Abstract
We have studied the effect of relative composition of γ-Oryzanol (γ-Or) on the liquid expanded–liquid condensed phase coexistence region in the mixed Langmuir monolayer of γ-Or and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) molecules at air–water interface. The surface manometry studies at a fixed temperature show that the mixture of γ-Or and DPPC forms a stable monolayer at air–water interface. As the relative composition of γ-Or increases the range of area per molecule over which the coexistence of liquid expanded (LE)–liquid condensed (LC) phases exists reduces. Although the LE–LC phase coexistence corresponds to the first-order phase transition, the slope of the surface pressure–area per molecule isotherm is non-zero. Earlier studies have attributed the non-zero slope in LE–LC phase coexistence region to the influence of the strain between the ordered LC phase and disordered LE phase. The effect of strain on the coexistence of LE–LC phases can be studied in terms of molecular density–strain coupling. Our analysis of the liquid condensed–liquid expanded coexistence region in the isotherms of mixed monolayers of DPPC and γ-Or shows that with the increase in the mole fraction of sterol in the mixed monolayer the molecular lateral density–strain coupling increases. However, at 0.6 mole fraction of γ-Or in the mixed monolayer the coupling decreases. This is corroborated by the observation of minimum Gibb’s free energy of the mixed monolayer at this relative composition of γ-Or indicating better packing of molecules.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biological function of a cell membrane is closely related to lateral distribution and packing of lipids (Goldfine 1984; Clarke 2019). Sterols and phospholipids are the major constituents of cell membranes (Kessel et al. 2001; Trinh et al. 2020). Cholesterol and its derivatives form a class of sterols which interact with the saturated lipids in the cell membrane to form aggregates called membrane domains or rafts. The interaction between sterols and lipids in a cell membrane can be through the formation of hydrogen bond between the \(-OH\) and the ester carbonyl group in lipids and hydrophobic interactions between cholesteryl moiety and lipid tails (Bhattacharya and Haldar 2000; Pandit et al. 2004). However, the mutual strength of the hydrogen bonding and hydrophobic interactions and the role played by each of these interactions in deciding the properties of the cell membranes remain unclear. While hydrophobic interactions are important in deciding the membrane properties (like lipid order, bilayer widths, etc) Bhattacharya and Haldar (2000), the formation of rafts (or ordered domains) in the membrane is associated with the formation of cholesterol–lipid complex due to the hydrogen bonding (Pandit et al. 2004). In addition, other factors like the lipid saturation and tail length affect the packing geometry which in turn also affects the extent of complex formation between sterols and lipids. Various models describing the cholesterol–lipid interactions have been proposed to explain the observed experimental properties of the model membranes. These interactions lead to the formation of rafts, which are cholesterol-rich ordered lipid domains (Botet-Carreras et al. 2019; Hung et al. 2007; Jaroque et al. 2020) coexisting with the disordered lipid bilayer (Maria et al. 2009). The coexistence of the ordered domains and the disordered lipid phase was found for a range of mole fraction of cholesterol (\(0.13 - 0.85\) in DPPC monolayer) in the mixed monolayer of sterols and lipids (Ivankin et al. 2010). X-ray studies have shown that the cholesterol molecules in the rafts are orderly arranged, whereas they were found to be arranged in a pair-wise binding in the disordered phase (Toppozini et al. 2014). The difference between the molecular organization in the condensed lipid ordered domains and the disordered domains would lead to the development of 2D strain in the condensed domains. It would be interesting to study the stability of coexistence of ordered–disordered domains with different molecular densities and the energetics of the self-assembly of lipid molecules.
Langmuir monolayers are mono-molecular films formed at air–water interface by amphiphilic molecules and can be used as model membranes to study the cell membrane properties. Studies on the coexistence of condensed lipid domains (usually referred as liquid condensed phase) along with the low-density disordered lipid regions (referred as liquid expanded phase) have shown that the difference in the lateral molecular density and chain tilt angle between the condensed region and disordered region contributes significantly towards the stability of the LE–LC coexistence region (Hatta and Nihei 2019). Although the transition from liquid condensed phase to liquid expanded phase is a first-order phase transition, it is usually observed that the surface pressure linearly increases with the increase in the lateral density of the molecules. Such a behaviour can be understood by considering the dependence of free energy of the film on the coupling between the lateral density of the molecules (\(\rho\)) and the strain (\(\epsilon _s\)) in the liquid condensed phase coexisting with the liquid expanded phase (Hatta and Nihei 2019) (\(\rho - \epsilon _s\) coupling). Most of the studies to understand the LE–LC transition are limited to the mono-molecular phospholipid films and the effect of sterol on the energetics of coexisting regions in lipid films is not understood.
