Abstract
The obtained results demonstrated an influence of PEF on increase in accumulation of various ions in S. cerevisiae cells. Optimization of particular PEF parameters and ions concentrations in the medium caused twofold increase in accumulation of magnesium and zinc ions and 3.5-fold higher accumulation of calcium ions in the cells. In the case of ion couple, accumulation of magnesium and zinc was, respectively, 1.5-fold and twofold higher in comparison to the control cultures. Yeast cells biomass enriched with Mg2+, Zn2+, Ca2+ as well as Mg2+ and Zn2+ (simultaneously) may be an alternative for pharmacological supplementation applied in deficiency of these cations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development of dietetics and nutrition sciences caused an increase in awareness of well-balanced diet including bioelements essential for living organisms. Magnesium, zinc, calcium, and selenium regulate cell metabolism as coenzymes. In recent years, deficiency symptoms of these elements have been observed which direct attention to yeasts as a potential source of not only valuable proteins but also deficient bioelements. Yeasts bind metal ions from the environment and permanently incorporate them into the cell structures. This is the way how biocomplexes or metalloproteins are formed (Liu et al. 2002). Previous research showed that these kind of complexes are better assimilated by the human body than mineral preparations (De Nicola and Walker 2009). Yeast cells biomass enriched with Mg2+, Zn2+, Ca2+ as well as Mg2+ and Zn2+ (simultaneously) became an alternative to pharmacological supplementation applied in deficiency of these cations. Bioelements provided in the form of metalloproteins are better assimilated by the body in comparison to the pharmacological preparations composed on the basis of organic or inorganic salts of these elements. Food products contained yeasts enriched with the above-mentioned elements could constitute an additional source of them in a diet (Vinopal et al. 2007; Cha and Cho 2009).
There are a few mechanisms of intake and accumulation of metal ions by microorganisms. These ions can be accumulated in an unspecified way on the surface of the cells. Sorption of ions and their binding by polymers of the cell wall, extracellular envelope, and mucus can also be responsible for accumulation. A common phenomenon is a specific active transport of some ions, e.g., sodium, potassium, magnesium, and manganese, to the cells of microorganisms. In the specific cases, biosynthesis and secretion of chelating compounds and ionophores enabling intake of an ion by the cells can take place. Accumulation of metal ions by microorganisms with the use of the above-mentioned mechanisms is now investigated by numerous scientists. In our studies, I used electroporation (Stehlik-Tomas et al. 2004) to enhance accumulation of the selected ions by Saccharomyces cerevisiae cells.
Treatment of the cells with pulsed electric field (PEF) is called electroporation or electroperforation and is based on an action of alternating current on the cells. In the cell treated with PEF, the induced transmembrane potential causes pore formation in the membrane and leads to an increase of its permeability. According to the model proposed by Zimmermann (1986) charges of opposite sign are induced by the electric field on the outer and inner surface of the cell membrane. Once the transmembrane potential reaches the critical value, bilateral attraction of charges leads to the formation of the large number of pores. Permeability of the cell membrane can increase to the level that allows such molecules as DNA or metal ions to enter the cell. When an action of the electric field stops, pores are sealed and the cells retain introduced molecules or ions.
The cell membrane loses its continuity when the membrane potential exceeds 0.5–1.0 V. Electroporation can be a reversible process when pores are sealed again. It depends on intensity and exposition time of the external field. Process is reversible when electric impulses of the field intensity in the range 1–20 kV/cm last from micro- to milliseconds. Pores open during a few microseconds and close after various time, depending on temperature, from a few seconds at 37 °C to several minutes at 4 °C (Torregrosa et al. 2006). When the electric field intensity exceeds considerably the critical value, pore formation can be irreversible and leads to destruction of a cell. Therefore, it is crucial that electroporation should not cause a serious damage to the cell and disturb its fundamental physiological processes.
It is also possible to perforate cells which compose organs e.g., islets of Langerhans in rat’s pancreas secrete more insulin after electroporation (Yaseen et al. 1982).
Electroporation is an easy, non-toxic, and cheap method of introduction of ions and macromolecules to the cells. It consists in formation of pores in the cell membrane that are big enough to permit the molecules enter cytoplasm and, at the same time, small enough to be sealed after some time in the way characteristic for the given type of the cells. Electroporation can be used in the case aqboth animal and plant cells (Aronsson et al. 2005). Most commonly PEF is applied for introducing DNA fragments, histones (20 kDa), ovoalbumins (45 kDa), immunoglobulins G (150 kDa), catalase (240 kDa), ferritin (445 kDa), molecules of colloidal gold diameter (5–20 nm), and latex (diameter 0.1 µm) to the cells.
