… results argue against the presence of an inductive element having a constant value but favor the suggestion that the apparent inductance has the nature
of ‘delayed rectification’…(Weidmann 1950).
Abstract
Effects of ibutilide, a class III antiarrhythmic drug, on delayed rectifier potassium currents (IK) in freshly isolated guinea pig ventricular myocytes were studied. Experiments were performed using the whole-cell configuration of patch-clamp technique under blockade of L-type calcium currents (Cav1). Ibutilide at concentrations ranging between 10 nM and 100 µM inhibited IKr in dose-dependent manner with a half maximal effective concentration of 2.03 ± 0.74 µM (n = 5–10). The amplitude of tail currents activated by prepulse to + 20 mV was decreased from 253 ± 52 to 130 ± 25 pA (n = 8, p < 0.01) in the presence of 1 µM ibutilide. The envelope test revealed time-dependent changes in ratio of IK-tail/ΔIK during 0.2–2 s pulse durations in the absence of drug. With ibutilide, regardless of pulse duration, a relatively constant ratio was estimated, indicative of predominant involvement of IKr component. The slow IKs persisted to greater extent even at 100 μM ibutilide revealing a distinguishable selectivity toward the IKr component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ibutilide is an effective class III antiarrhythmic agent when tested in dogs (Buchanan et al. 1992), humans (Jung and DiMarco 1996), and rabbits (Cimini et al. 1992). Major changes are manifested in physiological properties of an AP—action potential (Sasaki et al. 2014). In mouse atrial tumor myocytes (AT-1 cells) ibutilide blocks the rapid component (IKr) of delayed rectifier K+ channels (Yang et al. 1995). Ibutilide has been found to prolong action potential duration (APD) in guinea pig ventricular (Lee 1992) and human atrial cells (Lee and Lee 1998). Prolongation of APD by ibutilide occurred due to activation of a slow inward Na+ current (Lee 1992). Moreover, there is a likelihood that the inward Na+ current activated by ibutilide flow through L-type Ca2+ channels (Lee and Lee 1998). However, IKr—the principal target for most newcomer drugs especially those with class III antiarrhythmic properties—is mainly responsible for the repolarization phase of cardiac AP (DeFelice et al. 1990). The rapid component of delayed rectifier also in guinea pig ventricular cells chiefly contributes to Phase 3—repolarization of AP (Hiraoka et al. 1986). Similar counterpart is HERG K+ channel—human ether à-go–go related gene, which is critical for proper maintaining the rhythmicity of our hearts (Curran et al. 1995; Sanguinetti et al. 1995). Note that reminiscent of many other substances also antiarrhythmic drugs have ‘side effects’ which sometimes are beneficial for the heart as in case of E-4031 (Kodirov et al. 2014; Sano et al. 1990). The latter is both antiarrhythmic and cardio-protective, but does not influence the heart rate in mongrel dogs. It is perhaps due to simultaneous induction of both ventricular arrhythmia and myocardial ischemia by a single source and occurred when the ‘left anterior descending coronary artery was occluded for 2 h’ (Sano et al. 1990).
Despite its clinical use, ibutilide related basic studies are scarce in general and those addressing the ventricle in particular (Sendra-Ferrer and Gonzalez 2019). However, there are numerous patents and studies continuingly covering effects of ibutilide on atrial fibrillation and flutter as it was initially found to be effective when delivered intravenously (Ellenbogen et al. 1996). It increases QT interval in humans, but does not alter the PR interval or QRS duration (Stambler et al. 1997). Since ibutilide is the class III antiarrhythmic agent, we have investigated the effect of ibutilide on IKr in the present study. In order to differentiate IKr and slowly activating component of delayed rectifier (IKs) in guinea pig ventricular cells, the envelope of tails test was employed (Noble and Tsien 1969).
Methods
Cells and Solutions
Guinea pig (250–300 g) left ventricular myocytes were enzymatically dissociated using the Langendorff perfusion technique as previously described (Mitra and Morad 1985). Cells were stored at room temperature (23–27 °C) in ‘KB’-solution until use (Isenberg and Klockner 1982). For recordings only quiescent and rod-shaped cells with a clear cross striation on membrane were selected.