We have studied the stability of the mixed monolayer of gamma-oryzanol (γ-Or) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) at the air–water interface. We have further analysed the role of relative composition of the γ-Or on the \(\rho - \epsilon _s\) coupling. γ-Or is a phytosteryl ferulate, usually extracted from the rice bran, is used in cosmetic industry as an active ingredient in sunscreen products (Juliano et al. 2005). Earlier studies have established the role of γ-Or on the suppression of cell apoptosis (Huanga et al. 2020) and tumour cells(Yu et al. 2019). The antioxidative property of the ferulic acid moiety in the γ-Or has many biological effects and medicinal properties(Ramazani et al. 2021).
Experimental Section
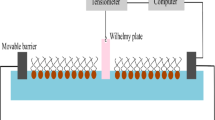
DPPC was procured from Avanti polar lipids and the γ-Or was procured from Tokyo Chemical Industry (TCI). The molecular structure of the material γ-Or is shown in Figure 1. The stock solutions of γ-Or and DPPC were prepared in HPLC-grade chloroform (concentration 1 mg/ml) and stored in dark (to reduce photo-isomerization of γ-Or molecules due to stray light). Mixtures of γ-Or and DPPC were prepared from the stock solutions to get 20%, 40%, 60% and 80% of mole fraction of γ-Or in DPPC. The surface manometry studies on mixed Langmuir monolayers of γ-Or and DPPC were carried out using Langmuir trough (Apex instruments). The trough was enclosed in an opaque cabinet to avoid light and air current. The surface pressure of the monolayer was measured using Wilhelmy method. The Langmuir monolayers were formed by spreading 25 μl of solution onto the sub-phase using Hamilton’s syringe. The surface pressure (\(\pi\))–mean area per molecule (\({A}_{m}\)) isotherms of DPPC and γ-Or mixed monolayer were obtained by compressing the monolayer between two barriers at a speed of 7 mm/min. All the experiments were carried out at \(21~^\circ\)C using refrigerated/heated circulator (Julabo, Model: F25).
Results and Discussion
The properties of Langmuir monolayer of pure DPPC and pure γ-Or are well studied (Ma and Allen 2007; Raghavendra and Kumar 2021). The surface manometry studies are carried out to understand the properties of mixed monolayers of DPPC and γ-Or. The \(\pi -A_m\) isotherms of the mixtures were obtained at \(21~^\circ\)C (shown in Figure 2).
To characterize the phases exhibited by the mixed monolayers, the elastic (compressional) modulus of mixed monolayer was calculated for different mole fractions of γ-Or in DPPC from the \(\pi\)-\(A_m\) isotherms using the following equation: \(|E| = A_m\frac{d\pi }{dA_m}.\) Figure 3 shows the variation of |E| as a function of \(A_m\) for different mole fractions of γ-Or \((X_{\gamma -Or})\) in mixed monolayer. From \(\pi -A_m\) isotherms and \(|E|-A_m\) plots, we infer that at high \(A_m\), the mixed monolayers exhibited gaseous phase (\(\pi \approx 0\) mNm\(^{-1})\)) and on compression the mixed monolayers exhibit liquid expanded phase \((|E|>50\) mNm\(^{-1})\). On further compression, the monolayers exhibit liquid condensed phase (\(|E|>100\) mNm\(^{-1}\)) before collapsing. This behaviour was found for all γ-Or compositions in the monolayer. The region corresponding to the transition from LE to LC phase showed a plateau in the \(\pi -A_m\) isotherm.
