the clinical significance of LRD in encephalitis, and our results suggested that patients having LRDs had poorer outcome than those without LRDs.
Concerning imaging score, multivariate logistic regression analysis showed imaging score to be a significant risk factor for unfavorable outcome only at 12th month follow-up (P < 0.043), in which subgroup III had a higher risk for unfavorable outcome (OR, 4.70; P = 0.20) when compared with subgroup I (Table 2). In the LRD (+) group, significant negative correlation was found between imaging score and GOS at both 1st month and 12th month follow-up (Fig. 4). On the other hand, in the LRD (−) group, no significant correlation was found between imaging score and GOS. Thus, our results suggest that the extent of abnormality, represented by imaging score, has greater clinical significance in the LRD (+) group than that in the LRD (−) group and also greater significance at long-term follow-up.
Time to imaging showed no significant difference between the LRD (+) group and the LRD (−) group. Although 95 % of our patients were imaged within 2 weeks after disease onset or at acute or subacute stage of the disease, more than 60 % of patients had no LRD. Previous reports of encephalitis revealed restricted diffusion in lesions at the acute stage of disease [6–9]. Having comparable time to imaging and more study subjects than in the reported series, our study did not find restricted diffusion in the lesions of more than 60 % of subjects. Moreover, in some cases of encephalitis such as JE, DWI occasionally did not show restricted water diffusion in the early stage [10]. In view of the fact that more than half of our patients did not show LRD at the acute stage, we suggested that LRD in acute encephalitis might not be completely explained by the time to imaging (that is, patients having LRD because they were imaged at the acute stage of disease) as has been previously reported. Serial follow-up imaging studies may help clarify the nature of LRD by showing the evolvement or evolution of LRDs.
The lack of significant correlation between the extent of abnormality and the clinical outcome in the LRD (−) group and that imaging score was not a significant risk factor for unfavorable outcome in 1st month on multivariate logistic regression, both suggest that the conventional MRI may not be effective in predicting the clinical outcome of patients with encephalitis, particularly at short-term follow-up. In contrast to the previous studies revealing an association of poor outcome with imaging abnormalities [2–5], our results did not support such an association in the majority of patients. This discrepancy may be explained by our use of a comparatively more systematic imaging scoring method covering the entire brain, various and different causative agents in our patients, and a relatively large number of patients in our cohort. The use of DWI and more frequent follow-up MR studies in patients with clinical progression may detect LRDs which are associated with poor outcome.
Diffusion imaging may help in the differential diagnosis of acute encephalopathy. Patients with acute disseminated encephalomyelitis (ADEM) can have neurologic symptoms and signs resembling those of encephalitis [23]. In ADEM, besides typical abnormalities in the periventricular and subcortical white matter, neuroimaging studies have revealed involvement of the gray matter in 50 % of patients and that of the brain stem in 30 % of patients [24, 25]; thus, ADEM may occasionally be difficult to differentiate from encephalitis. A recent study of diffusion imaging of ADEM [18] reported vasogenic edema, suggested by increased water diffusion, which was present in the majority (12 out of 16) of patients. This predominance of vasogenic edema may allow ADEM to be distinguished from encephalitis, particularly when the patients with encephalitis have cytotoxic edema.
Limitations
Our study was a collection of subjects who underwent MRI on 3 or 1.5 T scanners. A recent study of DWI using a phantom revealed that the ADC value and the lesion-to-background contrast ratio were different on 1.5 and 3 T scanners [26]. However, we believe that such differences were not large enough for an observer to recognize or to miss a lesion. Moreover, this study only focused on the presence of LRD, but not the ADC value of lesions which depends on the field strength of the scanner. We did not measure the amount of ADC reduction and did not correlate the extent of LRDs with patient outcome; otherwise, there would be too few subjects in each subgroup after stratification. Finally, our subjects had various or unknown etiologies of encephalitis. Less than 50 % of patients had identified causative agents.
Conclusion
Patients with LRD have a worse clinical outcome than those without LRD. In patients with LRD, those with a greater extent of abnormality have a poorer outcome.