In genetics, electroporation is used for introduction of nucleic acids into the cells. It is an alternative for viral vectors and chemical methods applied in genetic modifications. One of the advantages of electroporation is that it does not bring into environment any additional substances that may affect the cell in undesirable way.
Permeability of the cell membrane depends on as follows:
-
Kind and size of the cell,
-
Electric field intensity,
-
Impulse length,
-
Pulse width,
-
Field exposure time,
-
Time of culturing after which cell biomass is treated with PEF,
-
Chemical composition of the culture medium.
S. cerevisiae cells (GRAS) can constitute a source of deficient bioelements and vitamins in a diet. Accumulation of metal ions in the cells with the use of PEF is a point of the research. The reason for which I decided to undertake this subject was a lack of studies on effect of PEF on accumulation of selected ions in yeast cells in scientific literature. The literature data concern mainly an effect of PEF on survivability of microorganisms or changes of enzyme activity in liquid food products (Korolczuk et al. 2006). Moreover, the scientific reports only provide information on bioaccumulation of various ions without PEF participation (Tuszyński and Pasternakiewicz 2000; Gniewosz et al. 2006). The presented cycle of reports composing the accomplishment is a review of the publications documenting an effect of PEF on accumulation of various ions in Saccharomyces cerevisiae cells.
Electroporation of S. cerevisiae Cells’ Membrane at Increasing Concentrations of Selected Metal Ions
Accumulation of metal ions in S. cerevisiae cells depends on concentration of particular ions in the medium and their bioavailability. Accumulation of metal ions probably depends on intracellular transportation systems and on their chelating strength, by medium compounds and cellular substances. The majority of extracellular manganese, zinc, and magnesium is stored in vacuoles, where they can be bound by polyphosphates of a low molecular weight (Williams and Frausto da Silva 2000; Błażejak et al. 2004).
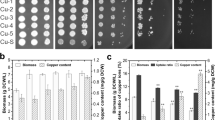
There is a small number of studies in which a new technique TEM-EDX is used for mapping of metal ions e.g., lead and potassium in Rhodotorula glutinis cells (Cho and Kim 2003), silver in Escherichia coli cells (Yamanaka et al. 2005) and ratio of Mn/P in Cladosporium cladosporioides cells (Shao and Sun 2007). Results and TEM-EDS analysis of S. cerevisiae cells enriched with zinc and magnesium (simultaneously) showed that in the case of the culture treated with PEF, accumulation of Zn2+ and Mg2+ in the cell wall is poorly visible, but noticeable. In cells from the cultures treated with PEF zinc ions are mainly distributed in cell organelles, whereas for Mg2+ the ions are observed in the cell wall (Pankiewicz et al. 2014).
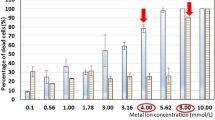
In the studies, I revealed the influence of concentration of Zn2+, Mg2+, Ca2+, and ion couple Zn2+ and Mg2+ in the medium on their accumulation in S. cerevisiae cells in the control cultures (not treated with PEF) as well as in the cultures treated with PEF. In the case of the control cultures, an increase of Ca2+ and Mg2+concentration in the whole range applied (10–100 µg/mL medium) did not affect significantly their accumulation in the cells. In the cultures treated with PEF, the effect of increasing concentrations of these ions in the medium on their accumulation in the cells was statistically significant (Pankiewicz and Jamroz 2010, 2013). In the case of Zn2+, initial increase of its concentration caused also a higher accumulation but when it was over 100 µg Zn2+/mL medium the opposite trend was observed both in the cultures treated and not treated with PEF (Pankiewicz and Jamroz 2011).
In the case of Ca2+, Zn2+, Mg2+, a higher accumulation in the cells was noted in the cultures treated with PEF in comparison to the control cultures in the whole range of concentrations (10–1000 µg/mL medium). At optimum concentration, 100 µg/mL medium, accumulation was higher sixfold for Ca2+, 1.5-fold for Zn2+, and twofold for Mg2+.