With the increase in the \(X_{\gamma -Or}\) within the mixed monolayer, the \(\pi -A_m\) isotherm monotonously shifts towards lower mean molecular area. In addition, the range of \(A_m\) corresponding to the LE–LC transition reduces with the increase in the γ-Or composition upto \(X_{\gamma -Or}=0.6\). If γ-Or and DPPC molecules are completely miscible in the mixed monolayer, then, for a fixed surface pressure (\(\pi _{o}\)), the mean molecular area \({A}_{ideal}\) is given by
Here, \({A}_{\gamma -Or}\) and \({A}_{DPPC}\) are the area per molecule corresponding to \(\pi _{o}\) in pure monolayers of γ-Or and DPPC, respectively, and \({X}_{\gamma -Or}\) and \({X}_{DPPC}\) are the mole fractions corresponding to the mixed monolayer of γ-Or and DPPC, respectively (Ohe et al. 2007; Savva and Acheampong 2009; Chou and Chang 2000). If \({A}_{12}\) is the experimentally obtained mean molecular area in the mixed monolayer at \(\pi _{o}\) corresponding to \({X}_{\gamma -Or}\), then the excess area (\({A}_{E}\)) is defined as, \({A}_{E} ={A}_{12}-{A}_{ideal}\). Any deviation of \({A}_{12}\) from \({A}_{ideal}\) will be due to the interactions between the γ-Or and DPPC molecules (Guzmán et al. 2015; Neunert et al. 2016). \({A}_{E}\) will be negative for the mixed monolayer in the case of a condensation of the monolayer and \({A}_{E}\) will be positive if there is an expansion of the monolayer.
Variation of excess area per molecule \(A_{E}\) as a function of \(X_{\gamma -Or}\) in the mixed monolayer at different surface pressures (5 mNm\(^{-1} (\bullet\)), 10 mNm\(^{-1} (\blacksquare\)), 15 mNm\(^{-1} (\blacklozenge\)), 20 mNm\(^{-1} (\blacktriangle\)), 25 mNm\(^{-1} (\blacktriangledown\)), 30 mNm\(^{-1} (\circ\)), 35 mNm\(^{-1} (\square\)) and 40 mNm\(^{-1} (\diamond\))
The variation of excess area as a function of \({X}_{\gamma -Or}\) at different surface pressures is shown in Fig. 4. For \({X}_{\gamma -Or}\ge 0.6\), \(A_E\) was negative at all the surface pressures. This suggests that, for \({X}_{\gamma -Or}\ge 0.6\), the monolayer condenses. For \({X}_{\gamma -Or}<0.6\), the monolayer condenses only at high \(\pi\) values (\(>30\) mNm\(^{-1}\)). This behaviour at high surface pressure can be attributed to the increased interactions between the γ-Or and DPPC resulting from high surface density of the monolayer. The excess free energy (\(\Delta {G}_{E}\)) for the mixed monolayer at a given surface pressure (see Figure 4) was obtained using equation 2.
Here, \({N}_{A}\) is the Avogadro number (Lehmler et al. 2000). The ideal free energy of mixing (\(\Delta {G}_{id}\)) and mixing free energy \((\Delta {G}_{mix})\) can be calculated from the following equations:
Here, R is universal gas constant and T is temperature (Hao et al. 2016; Rojewska et al. 2017; Slotte and Mattjus 1995).
Plot of variation of excess free energy \(\Delta G_{E}\) as a function of \(X_{\gamma -Or}\) in the mixed monolayer at different surface pressures of (5 mNm\(^{-1} (\bullet\)), 10 mNm\(^{-1} (\blacksquare\)), 15 mNm\(^{-1} (\blacklozenge\)), 20 mNm\(^{-1} (\blacktriangle\)), 25 mNm\(^{-1} (\blacktriangledown\)), 30 mNm\(^{-1} (\circ\)), 35 mNm\(^{-1} (\square\)) and 40 mNm\(^{-1} (\diamond\))
Figure 5 shows the variation of excess free energy \(\Delta {G}_{E}\) as a function of \(X_{\gamma -Or}\). The minimum value of \(\Delta {G}_{E}\) for all the surface pressures corresponding to LE, LC and LE–LC coexistence region was at \({X}_{\gamma -Or} = 0.6\), which implies that for the mole fraction \({X}_{\gamma -Or} = 0.6\), the monolayer is in a more stable thermodynamic state as compared to other compositions of γ-Or in the mixed monolayer (Gong et al. 2002). At high surface pressures, the monolayer condenses and the value of \(\Delta {G}_{E}\) takes more negative values due to the shorter inter-molecular distance. For low surface pressures, the value of \(\Delta {G}_{E}\) is positive for molar fraction \({X}_{\gamma -Or} < 0.6\). The value of \(\Delta {G}_{mix}\) is negative for all molar fraction \({X}_{\gamma -Or}\) (see Figure 6) and at all the surface pressures which implies that there is no phase separation in the mixed monolayer of DPPC and γ-Or.