Cardiomyocytes were transferred into the recording chamber with a volume of 0.8 ml and were superfused at a constant rate of 2.5 ml/min with an external solution maintained at room temperature. It contained (in mM): NaCl 140, KCl 3.5, MgCl2 1.5, CaCl2 1.5, and HEPES 10 (pH 7.4 adjusted with NaOH). Either 5 µM nifedipine or 1 mM CdCl2 was added to the external solutions in order to pharmacologically isolate the delayed rectifier from L-type Ca2+ currents (ICa-L). The pipette solution contained (in mM): KCl 140, MgCl2 1.5, CaCl2 0.5, EGTA 1, KATP 5, and HEPES 10 (pH adjusted to 7.4 using KOH).
Stock solutions of 10 mM ibutilide (Upjohn, Kalamazoo, MI) and 1 M CdCl2 were made using distilled water. Nifedipine was prepared as 10 mM stock solution in ethanol. All other chemicals were purchased from Sigma (St. Louis, MO, USA). Electrophysiological experiments were performed at room temperature (23–27 °C).
Data Acquisition and Analysis
Membrane currents in cardiomyocytes were measured using the whole-cell configuration of patch-clamp technique (Hamill et al. 1981). Voltage and current signals were digitized using a personal computer equipped with an analogue-to-digital converter (Digidata 1200, Axon Instruments, CA, USA). Data were acquired with an RK-400 patch and cell-clamp amplifier (Bio-Logic, Claix, France) and the pClamp 6.0.3 software package (Axon). The current signals were filtered at 1 kHz and stored for later off-line computer analyses. Microelectrodes were pulled from capillary tubes (WPI, Florida, USA) using the P87 puller (Sutter, CA, USA). Electrodes had resistances of between 1.5 and 4 MΩ when filled with intracellular solution. A calculated (AxoScope, Axon) junction potential between both standard external and internal solutions was 7.3 mV at 24 °C. At the beginning of an experiment the current output signal of the amplifier was set to zero with the pipette being immersed in the solution within the chamber. The whole-cell configuration was established by disrupting the membrane under a pipette by a suction pulse developed by the 20 cc glass syringe. Series resistance was compensated to minimize the duration of the capacitive currents. Compensation was made for not more than 50% to prevent excessive noise and oscillation.
Origin 4.0 (Microcal, MA, USA) and Microsoft Excel 5.0 software were used for data analyses and for drawing the figures. To obtain the dose–response relationship, a sigmoidal curve was fitted to data using the Logistic equation:
where the A1 and A2 denote the initial and final amplitude values, respectively; x0 is the concentration that produces the half maximal inhibition, p is power. Data are presented as the mean ± S. E. M. and n corresponds to the number of experiments/cells. Student’s paired t test was used for examination of the difference between data groups and values are indicated where appropriate.
Results
Separation of Two Components—Envelope of Tails Test
In most of guinea pig ventricular myocytes a significant outward delayed rectifier K+ currents were present (Fig. 1). Although, the use of L-type Ca2+ channel blockers may modify any sodium current flowing through these channels, the ICa-L in this and subsequent experiments was blocked by addition of 5 µM nifedipine to the extracellular solution.