Accumulation of magnesium at optimal concentration 100 µg/mL medium reached 4 mg/g d.m. which constituted 40 % Mg2+ added to the medium; for zinc it was as high as 13,29 mg/g d.m. (63 % Zn2+) and for calcium—3 mg/g d.m. (30 % Ca2+) (Pankiewicz and Jamroz 2010, 2011, 2013).
Results of the next experiments showed that concentration of ion couple, magnesium, and zinc, in the medium has a significant effect on their accumulation in yeast cells. At a few combinations of ions concentrations, a relatively high accumulation of one of the ions was noted and, in the case of the second one, it was two or more times lower than maximum value. Concentrations of 100 µg Mg2+/mL and 150 µg Zn2+/mL medium were assumed as optimal for both ions. The high accumulation of Mg2+ (2.32 mg/g d.m.) and Zn2+ (7.24 mg/g d.m.) was obtained at these concentrations in the cultures treated with PEF (Pankiewicz et al. 2014).
Optimization of PEF Parameters (Intensity, Pulse Width, Exposition Time, and PEF Treatment of the Cells in Various Stages of Growth)
The results demonstrated that electric fields strength between 0.1 and 1.0 kV/cm had no significant influence on ions accumulation in the cells which was similar to that in the control culture. Statistically significant changes of their accumulation were noted in the range of high values of electric fields strength from 2 to 6 kV/cm. At the optimal value, which was 5.0 kV/cm, the maximum accumulation was noted and it was two (Mg2+, ion couple Zn2+, and Mg2+) or three times higher in comparison to the control culture (Pankiewicz and Jamroz 2010, 2013; Pankiewicz et al. 2014). Electric fields strength above 3.0 kV/cm for Zn2+ and 5.0 kV/cm for Ca2+, Mg2+, and ion couple Zn2+ and Mg2+ caused a statistically significant decrease of ions accumulation in the cells or did not provide any changes as in the case of ion couple (Pankiewicz and Jamroz 2010, 2011, 2013; Pankiewicz et al. 2014) (Table 1).
The study revealed that pulse width had also a significant effect on accumulation of the examined ions in the cells. The range applied was 10–150 µs. Maximum accumulation of ions in yeast cells was reached at 10 or 20 µs and these values were assumed to be optimal (Table 1). At pulse width of 10 µs the maximum accumulation of zinc in the yeast biomass (15 mg/g d.m) was reached and it was over twofold higher in comparison to the culture not treated with PEF (Pankiewicz and Jamroz 2011). In the case of Mg2+, Ca2+, and ion couple Zn2+ and Mg2+ the highest accumulations, respectively 4, 2.5, 11.42, and 2.89 mg/g d.m., were noted at pulse width of 20 µs (Pankiewicz and Jamroz 2010, 2013; Pankiewicz et al. 2014). Accumulation of Mg2+ and Ca2+ was, respectively, 2 and fivefold higher than in the culture not treated with PEF (Pankiewicz and Jamroz 2010, 2013) (Table 2). Statistically significant decrease in accumulation of all analyzed ions (e.g., over twofold for Ca2+) in the cells was noted when pulse width was higher than optimal one (Pankiewicz and Jamroz 2010, 2011, 2013; Pankiewicz et al. 2014).
It was proved in numerous experiments that PEF exposition time is a very important parameter that influences ions accumulation in the yeast biomass. Cells were treated with PEF for 5, 10, 15, 20, and 25 min. After 5 and 10 min of exposition to PEF, accumulation of ions was higher when compared to the control culture but significantly lower than at optimal exposition time determined for each ion (Pankiewicz and Jamroz 2010, 2011, 2013; Pankiewicz et al. 2014). 15 and 20 min were regarded as optimal PEF exposition time for the highest accumulation of ions in the cells (Table 1). After 15 min of PEF exposition, accumulation of Mg2+, Zn2+, and ion couple Mg2+ and Zn2+ was maximum and for Ca2+ it was reached after 20 min. Prolonging of exposition time to 20 and 25 min caused a statistically significant decrease in accumulation of all ions except Mg2+ for which no effect was observed (Pankiewicz and Jamroz 2010, 2011, 2013; Pankiewicz et al. 2014).