Plot of free energy of mixing (\(\Delta {G}_{mix}\)) as a function of mole fraction of γ-Or in the γ-Or/DPPC mixed Langmuir monolayer at various surface pressures of (5 mNm\(^{-1} (\bullet\)), 10 mNm\(^{-1} (\blacksquare\)), 15 mNm\(^{-1} (\blacklozenge\)), 20 mNm\(^{-1} (\blacktriangle\)), 25 mNm\(^{-1} (\blacktriangledown\)), 30 mNm\(^{-1} (\circ\)), 35 mNm\(^{-1} (\square\)) and 40 mNm\(^{-1} (\diamond\))
From \(\Delta A_E\) and \(\Delta G_{mix}\), we deduce that the mixed monolayer is most stable for \(X_{\gamma -Or}\approx 0.6\). The free energy of \(\Delta G_{mix}\) depends on the inter-molecular interactions and the entropy of mixing. Since the entropic contribution is maximum for \(X_{\gamma -Or}\approx 0.5\), the reduction in the \(\Delta G_{mix}\) near \(X_{\gamma -Or}=0.6\) can be attributed to the increased molecular interactions resulting from the molecular organization.
This is further supported by the plot of collapse pressure as a function of \(X_{\gamma -Or}\). We find that the collapse pressure of mixed monolayer initially decreases with the increase in the γ-Or mole fraction in DPPC up to \(X_{\gamma -Or}=0.6\) and is followed by an increase in the collapse pressure with further increase in γ-Or composition (see Figure 7). The initial decrease in collapse pressure can be understood in terms of decreasing excess area indicating condensation of the monolayer. Earlier studies on DPPC-cholesterol monolayer have shown that for mole fraction of cholesterol in the range of \(0.09 - 0.20\), the lower values for the excess area and elasticity modulus are due to specific packing of the DPPC and cholesterol molecules (Miyoshi and Kato 2015) and the monolayer was said to be in LC* phase which is different from LE and LC phases. In the present case, we find that elastic modulus increases in the coexistence of LE–LC region with the increase in γ-Or composition for \(0<X_{\gamma -Or}<0.4\), it reduces for \(0.4<X_{\gamma -Or}<0.6\) and again increases for \(X_{\gamma -Or}>0.6\) (see Figure 8). This low elastic modulus region for \(X_{\gamma -Or}\) between 0.4 and 0.6 is similar to the LC* phase observed in cholesterol–DPPC mixed monolayers. γ-Or has a ferulic acid moiety attached to the cholesteryl moiety. The presence of ferulic acid moiety affects the stearic hindrances in closely packed mixed monolayer. This results in the reduction of the elastic modulus of the mixed monolayer (Baoukina et al. 2014).
To further understand the effect of γ-Or on the elastic modulus and the strain in the LC phase coexisting with LE phase, we have investigated the LE–LC coexistence region in the isotherm. Since LE–LC phase transition is a first-order phase transition, the surface pressure is not expected to change with the decrease in area per molecule (Schrader et al. 2015). The non-horizontal linear curve in the isotherm can be due to long-range dipole interaction (Leblanc 2006; Kaganer et al. 1999), impurities in the materials and sub-microscopic clusters of amphiphilic molecules (Hifeda and Rayfield 1992; Moore et al. 1990; Milner et al. 1989).