The envelope of tails test. a K+ currents evoked under control conditions, during 50 µM ibutilide application to the external surface of the membrane (b) and after its wash-out (c). d Outward currents were activated by 0.2 to 2 s steps to + 40 mV from a holding potential of − 40 mV. In this and subsequent experiments the L-type Ca2+-current was blocked by 1 mM CdCl2 in bath solution. Time of exposure to the drug was 2 min. Magnitudes of tails during 1st step are indicated to reveal blockade and a slight reversibility. Time and amplitude scales are identical and are shown in (b) only
The effect of ibutilide on rapid (IKr) and slow (IKs) cardiac delayed rectifier were examined using the envelope of tails test (Noble and Tsien 1969), which enabled the separation of two components without using the selective blockers (Sanguinetti and Jurkiewicz 1990, 1991). The envelopes of tails were enabled by the protocol illustrated in Fig. 1d. Currents were evoked under control conditions, in presence of 50 µM ibutilide, and after the wash-out of drug. In this experiment, a high concentration of ibutilide substantiates the relative insensitivity of IKs component. The duration of depolarizing steps was consistently varied from 0.2 to 2 s. Upon the depolarization, the outward current increased in a time-dependent manner (Fig. 1a). This figure illustrates also tail currents upon return to a HP—holding potential of − 40 mV after depolarizing pulses to + 40 mV. Ibutilide significantly decreased the amplitude of IK-tail (Fig. 1b). This effect was more pronounced after short activating pulses (< 500 ms). Note that the tail current activated during 200 ms voltage step was almost obliterated. The inhibition by ibutilide was not completely reversible with wash-out (Fig. 1c). In contrast to its depressive effect on IK-tail, in some experiments, ibutilide slightly enhanced the amplitude of time-independent current (Fig. 1b). However, see Fig. 3c. The origin of this current was not investigated, but as it tends to be reversible with wash-out (Fig. 1c), it is not an artifact and most plausibly of delayed rectifier nature.
In Fig. 2 the ratio of the peak tail current to the time-dependent one (IK-tail/ΔIK) was estimated. Amplitudes of outward currents evoked during a depolarizing pulse were plotted against the duration of the tested pulses. The ratio of IK-tail/ΔIK in the absence (open circles) and the presence of ibutilide (solid) were compared. The data under control conditions (Fig. 2, open circles) are in agreement with the previous observations that the delayed rectifier potassium currents of ventricular myocytes from a guinea pig consists of two components, i.e., IKr and IKs (Sanguinetti and Jurkiewicz 1990). In the absence of the drug (open circles) the ratio of IK-tail/ΔIK had relatively large values during shorter pulses (< 0.5 s, Fig. 2). Thus, under control conditions, the average ratio had a value of 1.42 ± 0.39 (n = 3) at 0.2 s pulse. When the pulse duration was increased, the ratio after a 1 s pulse decreases to a steady-state value of 0.57 ± 0.01 (n = 3). The decrease was exponential between 0.2 and 1 s (Kodirov, 1998). In the presence of ibutilide (solid circles), however, the ratio was relatively constant independent from the pulse duration, indicating a prevailing contribution of single residual component to outward currents. Since application of 50 µM ibutilide decreased the ratio of IK-tail/ΔIK with more effectiveness at the shorter pulses, these results confirm the selectivity of drug toward IKr compared to IKs component. The IKs was resistant even to 100 μM ibutilide (see further).
Separation of two components. The ratio of IK-tail/ΔIK was plotted in the absence and presence of ibutilide. In the absence of the drug (open circles) the ratio had larger values at shorter pulse durations (predominantly of IKr), while at longer ones it gradually decreased reaching the steady-state (IKs). In the presence of ibutilide (solid) the ratio was relatively linear and decreased to a constant value. Where standard error bars are not seen, they are smaller than the size of the symbol. Note the theoretical contribution of IKs to the total outward tail currents (Relative slow tail)
Ibutilide-Sensitive Currents—The Resistance of I Ks Component
Next, similar to experiments in Fig. 1 the IKr and IKs components of delayed rectifier potassium currents were activated in the same cells (Fig. 3). During these experiments only two depolarizing 0.2 and 2 s voltage steps to + 40 mV were applied, since former prevailingly activates the IKr while the latter IKs. The pulse protocol is shown in Fig. 3a. A holding potential was set to − 40 mV to inactivate both T-type Ca2+ channel and fast Na+ current. In order to analyze the ibutilide-sensitive current shown in Fig. 3d, control measurements were performed in standard extracellular solution (Fig. 3b). Subsequently, the class III antiarrhythmic drug, ibutilide, was extracellularly applied (Fig. 3c). Ibutilide-sensitive component was obtained by subtracting the current in the presence of 100 µM ibutilide from those in the absence of the agent (Fig. 3c). The ibutilide-sensitive current activates rapidly and shows relatively large IK-tail (Fig. 3d, rapid tail). The amplitude of ibutilide-sensitive IK-tail activated by 0.2 s pulses was measured upon the repolarization to − 40 mV, which is greater then those activated by 2 s (Fig. 3d, slow). Thus, the tail currents were blocked by ibutilide with different affinity revealing the IKr as a prime target. The latter notion could be substantiated by the fact that tails of IKs are obviously higher in amplitude under control conditions and if there was a significant contamination by IKr at 2 s pulse then ibutilide-sensitive currents should be of at least equal magnitude as at 0.2 s. At this concentration also two other effects were observed i.e., an increase in instantaneous component (IINST) and time-dependent outward currents (IK).