It was also revealed that time of culturing, after which cells were treated with PEF, affected significantly ions accumulation. Yeast biomass was treated with PEF after 8, 12, 16, 20, and 24 h of culturing. Maximum accumulation of ions was reached after PEF treatment of the 20 h cultures (Pankiewicz and Jamroz 2010, 2011, 2013; Pankiewicz et al. 2014) (Table 1). In the case of zinc, its accumulation (about 15.5 mg/g d.m.) was about 72 or 57 % higher than in the cultures treated with PEF after 8 or 24 h of cells multiplication (Pankiewicz and Jamroz 2011). The results obtained for magnesium (about 4 mg/g d.m.) (Pankiewicz and Jamroz 2010) and for calcium (3 mg/g d.m) (Pankiewicz and Jamroz 2013) were also higher by, respectively, 34 or 23 % and 150 or 8 %. Maximum accumulation of Zn2+ (11.41 mg/g d.m.) and Mg2+ (2.85 mg/g d.m.) was observed after treatment of 20 h culture with PEF. Zinc concentration in the cells was about 30 % higher in comparison to the cultures treated with PEF after 8 or 24 h of cells multiplication. Accumulation of magnesium in the cells did not increase statistically significantly as a result of electroporation of the cultures after various times of culturing (Pankiewicz et al. 2014).
In the cells from the cultures treated with PEF after 8, 12, 16 h of culturing accumulation of ions was lower, whereas after 24 h of culturing—a statistically significant decrease was noted in comparison to the culture electroporated after 20 h of culturing.
In the cultures enriched with MgCl2·6H2O (1.25 g Mg2+/L), after 48 h Błażejak et al. (2004) collected 5.77 g dw/L biomass with washing. In 48 h culturing S. cerevisiae 0.5 g Mg2+/L added, (Duszkiewicz-Reinhard et al. 2002) noted 3.13 mg Mg/g dm in the rinsed biomass. That accumulation was by 30 % higher than in the control culture, which was not supplemented with magnesium.
Walker and Maynard (1996) reached maximum accumulation of magnesium, 450 µM, after 40 h culturing in the medium containing 347 µM magnesium. Higher concentration of magnesium, 496 µM, provided 300 µM of accumulated element after 58 h culturing. After 17 h culturing in the broth with 30 mM magnesium, Blackwell et al. (1997) found magnesium accumulation on the level of 4000 nmol (109 cells)−1.
Stehlik-Tomas et al. (2004) enriched S. cerevisiae cells with zinc, copper, and manganese. The highest concentration of zinc in dry matter (700 µg/g) was achieved in anaerobic conditions at medium pH 4.
Cha and Cho (2009) the highest accumulation of zinc was obtained in a medium containing 2.0 %(w/v) glucose, 5.0 % yeast extract, and 0.05 % ZnSO4 after cultivation at 110 rpm, 30 ∘C, 72 h. Under these conditions, the 31,932 ppm zinc represented a 93-fold increase over the 339 ppm in the basal medium.
Bonin and Ślusarska (2007) obtained the highest increase of biomass (9.2 g d.m./L) for the sample enriched with 40 mg Ca2+/L after 72 h of culturing Saccharomyces bayanus yeasts, and the lowest (7.7 g d.m./L)—for the culture supplemented with 400 mg Ca2+/L.
Pasternakiewicz and Tuszyński (1997) experiments confirmed the beneficial effect of calcium ions on yeast growth. However, the studied strains differed in their sensitivities to these ions. For instance, the Mautner strain growth was reduced by the calcium concentrations higher than 20 mmol/l after 16 h cultivation, whereas the YT strains did not negatively respond even to 50 mmol/L concentrations within the whole culturing period. Lotan et al. (1976) demonstrated that cells of Saccharomyces carlsbergensis accumulated relatively low amounts of calcium isotope. Other investigations (Mochaba et al. 1996a, b) demonstrated the ability of yeast cell walls to bind Ca2+ in the amount of 2.5–7.5 mol per 100 g of dry matter.
Multiplicity of PEF Exposition and Way of Salt Dosage During Culturing
In the next experiment, I treated yeast cultures fourfold with PEF after 8, 12, 16, 20 h from the beginning of culturing. I wanted to find out what is a relation between level of ions accumulation in the cells and multiple exposition of cells at different stages of growth to PEF. Cultures were conducted at optimized concentrations of ions in the medium and PEF parameters. In the case of ions: Zn2+, Mg2+, Ca2+, and ion couple Zn2+ and Mg2+, multiple exposition to PEF did not cause a rise of their accumulation. It was even lower by 47 % (Zn2+) and 125 % (Ca2+) in comparison to the cultures after single PEF treatment (Pankiewicz and Jamroz 2011, 2013). Accumulation of ion couple Mg2+ and Zn2+was 2.5 and 3.5-fold lower in comparison to the maximum accumulation obtained after a single PEF treatment of the culture (Pankiewicz et al. 2014). Only in the case of Mg2+, its accumulation in the cells treated with PEF fourfold increased by 5 % compared to the cells biomass treated with PEF once (Pankiewicz and Jamroz 2010).