LE−LC Coexistence Region
The reason for \(\Delta G_{mix}\) corresponding to the LE–LC coexistence region to be minimum at \(X_{\gamma -Or} \approx 0.6\) can be understood from the following analysis. Eiji Hatta et.al have studied the non-zero slope of \(\pi - A_m\) isotherm of DPPC monolayer corresponding to \(A_m\) for LE–LC coexistence phase taking into account the 2D strain field and tilt angle of the molecules (Hatta and Nihei 2019). The Landau free energy expansion obtained by including the coupling between molecular lateral density (\(\rho\)) and collective chain tilt angle (\(\theta\)) and 2D strain (\(\epsilon _s\)) for the LE–LC coexistence phase is given by equation 3. The magnitudes of \(\epsilon _s\) and \(\theta\) in the LC domain depend on the extent of LE to LC transition. The total free energy change \(\Delta {G}={G}_{LC}-{G}_{LE}\) is given by the sum of standard Landau potential, excess chain tilt, strain energy and coupling between \(\rho - \theta\) and \(\rho - \epsilon _s\) (Hatta and Nihei 2019).
Here, a, A, B and C are the coefficients (\(a, C > 0, B < 0)\) assumed to be independent of surface pressure and temperature. \(K_0\) and \(K_{\theta }\) are the elastic (or compressional modulus) and orientational elastic constant of LE phase, respectively. d expresses coupling strength between \(\rho\) and \(\theta\) and e expresses the coupling strength between \(\rho\) and \(\epsilon _s\). \(\nu\) is the ratio of area fraction of LC phase to the total area. In the case of the coexistence region, \(\nu =0\) corresponds to the molecular area of LE phase at the onset of the coexistence region and \(\nu =1\) corresponds to the molecular area of the LC phase at the end of the coexistence region. The standard Landau potential corresponding to the density order parameter \(\Delta {G}_{\rho }\) (first three terms on the right-hand side in Equation 3) is a function of temperature.
We have modified the Landau potential for the mixed monolayer. The free energy difference between the LE and the LC phases of the mixed monolayer of γ-Or and DPPC can be written as
Here, \(G^{DPPC}_{LC}\) and \(G^{DPPC}_{LE}\) denote the free energy of DPPC lipid molecules in LC and LE phases, respectively, N represents total number of DPPC and γ-Or molecules in the mixed monolayer, \(k_B\) is Boltzmann’s constant and T is the absolute temperature of the mixed monolayer. \(G^{\gamma -Or}_{LC}\) and \(G^{\gamma -Or}_{LE}\) represent the free energy of γ-Or in the LC and LE phases, respectively. The composition variable \(X_{\gamma -Or}\) specifies the mole fraction of the respective component. \(\Delta ~G^{DPPC}\) and \(\Delta ~G^{\gamma -Or}\) indicate the free energy difference between the LC and LE phases in the respective pure components of DPPC and γ-Or, as given below.
The last term in Equation 4 is the contribution to the free energy difference \(\Delta G\), due to the mixing entropy (\(\Delta S_{\text {mix}}\)) at temperature T. The total number of molecules \(N = n^{DPPC} + n^{\gamma -Or}\). Note that the contribution to the enthalpy because of the interactions between the two components (DPPC & γ-Or) is neglected. In order to find the value of \(X_{\gamma -Or}\) that minimizes \(\Delta G\),
From the above equation one can extract the value of \(X_{\gamma -Or}\) to be
where \(\Delta G^{'}_{mix}=(\Delta G^{DPPC}-\Delta G^{{\gamma{\text{-}}Or}})/N\) and \(\beta ={1}/{k_{B} T}\).
- case (i):
-
\(\Delta G^{'}_{mix} > 0\), or \(\Delta G^{DPPC} > \Delta G^{\gamma -Or}\)
Then, for small values of \(\Delta G^{'}_{mix}\), the value of \(\displaystyle e^{-\beta \Delta G^{'}_{mix}}\) can be approximated to the first order as
$$\begin{aligned} e^{-\beta \Delta G^{'}_{mix}} \approx 1 - \beta \Delta G^{'}_{mix} ~. \end{aligned}$$(9)Hence, from Equation 8 we have
$$\begin{aligned} X_{\gamma{\text{-}}Or} = \frac{1}{2 - \beta ~\Delta G^{'}_{mix}} > 0.5~. \end{aligned}$$(10) - case (ii):
-
\(\Delta G^{'}_{mix} < 0\), or \(\Delta G^{DPPC} < \Delta G^{\gamma -Or}\)
\(\Delta G^{'}_{mix} < 0 \implies \Delta G^{'}_{mix} = -|\Delta G^{'}_{mix}|\). Now, from equation 8 we have
$$\begin{aligned} X_{\gamma{\text{-}}Or} = \frac{1}{2 + \beta ~|\Delta G^{'}_{mix}|} < 0.5~. \end{aligned}$$(11)One can immediately realize from Equation 10 and Equation 11 that, for the components with the equal free energy differences between the respective LC and LE phases, the critical composition value becomes \(X_{\gamma -Or} = 0.5\), which is expected.