Relative resistance of IKs to ibutilide. a Example of whole-cell measurement including pulse protocol (a), recordings under control conditions (b) and after 100 µM ibutilide wash-in (c). The time-independent outward current in this experiment, at both 0.2 and 2 s depolarizing pulses, was slightly increased. Note a complete inhibition of tail current by drug only in the case of 200 ms activating pulse revealing selectivity toward the rapid component. This notion is substantiated by the waveform of ibutilide-sensitive component obtained by subtracting the current in the presence of 100 µM ibutilide from those in the absence of drug. The IK-tail (tail) activated during 0.2 s test pulse (fast) was more sensitive to ibutilide than those activated by 2 s (slow). The tail currents were enabled upon return to − 40 mV. Time scales are identical for all traces, while the amplitude one for b and c
Concentration-Dependent Block of I Kr by Ibutilide
As presented above, the application of ibutilide to guinea pig cardiomyocytes resulted in the inhibition of potassium tail current. The dependence of inhibition on the concentration of ibutilide is illustrated in Fig. 4. The inhibitory effects of ibutilide on the delayed rectifier were similarly examined in the presence of either Cd2+ or nifedipine and after obtaining stable control recordings. This figure summarizes the effect of ibutilide in concentrations ranging from 10 nM to 100 µM in different cells i.e., it is not a cumulative increase. The relative peak of IK-tail activated at + 40 mV (the duration of pulses were 0.2 s) in the absence and presence of various extracellular ibutilide concentrations, were measured upon repolarization to − 40 mV. The concentration-dependent block of IKr was determined by normalizing the current as Idrug/Icontrol in the same cell. A single Logistic distribution of sigmoidal curve was fitted to the data (see equation in “Methods”). Ibutilide induced inhibition of IKr was dose-dependent and half maximal inhibition of tail current (IKr) was achieved at 2.03 ± 0.74 µM concentration (n = 5–10).
Concentration dependence of ibutilide effects in guinea pig cardiomyocytes. Dose–response relationship for IK-tail current was obtained at various extracellular ibutilide concentrations. This current was activated by +40 mV depolarizing pulses during 0.2 s from a holding potential of − 40 mV. Membrane potential was then stepped from this activating pulse back to − 40 mV that mimics repolarization. Concentration-dependent effect is represented as the ratio of current amplitude before and after application of agent. A sigmoidal curve using the Logistic equation (see Methods) was fitted to the data in order to obtain the dose–response curve. The amplitude of tail current was decreased with the half maximal effective concentration (EC50) of 2.03 ± 0.74 µM. The numbers of experiments (n) for each tested concentration are shown in parentheses. Each symbol represents the mean ± SEM
Discussion
To our knowledge there are only two articles directly addressing the ibutilide and delayed rectifier K+ channels. One reveals an inhibition of IKr in AT-1 cells (Yang et al. 1995) and another the most effective concentration at 300 nM on HERG in oocytes (Perry et al. 2004). Both studies are not conducted in native cardiomyocytes and do not elucidate the IKs either in isolation or simultaneously. The latter constitute the novelty of current experiments in ventricular cells of mammals. The aim was accomplished by using envelope of tails test and observing simultaneously the IKr and IKs components.