A method of salt dosage to the medium seemed to be important with respect to rising of ionic strength of environment or changes of the osmotic pressure. Total dose of salt was added in four portions to the cultures conducted at optimized PEF parameters and optimal concentration of the examined ions in the medium after 8, 12, 16, and 20 h of culturing. Salt dosage did not increase ions accumulation in the cells. In the case of Mg2+, Ca2+, and Zn2+, it was even lower by, respectively, 30 and 57 % (for the last two ions) in comparison to the culture which was supplemented with the whole dose of these ions before culturing was started (Pankiewicz and Jamroz 2010, 2011, 2013). Addition of the whole dose of ion couple, magnesium and zinc, to the medium in four portions reduced accumulation by, respectively, 22 and 74 % (compared to the culture which was supplemented with the whole dose before beginning of culturing) (Pankiewicz et al. 2014).
Changes of Cells Viability and Biomass Yield After Treatment with PEF at Increasing Concentrations of Metal Ions
In the succeeding experiments, changes of yeast cells viability in stress conditions such as increasing concentration of metal ions (10–1000 µg/mL) or various range of electric fields strength (1–6 kV/cm) and pulse width (10–150 µs) were studied. Increasing concentration of Mg2+ and Ca2+ in the medium affected a number of dead cells in a much lesser degree than in the case of Zn2+ (Pankiewicz and Jamroz 2010, 2011, 2013). At optimal concentration of Ca2+ (100 µg/mL medium) and Mg2+ (100 µg/mL medium) contribution of dead cells reached, respectively, 17 and 10 %. Further increase of ions concentration in the medium to 1000 µg/mL led to duplication of a dead cells number to about 35 % (Pankiewicz and Jamroz 2010, 2011, 2013). In the case of Zn2+, an increase of its concentration in the medium had a significant effect on cells viability. At optimal concentration of 100 µg Zn2+/mL medium, number of dead cells constituted 15 % but when concentration increased to 1000 µg/mL this number suddenly rose to 78 % (Pankiewicz and Jamroz 2011).
In the case of ion couple Zn2+ and Mg2+, much lesser influence of magnesium on cells viability in comparison to zinc was observed. Increasing concentration of magnesium from 20 to 500 µg/mL medium (at constant zinc concentration of 100 µg/mL medium) caused an increase of dead cells number by 52 % (from 19 to 29 %, respectively). Effect of increasing concentration of zinc in medium (at constant magnesium concentration of 100 µg/mL medium) on dead cells number was significant. For the range of 20–100 µg Zn2+/mL medium, number of dead cells increased threefold from 6 to 18 %. At concentrations higher than 150 µgZn2+/mL medium, dead cells constituted over 50 % of the culture reaching 68 % at 500 µgZn2+/mL medium. At optimal concentration (100 µg Mg2+/mL and 150 µg Zn2+/mL medium), under conditions of maximum accumulation for both ions, contribution of dead cells amounted to 33 % (Pankiewicz et al. 2014).
Changes of electric field strength and pulse width had significant effect on cells viability in the case of all analyzed ions. In order to demonstrate an influence of electric field strength, investigations were conducted for the range 1.0–6.0 kV/cm. In the case of Zn2+, Mg2+, and Ca2+, an increase of this parameter from 2.0 to 5.0 kV/cm led to duplication of dead cells number. At 5.0 kV/cm this number amounted to 12, 17, and 24 %, respectively, for Mg2+, Ca2+, and Zn2+ (Pankiewicz and Jamroz 2010, 2011, 2013). In the case of ion couple Zn2+ and Mg2+, contribution of dead cells in the culture at electric field strength of 5.0 kV/cm reached 46 % and was higher by about 20 % than that observed at 2.0 kV/cm (Pankiewicz et al. 2014). Experiments conducted in order to prove an effect of pulse width on cells viability showed that in the range of pulse width from 10 to 150 µs, number of dead cells increased together with increasing value of this parameter in the case of all ions. At pulse width of 20 µs contribution of dead cells reached 10, 15, and 17 %, respectively, for the cultures containing Mg2+, Zn2+, and Ca2+. An increase of pulse width to 125 µs caused that dead cells number rose 2- (for Mg2+ and Ca2+) or even fourfold (for Zn2+) Pankiewicz and Jamroz (2010, 2011, 2013). In the case of ion couple Zn2+ and Mg2+ an increase of pulse width from 10 to 40 µs (at electric fields strength of 4 kV/cm) caused 2.5-fold rise of dead cells number. Under optimal conditions for accumulation of both ions (pulse width of 20 µs and electric field strength of 5.0 kV/cm), contribution of dead cells amounted to 55 % (Pankiewicz et al. 2014).