We have calculated the values of \(\Delta G^{DPPC}\) and \(\Delta G^{\gamma -Or}\) from the corresponding isotherms and have obtained \(\Delta G^{'}_{mix}\). Substituting \(\Delta G^{'}_{mix}\) in equation 8 we get the mole fraction of \(\gamma -\)Or corresponding to the minimum \(\Delta G\) at \(X_{\gamma -Or} = 0.54\) which is close to the value we have obtained for the stable \(\Delta G_{\text{ m }ix}\).
In order to further understand the LE–LC coexistence region, we have analysed the isotherms. The external intensive variable temperature can be converted to surface pressure to get rescaled surface pressure (see reference Hatta and Nihei 2019 for more details):
Equation 12 can be further written in terms of rescaled order parameter \(\xi =\Big (\frac{C}{|B|}\Big )^\frac{1}{2}\rho\), as follows (Hatta and Nihei 2019):
For \(\gamma _0 \ll 1\), Equations 12 and 13 give
Equation 14 gives a linear relationship between \(\pi\) and \(\nu\) equation with slope of \(m=\frac{ B\beta \rho ^2}{a\frac{dT_c}{d\pi _c}}\). Substituting \(\beta =\frac{2e^2}{|B|K_0}\), we get \(m=\frac{2\rho ^2e^2}{a\frac{dT_c}{d\pi _c}K_0}\). Equation 14 is fitted to the experimental data corresponding to the LE–LC coexistence phase and \(\frac{e^2}{a}\) was calculated for mixed monolayers with different \(X_{\gamma -Or}\) using Equation 15 as shown in Figure 9.
Albrecht et al. reported the value of \(\frac{dT_c}{d\pi _c}=2.35\) mNm\(^{-1}\) for DPPC monolayer from the phase diagrams of the isotherm (Albrecht et al. 1978). We have obtained the values of \(K_o\) and \(\rho\) for different mixtures from \(\pi -A_m\) isotherms and are tabulated in Table 1. Figure 10 shows the plot of \(\frac{e^2}{a}\) for different moles of \(X_{\gamma - Or}\) in the mixed monolayer. The \(\rho -\epsilon _s\) coupling constant e depends on the composition of the mixed monolayer. The decrease in \(e^2/a\) for \(X_{\gamma -Or}\) between 0.4 and 0.6 can be attributed to the improved molecular packing which reduces the strain between LE and LC phases. However, for \(X_{\gamma -Or}<0.4\) and \(X_{\gamma -Or}>0.6\), \(e^2/a\) increases due to strong \(\rho -\epsilon _s\) coupling resulting from the excess γ-Or molecules in the monolayer. Hence, the free energy increase for the mixed monolayer with \(X_{\gamma -Or}>0.6\) can be attributed to the increase of strain in the LC phase. These results indicate that the stability of the mixed monolayers of γ-Or and DPPC at \(X_{\gamma -Or}=0.6\) is due to the reduction in the LC strain, which in turn can be attributed to the better molecular packing of the molecules in the mixed monolayer of γ-Or and DPPC.
Conclusions
To conclude, we find that at a specific concentration of \(X_{\gamma -Or}\approx 0.6\), the mixed monolayer of γ-Or-DPPC exhibits a lower value of compressional modulus as compared to the other relative compositions of γ-Or. \(\Delta G_{mix}<0\) for all values of \(X_{\gamma -Or}\) suggests that there is no phase separation of γ-Or and DPPC in the mixed monolayer. Our studies show that the coexistence of the LE and LC phases depends on the relative composition of sterol and lipids. For γ-Or and DPPC mixed monolayer the LE–LC coexistence region was most stable for the mixed monolayer with 0.6 mole fraction of γ-Or.