The delayed rectifier potassium outward currents modulate the repolarization phase of cardiac action potentials (Nerbonne 2016). In most species multiple components of outward K+ currents are concurrently activated during depolarization and they are subsequently dissected by designated pulse protocols, changing the HP, or using antagonists (Kodirov et al. 2003, 2004a, b). Also simulated AP and 11 major underlying currents have been described for a guinea pig ventricular cell model (Noble et al. 1998). In this model the delayed rectifier channels have in turn 4 components: total IK, IKr1, IKr2, and IKs. In contrast to real channels none of these four simulated currents exhibit tail component upon the repolarization of AP. But the IKr tail contributes to simulated AP of ventricle in human (Dutta et al. 2017).
In guinea pig ventricular cardiomyocytes robust tails observed after activation of IK (Figs. 1 and 3). Designated protocol—envelope of tails test enables the quasi separation of IKr and IKs components. Under control conditions, the ratio of the tails to the time-dependent currents (IK-tail/ΔIK) was not constant in respect to pulse duration. When the ratio is constant over entire pulse durations, the delayed rectifier consists of only one component in given preparation/conditions, while the altered ratio indicates that more than one components of currents are involved (Noble and Tsien 1969). In current experiments when the duration of depolarizing pulse to + 40 mV was prolonged, the amplitude of both time-dependent outward and tail current was increased. Our finding indicates that the short pulses activate IKr mainly, whereas IKs requires much longer pulse duration to be activated. Therefore, the ratio should be large because ΔIK is minimal at + 40 mV while IK-tail at − 40 mV is large due to the strong inward rectification property of IKr component. With longer pulses, this ratio decreases and after ~ 1 s the ratio reaches its constant value.
The class III antiarrhythmic agent, E-4031, has been characterized previously as a selective IK blocker with a drug sensitive (IKr) and drug resistant (IKs) components in ventricular myocytes (Follmer and Colatsky 1990). The application of E4031 (1-10 µM) blocks the IKr activity. This rapidly activating component showed characteristics similar to those of IKr as described in rabbit and canine ventricular cells (Cimini et al. 1992; Cordeiro et al. 2013). The major physiological feature of IKr is a rapid activation and a prominent inward rectification, which occurs due to C-type inactivation (Smith et al. 1996; Spector et al. 1996).
The human ether-à-go–go related gene (HERG) encodes the rapid component of IK in cardiac cells (Curran et al. 1995; Sanguinetti et al. 1995; Trudeau et al. 1995). Multiple parameters of these channels and their currents may contribute to long QT syndrome as revealed for the deactivation rate (McBride et al. 2013). The slow component (IKs) of delayed rectifier is a result of coassembly of two proteins, KvLQT1 and minK (Barhanin et al. 1996; Sanguinetti et al. 1996). The single channel properties of IKs have been also established (Sesti and Goldstein 1998).
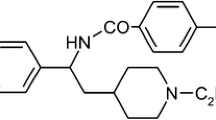
Our results show that ibutilide, known also as an activator of slow inward Na+ currents occurring in a non-selective way via L-type Ca2+ channels (Lee 1992; Lee and Lee 1998), selectively blocks IKr (Fig. 2). The latter effect constitutes a mechanism by which ibutilide modulates the action potential duration in guinea pig ventricular myocytes (Scheme 1). Since amplitudes of tails during 0.2 s in our preparation were relatively small (compared to cloned HERG), the half maximal effective concentration (EC50) of ~ 2 µM was revealed at + 40 mV (Fig. 4). This could be considered as shortcoming, since often pulses to about ± 0 mV is preferred, where IKs contribution to the family of outward currents to be less whereas its activation along the IKr is possibly similar at + 40 mV. However, resistance of tails to highest concentration of ibutilide at longer pulses substantiates marginal involvement of IKs under our conditions. Nevertheless, we suggest that whichever way is chosen to separate these components it is difficult to avoid an overlapping activities of either IKr or IKs and underlying channels in native cells (Kodirov 2019).