References
Simon DW, Da Silva YS, Zuccoli G, Clark RS (2013) Acute encephalitis. Crit Care Clin 29(2):259–277. doi:10.1016/j.ccc.2013.01.001
Schleede L, Bueter W, Baumgartner-Sigl S, Opladen T, Weigt-Usinger K, Stephan S, Smitka M, Leiz S, Kaiser O, Kraus V, van Baalen A, Skopnik H, Hartmann H, Rostasy K, Lucke T, Schara U, Hausler M (2013) Pediatric herpes simplex virus encephalitis: a retrospective multicenter experience. J Child Neurol 28(3):321–331. doi:10.1177/0883073812471428
Okumura A, Kidokoro H, Tsuji T, Suzuki M, Kubota T, Kato T, Komatsu M, Shono T, Hayakawa F, Shimizu T, Morishima T (2009) Differences of clinical manifestations according to the patterns of brain lesions in acute encephalopathy with reduced diffusion in the bilateral hemispheres. AJNR Am J Neuroradiol 30(4):825–830. doi:10.3174/ajnr.A1431
Nakka P, Amos GJ, Saad N, Jeavons S (2012) MRI findings in acute Hendra virus meningoencephalitis. Clin Radiol 67(5):420–428. doi:10.1016/j.crad.2011.10.008
Wong AM, Simon EM, Zimmerman RA, Wang HS, Toh CH, Ng SH (2006) Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol 27(9):1919–1923
Tsuchiya K, Katase S, Yoshino A, Hachiya J (1999) Diffusion-weighted MR imaging of encephalitis. AJR Am J Roentgenol 173(4):1097–1099
Lian ZY, Huang B, He SR, Liang CH, Guo YX (2012) Diffusion-weighted imaging in the diagnosis of enterovirus 71 encephalitis. Acta Radiol 53(2):208–213. doi:10.1258/ar.2011.110407
Kiroglu Y, Calli C, Yunten N, Kitis O, Kocaman A, Karabulut N, Isaev H, Yagci B (2006) Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology 48(12):875–880. doi:10.1007/s00234-006-0143-7
Prakash M, Kumar S, Gupta RK (2004) Diffusion-weighted MR imaging in Japanese encephalitis. J Comput Assist Tomogr 28(6):756–761
Sawlani V (2009) Diffusion-weighted imaging and apparent diffusion coefficient evaluation of herpes simplex encephalitis and Japanese encephalitis. J Neurol Sci 287(1–2):221–226. doi:10.1016/j.jns.2009.07.010
Le Bihan D (2013) Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 268(2):318–322. doi:10.1148/radiol.13130420
McKeever PE (2012) Pathologic basis of central nervous system infections. Neuroimaging Clin N Am 22(4):773–790. doi:10.1016/j.nic.2012.06.001
Shankar SK, Mahadevan A, Kovoor JM (2008) Neuropathology of viral infections of the central nervous system. Neuroimaging Clin N Am 18(1):19–39. doi:10.1016/j.nic.2007.12.009
Vossough A, Zimmerman RA, Bilaniuk LT, Schwartz EM (2008) Imaging findings of neonatal herpes simplex virus type 2 encephalitis. Neuroradiology 50(4):355–366. doi:10.1007/s00234-007-0349-3
Albayram S, Bilgi Z, Selcuk H, Selcuk D, Cam H, Kocer N, Islak C (2004) Diffusion-weighted MR imaging findings of acute necrotizing encephalopathy. AJNR Am J Neuroradiol 25(5):792–797
Axer H, Ragoschke-Schumm A, Bottcher J, Fitzek C, Witte OW, Isenmann S (2005) Initial DWI and ADC imaging may predict outcome in acute disseminated encephalomyelitis: report of two cases of brain stem encephalitis. J Neurol Neurosurg Psychiatry 76(7):996–998. doi:10.1136/jnnp.2004.045500
Sener RN (2001) Herpes simplex encephalitis: diffusion MR imaging findings. Comput Med Imaging Graph 25(5):391–397
Zuccoli G, Panigrahy A, Sreedher G, Bailey A, Laney EJt, La Colla L, Alper G (2014) Vasogenic edema characterizes pediatric acute disseminated encephalomyelitis. Neuroradiology. doi:10.1007/s00234-014-1379-2
Mailles A, De Broucker T, Costanzo P, Martinez-Almoyna L, Vaillant V, Stahl JP (2012) Long-term outcome of patients presenting with acute infectious encephalitis of various causes in France. Clin Infect Dis 54(10):1455–1464. doi:10.1093/cid/cis226
Basumatary LJ, Raja D, Bhuyan D, Das M, Goswami M, Kayal AK (2013) Clinical and radiological spectrum of Japanese encephalitis. J Neurol Sci 325(1–2):15–21. doi:10.1016/j.jns.2012.11.007
Chou IJ, Wang HS, Lin JJ, Kuo CF, Lin KL, Chou ML, Hung PC, Hsieh MY, Lin YT (2013) Limbic encephalitis in Taiwanese children and adolescence: a single center study. Pediatr Neonatol 54(4):246–253. doi:10.1016/j.pedneo.2013.01.016
Goo HW, Choi CG, Yoon CH, Ko TS (2003) Acute necrotizing encephalopathy: diffusion MR imaging and localized proton MR spectroscopic findings in two infants. Korean J Radiol 4(1):61–65
Parmar H, Ibrahim M (2012) Pediatric intracranial infections. Neuroimaging Clin N Am 22(4):707–725. doi:10.1016/j.nic.2012.05.016
Baum PA, Barkovich AJ, Koch TK, Berg BO (1994) Deep gray matter involvement in children with acute disseminated encephalomyelitis. AJNR Am J Neuroradiol 15(7):1275–1283
Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG (2000) Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 123(Pt 12):2407–2422
Lavdas I, Behan KC, Papadaki A, McRobbie DW, Aboagye EO (2013) A phantom for diffusion-weighted MRI (DW-MRI). J Magn Reson Imaging 38(1):173–179. doi:10.1002/jmri.23950
Ethical standards and patient consent
We declare that all human studies have been approved by the Institutional Review Board of Chang Gung Medical Foundation and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients or their guardians gave informed consent prior to magnetic resonance examination in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, A.M., Lin, JJ., Toh, CH. et al. Childhood encephalitis: relationship between diffusion abnormalities and clinical outcome. Neuroradiology 57, 55–62 (2015). https://doi.org/10.1007/s00234-014-1449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1449-5