Data Availability
Data will be provided if requested.
Code Availability
Not applicable
References
Albrecht O, Gruler H, Sackmann E (1978) Polymorphism of phospholipid monolayers. J de Physique 39(3):301–313
Baoukina S, Rozmanov D, Mendez-Villuendas E, Tieleman DP (2014) The mechanism of collapse of heterogeneous lipid monolayers. Biophys J 107(5):1136–1145
Bhattacharya S, Haldar S (2000) Interactions between cholesterol and lipids in bilayer membranes. role of lipid headgroup and hydrocarbon chain-backbone linkage. Biochimica et Biophys Acta(BBA)-Biomembranes 1467(1):39–53
Botet-Carreras A, Montero MT, Domènech Ò, Borrell JH (2019) Effect of cholesterol on monolayer structure of different acyl chained phospholipids. Colloids Surf B: Biointerfaces 174:374–383
Chou TH, Chang CH (2000) Thermodynamic behavior and relaxation processes of mixed dppc/cholesterol monolayers at the air/water interface. Colloids Surf B: Biointerfaces 17(2):71–79
Clarke RJ (2019) In Cholesterol Modulation of Protein Function. Springer, Germany, pp 135–154
Goldfine H (1984) Bacterial membranes and lipid packing theory. J Lipid Res 25(13):1501–1507
Gong K, Feng SS, Go ML, Soew PH (2002) Effects of ph on the stability and compressibility of dppc/cholesterol monolayers at the air-water interface. Colloids Surf A: Physicochem Eng Aspects 207(1–3):113–125
Guzmán E, Ferrari M, Santini E, Liggieri L, Ravera F (2015) Effect of silica nanoparticles on the interfacial properties of a canonical lipid mixture. Colloids Surf B: Biointerfaces 136:971–980
Hao C, Liu Q, Li Q, Zhang J, Sun R (2016) Thermodynamic and structural studies of dmpc and dspc with dotap mixed monolayers at the air-water interface. Russ J Phys Chem A 90(1):214–219
Hatta E, Nihei K (2019) Emergence of a linear slope region of the isotherm in the first-order liquid-expanded-liquid-condensed phase transition in langmuir monolayers. Phys Rev E 100(2):022801
Hifeda Y, Rayfield G (1992) Evidence for first-order phase transitions in lipid and fatty acid monolayers. Langmuir 8(1):197–200
Huanga L, Jianga W, Zhua L, Maa C, Oua Z, Luoa C, Wua J, Wena L, Tana Z, Yi J (2020) Gamma-oryzanol suppresses cell apoptosis by inhibiting reactive oxygen speciesmediated mitochondrial signaling pathway in h2o2-stimulated l02 cells. Biomed Pharmacother 23:109554
Hung WC, Lee MT, Chen FY, Huang HW (2007) The condensing effect of cholesterol in lipid bilayers. Biophys J 92(11):3960–3967
Ivankin A, Kuzmenko I, Gidalevitz D (2010) Cholesterol-phospholipid interactions: New insights from surface x-ray scattering data. Phys Rev Lett 104:108101
Jaroque GN, Sartorelli P, Caseli L (2020) The effect of the monocyclic monoterpene tertiary alcohol γ-terpineol on biointerfaces containing cholesterol. Chem Phys Lipids 230:104915
Juliano C, Cossu M, Alamanni MC, Piu L (2005) Antioxidant activity of gamma-oryzanol: mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int J Pharm 299(1–2):146–154
Kaganer VM, Möhwald H, Dutta P (1999) Structure and phase transitions in langmuir monolayers. Rev Mod Phys 71(3):779
Kessel A, Ben-Tal N, May S (2001) Interactions of cholesterol with lipid bilayers: the preferred configuration and fluctuations. Biophys J 81(2):643–658
Leblanc RM (2006) Molecular recognition at langmuir monolayers. Curr Opin Chem Biol 10(6):529–536
Lehmler HJ, Jay M, Bummer PM (2000) Mixing of partially fluorinated carboxylic acids and their hydrocarbon analogues with dipalmitoylphosphatidylcholine at the air- water interface. Langmuir 16(26):10161–10166
Ma G, Allen HC (2007) Condensing effect of palmitic acid on dppc in mixed langmuir monolayers. Langmuir 23(2):589–597