Furthermore, the effect of the drug was concentration dependent in a ‘classical’ way compared to the ‘bell-shaped’ manner as in the case of Na+ current (Lee 1992). The fact that the blockade increases over a voltage range in which the probability of channel opening also increases suggests that the drug binds when the channel is open (Balser et al. 1991). The effect of ibutilide on IKr was not completely reversible with wash-out that is similar to data in AT-1 cells (Yang et al. 1995).
In dog model of CAVB—chronic atrioventricular block ibutilide prolongs the duration of MAP—monophasic action potentials (Scheme 1) and triggers TdP—Torsades de Pointes in LV and RV, but the EAD—early after depolarization is observed in LV only (Vos et al. 2001). The AP phenotypes of LV vary and sometimes exhibit the MDP—maximal diastolic potential that is an unstable RMP (Verduyn et al. 2004). However, it is not clear which of conditions are contributing to, i.e., endocardium vs. mid-myocardium or in vitro vs. in vivo. Ibutilide also decreases the amplitude of MAP that is, however, reversed during cumulative concentrations (Vos et al. 2001). Importantly, the QT interval is not modulated by only one type of channel (Kodirov 2015). Therefore, perhaps other alternatives for the therapy are considered as advantageous (Donahue 2012; Kodirov et al. 2003).
We conclude that IKr blockade contributes to QT interval prolongation by ibutilide, which thereby shows a class III antiarrhythmic action. Although, the ibutilide effects are comparable with other drugs, it may also trigger TdP which underlie extrasystoles and QT prolongation as shown in rabbits (Amos et al. 2001). The envelope of tails test not only enabled the distinction of IKr and IKs components, but also revealed targeted effects of ibutilide toward former. Moreover, since even 100 μM ibutilide did not obliterate the IKs it can be used as both an experimental tool and negative control along the selective blockers.
Abbreviations
- AF:
-
Atrial fibrillation
- AP:
-
Action potential
- APD:
-
Action potential duration
- APD90 :
-
Action potential duration at 90% repolarization
- Cav1:
-
Voltage-dependent calcium channel responsible for L-type currents
- EAD:
-
Early afterdepolarization
- ECG:
-
Electrocardiogram
- I K :
-
Delayed rectifier potassium currents
- I K-tail :
-
Delayed rectifier potassium currents upon repolarization
- I Kr :
-
Rapid component of delayed rectifier potassium currents
- I Ks :
-
Slow component of delayed rectifier potassium currents
- LV:
-
Left ventricle
- MDP:
-
Maximum diastolic potential, the counterpart of RMP in pacemaker cells
- PR:
-
Duration of PR waves reflects the electrical conductance from atria to ventricle
- QRS:
-
Timing of QRS complex reflects the depolarization phase of ventricular AP
- QT:
-
Interval between the Q and T waves in ECG
- RMP:
-
Resting membrane potential
- RV:
-
Right ventricle
- TdP:
-
Torsades de Pointes
References
Amos GJ, Abrahamsson C, Duker G, Hondeghem L, Palmer M, Carlsson L (2001) Potassium and calcium current blocking properties of the novel antiarrhythmic agent H 345/52: implications for proarrhythmic potential. Cardiovasc Res 49:351–360
Balser JR, Bennett PB, Hondeghem LM, Roden DM (1991) Suppression of time-dependent outward current in guinea pig ventricular myocytes. Actions of quinidine and amiodarone. Circ Res 69:519–529
Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G (1996) K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384:78–80
Buchanan LV, Kabell G, Turcotte UM, Brunden MN, Gibson JK (1992) Effects of ibutilide on spontaneous and induced ventricular arrhythmias in 24-hour canine myocardial infarction: a comparative study with sotalol and encainide. J Cardiovasc Pharmacol 19:256–263
Cimini MG, Brunden MN, Gibson JK (1992) Effects of ibutilide fumarate, a novel antiarrhythmic agent, and its enantiomers on isolated rabbit myocardium. Eur J Pharmacol 222:93–98
Cordeiro JM, Panama BK, Goodrow R, Zygmunt AC, White C, Treat JA et al (2013) Developmental changes in expression and biophysics of ion channels in the canine ventricle. J Mol Cell Cardiol 64:79–89
Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT (1995) A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80:795–803
DeFelice LJ, Goolsby WN, Mazzanti M (1990) Potassium channels and the repolarization of cardiac cells. Ann N Y Acad Sci 588:174–184
Donahue JK (2012) Gene therapy for ventricular tachyarrhythmias. Gene Ther 19:600–605
Dutta S, Mincholé A, Quinn TA, Rodriguez B (2017) Electrophysiological properties of computational human ventricular cell action potential models under acute ischemic conditions. Prog Biophys Mol Biol 129:40–52
Ellenbogen KA, Clemo HF, Stambler BS, Wood MA, VanderLugt JT (1996) Efficacy of ibutilide for termination of atrial fibrillation and flutter. Am J Cardiol 78:42–45
Follmer CH, Colatsky TJ (1990) Block of delayed rectifier potassium current, I K, by flecainide and E-4031 in cat ventricular myocytes. Circulation 82:289–293
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100
Hiraoka M, Sawada K, Kawano S (1986) Effects of quinidine on plateau currents of guinea-pig ventricular myocytes. J Mol Cell Cardiol 18:1097–1106
Isenberg G, Klockner U (1982) Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium”. Pflugers Arch 395:6–18
Jung F, DiMarco JP (1996) Antiarrhythmic drug therapy in the treatment of atrial fibrillation. Cardiol Clin 14:507–520
Kodirov SA. 1998. Patch-Clamp-Untersuchungen zur Wirkungsweise von Ibutilide auf Ionenkanäle an Herzmuskelzellen des Meerschweinchens [Ph. D.]. Heidelberg: Ruprecht-Karls-Universität Heidelberg. 98 p
Kodirov SA (2015) LQT, HCN and epilepsy model. Epilepsia 56:1855
Kodirov SA. 2019. Tale of tail current. Prog Biophys Mol Biol: In Press
Kodirov SA, Brunner M, Busconi L, Koren G (2003) Long-term restitution of 4-aminopyridine-sensitive currents in Kv1DN ventricular myocytes using adeno-associated virus-mediated delivery of Kv1.5. FEBS Lett 550:74–78
Kodirov SA, Brunner M, Nerbonne JM, Buckett P, Mitchell G, Koren G (2004a) Attenuation of IK, slow1 and IK, slow2 in Kv1/Kv2DN mice prolongs the APD and QT intervals but does not suppress spontaneous or inducible arrhythmias. Am J Physiol Heart Circ Physiol 286:H368–H374
Kodirov SA, Zhuravlev VL, Pavlenko VK, Safonova TA, Brachmann J (2004b) K+ channels in cardiomyocytes of the pulmonate snail Helix. J Membr Biol 197:145–154
Kodirov SA, Wehrmeister M, Colom LV (2014) Modulation of HCN channels in lateral septum by nicotine. Neuropharmacology 81:274–282
Lee KS (1992) Ibutilide, a new compound with potent class III antiarrhythmic activity, activates a slow inward Na+ current in guinea pig ventricular cells. J Pharmacol Exp Ther 262:99–108
Lee KS, Lee EW (1998) Ionic mechanism of ibutilide in human atrium: evidence for a drug—induced Na+ current through a nifedipine inhibited inward channel. J Pharmacol Exp Ther 286:9–22
McBride C, Smith A, Smith J, Reloj A, Velasco E, Powell J et al (2013) Mechanistic basis for type 2 long QT syndrome caused by KCNH2 mutations that disrupt conserved arginine residues in the voltage sensor. J Membr Biol 246:355–364
Mitra R, Morad M (1985) A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol 249:H1056–H1060
Nerbonne JM (2016) Molecular basis of functional myocardial potassium channel diversity. Card Electrophysiol Clin 8:257–273
Noble D, Tsien RW (1969) Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol 200:205–231
Noble D, Varghese A, Kohl P, Noble P (1998) Improved guinea-pig ventricular cell model incorporating a diadic space, I Kr and I Ks, and length- and tension-dependent processes. Can J Cardiol 14:123–134