Maria EYC, Ratajczak K, Frey SL, Cao KD, Luther LM, Lee KYC, Majewski J (2009) Ordered nanoclusters in lipid-cholesterol membranes. Phys Rev Lett 103:028103
Milner ST, Joanny J, Pincus P (1989) Buckling of langmuir monolayers. EPL (Europhysics Letters) 9(5):495
Miyoshi T, Kato S (2015) Detailed analysis of the surface area and elasticity in the saturated 1, 2-diacylphosphatidylcholine/cholesterol binary monolayer system. Langmuir 31(33):9086–9096
Moore BG, Knobler CM, Akamatsu S, Rondelez F (1990) Phase diagram of langmuir monolayers of pentadecanoic acid: quantitative comparison of surface pressure and fluorescence microscopy results. J Phys Chem 94(11):4588–4595
Neunert G, Makowiecki J, Piosik E, Hertmanowski R, Polewski K, Martynski T (2016) Miscibility of dl-α-tocopherol β-glucoside in dppc monolayer at air/water and air/solid interfaces. Mater Sci Eng: C 67:362–368
Ohe C, Sasaki T, Noi M, Goto Y, Itoh K (2007) Sum frequency generation spectroscopic study of the condensation effect of cholesterol on a lipid monolayer. Anal Bioanal Chem 388(1):73–79
Pandit SA, Bostick D, Berkowitz ML (2004) Complexation of phosphatidylcholine lipids with cholesterol. Biophys J 86(3):1345–1356
Raghavendra Kumar B (2021) Studies on kinetics of isomerization of gamma oryzanol at air-water interface. Thin Solid Films 732:138764
Ramazani E, Akaberi M, Emami SA, Tayarani-Najaran Z (2021) Biological and pharmacological effects of gamma-oryzanol: An updated review of the molecular mechanisms. Curr Pharm Des 27(19):2299–2316
Rojewska M, Skrzypiec M, Prochaska K (2017) Surface properties and morphology of mixed poss-dppc monolayers at the air/water interface. Colloids Surf B: Biointerfaces 150:334–343
Savva M, Acheampong S (2009) The interaction energies of cholesterol and 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine in spread mixed monolayers at the air- water interface. J Phys Chem B 113(29):9811–9820
Schrader AM, Donaldson SH, Song J, Cheng CY, Lee DW, Han S, Israelachvili JN (2015) Correlating steric hydration forces with water dynamics through surface force and diffusion nmr measurements in a lipid-dmso-h2o system. Proc Natl Acad Sci 112(34):10708–10713
Slotte JP, Mattjus P (1995) Visualization of lateral phases in cholesterol and phosphatidylcholine monolayers at the air/water interface-a comparative study with two different reporter molecules. Biochimica et Biophys Acta-Lipids Lipid Metab 1254(1):22–29
Toppozini L, Meinhardt S, Armstrong CL, Yamani Z, Kučerka N, Schmid F, Rheinstädter MC (2014) Structure of cholesterol in lipid rafts. Phys Rev Lett 113:228101
Trinh MN, Brown MS, Goldstein JL, Han J, Vale G, McDonald JG, Seemann J, Mendell JT, Lu F (2020) Last step in the path of ldl cholesterol from lysosome to plasma membrane to er is governed by phosphatidylserine. Proc Natl Acad Sci 117(31):18521–18529
Yu Y, Zhang J, Wang J, Sun B (2019) The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv 9:18060
Acknowledgements
B. Kumar acknowledges the DST-SERB Early Career Research award for financial support.
Funding
BK acknowledges the funding from SERB for ECR grants.
Author information
Authors and Affiliations
Contributions
Raghavendra has carried out all the experiments and has written the paper. BK has planned the work and has designed the methodology. SNC has given inputs and has contributed in writing the paper. All the authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raghavendra, Kumar, B. & Chari, S.N. Effect of γ-Oryzanol on the LE–LC Phase Coexistence Region of DPPC Langmuir Monolayer. J Membrane Biol 256, 413–422 (2023). https://doi.org/10.1007/s00232-023-00288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-023-00288-8