Perry M, de Groot MJ, Helliwell R, Leishman D, Tristani-Firouzi M, Sanguinetti MC et al (2004) Structural determinants of HERG channel block by clofilium and ibutilide. Mol Pharmacol 66:240–249
Sanguinetti MC, Jurkiewicz NK (1990) Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96:195–215
Sanguinetti MC, Jurkiewicz NK (1991) Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am J Physiol 260:H393–H399
Sanguinetti MC, Jiang C, Curran ME, Keating MT (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the I Kr potassium channel. Cell 81:299–307
Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL et al (1996) Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384:80–83
Sano T, Sugiyama S, Taki K, Hanaki Y, Shimada Y, Ozawa T (1990) Effects of antiarrhythmic agents classified as class III group on ischaemia-induced myocardial damage in canine hearts. Br J Pharmacol 99:577–581
Sasaki N, Watanabe I, Kogawa R, Sonoda K, Takahashi K, Okumura Y et al (2014) Effects of intravenous amiodarone and ibutilide on action potential duration and atrial conduction kinetics in patients with persistent atrial fibrillation. Int Heart J 55:244–248
Sendra-Ferrer M, Gonzalez MD (2019) Ibutilide for the control of refractory ventricular tachycardia and ventricular fibrillation in patients with myocardial ischemia and hemodynamic instability. J Cardiovasc Electrophysiol. https://doi.org/10.1111/jce.13835
Sesti F, Goldstein SA (1998) Single-channel characteristics of wild-type I Ks channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol 112:651–663
Smith PL, Baukrowitz T, Yellen G (1996) The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379:833–836
Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC (1996) Fast inactivation causes rectification of the I Kr channel. J Gen Physiol 107:611–619
Stambler BS, Beckman KJ, Kadish AH, Camm JA, Ellenbogen KA, Perry KT et al (1997) Acute hemodynamic effects of intravenous ibutilide in patients with or without reduced left ventricular function. Am J Cardiol 80:458–463
Trudeau MC, Warmke JW, Ganetzky B, Robertson GA (1995) HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269:92–95
Verduyn SC, Jungschleger JG, Stengl M, Spätjens RL, Beekman JD, Vos MA (2004) Electrophysiological and proarrhythmic parameters in transmural canine left-ventricular needle biopsies. Pflugers Arch 449:115–122
Vos MA, van Opstal JM, Leunissen JDM, Verduyn SC (2001) Electrophysiologic parameters and predisposing factors in the generation of drug-induced Torsade de Pointes arrhythmias. Pharmacol Ther 92:109–122
Weidmann S (1950) Die Natur des induktiven Elements in biologischen Membranen. Experientia 6:53–54
Yang T, Snyders DJ, Roden DM (1995) Ibutilide, a methanesulfonanilide antiarrhythmic, is a potent blocker of the rapidly activating delayed rectifier K+ current (I Kr) in AT-1 cells. Concentration-, time-, voltage-, and use-dependent effects. Circulation 91:1799–1806
Acknowledgements
Technical support of Mrs. Klara Güth and comments and useful discussions by Drs. Armin Just and Johannes Vogel, Heidelberg University are gratefully acknowledged. We thank Mrs. Tania Simon, Heidelberg University and Mrs. Carrie Couper, Harvard University for critically reading the manuscript.
Funding
Deutsche Forschungsgemeinschaft and SFB320 ‘Herzfunktion und ihre Regulation’ project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the Authors have potential conflicts of interest.
Ethical Approval
The guidelines for the care and use of laboratory animals were strictly followed, and all procedures were approved by the local committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kodirov, S.A., Zhuravlev, V.L. & Brachmann, J. Prevailing Effects of Ibutilide on Fast Delayed Rectifier K+ Channel. J Membrane Biol 252, 609–616 (2019). https://doi.org/10.1007/s00232-019-00098-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-019-00098-x