In all experiments also, changes of yeast biomass after culturing were investigated. In the cultures enriched with Mg2+ and Ca2+, there was no effect of ions concentration in the medium or particular PEF parameter on changes of biomass, which was on the level of about 1 g d.m./100 mL (Pankiewicz and Jamroz 2010, 2013). Whereas zinc concentration in the medium had a significant influence on biomass. For concentrations up to 100 µg Zn2+/mL medium, no significant rise of biomass, which remained on the level of 0.8–0.9 g d.m./100 mL, was noted. But for the range of 200–1000 µgZn2+/mL medium a significant, even fivefold, drop of biomass was observed (respectively, 0.35 and 0.15 g d.m./100 mL). In the cultures enriched with Zn2+ PEF parameters did not influence yeast biomass (Pankiewicz and Jamroz 2011). In the case of ion couple, increasing Zn2+ concentration in the medium (10–500 µg/mL) at constant concentration of Mg2+ (100 µg/mL medium) caused a slight decrease of yeast biomass by 10 %, whereas increasing Mg2+ concentration in the medium (10-500 µg/mL) at constant Zn2+ concentration (100 µg/mL medium) led to an increase of biomass by 15 % (Pankiewicz et al. 2014).
References
Aronsson K, Rönner U, Borch E (2005) Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. Int J Food Microbiol 99:19–32
Blackwell KJ, Tobin JM, Avery SV (1997) Manganese uptake and toxicity in magnesium-supplemented and unsupplemented Saccharomyces cerevisiae. Appl Microbiol Biotech 47:180–184
Błażejak S, Duszkiewicz-Reinhard W, Gniewosz M, Mazurkiewicz B (2004) Distribution of magnesium in the Candida utilis ATCC 9950 yeast cells enriched in that element (in Polish). Acta Sci Pol Technol Aliment 3:95–110
Bonin S, Ślusarska M (2007) Influence of addition of magnesium and calcium salts to high-sugar musts on the process of wine fermentation and biomass growth (in Polish). Żywn Nauk Technol Ja 4:109–119
Cha JY, Cho YS (2009) Determination of optimal conditions for zinc hyperaccumulation by Saccharomyces cerevisiae FF-10. J Korean Soc Appl Biol Chem 52:227–233
Cho DH, Kim EY (2003) Characterization of Pb2 + biosorption from aqueous solution by Rhodotorula glutinis. Bioprocess Biosyst Eng 25:271–277
De Nicola R, Walker GM (2009) Accumulation and cellular distribution of zinc by brewing yeast. Enzym Microb Technol 44:2010–2016
Duszkiewicz-Reinhard W, Gniewosz M, Błażejak S, Bańkowski A (2002) Badania zdolności wiązania magnezu przez drożdże piekarskie Saccharomyces cerevisiae w hodowli stacjonarnej. Acta Sci Pol Technol Aliment 1:17–26
Gniewosz M, Błażejak S, Roman J, Duszkiewicz-Reinhard W (2006) A study on Saccharomyces cerevisiae and Candida utilis cell wall capacity for binding magnesium. Eur Food Res Technol 224:49–54
Korolczuk J, Mc Keag JR, Fernandez JC, Baron F, Grosset N, Jeantet R (2006) Effect of pulsed electric field processing parameters on Salmonella enteritidis inactivation. J Food Eng 75:11–20
Liu GJ, Martin DK, Gardner RC, Ryan PR (2002) Large Mg2+—dependent currents are associated with the increased expression of ALR1 in Saccharomyces cerevisiae. Microbiol Lett 213:231–237
Lotan R, Berdicevsky I, Merzbach D, Grossowicz N (1976) Effect of calcium ions on growth and metabolism of Saccharomyces carlsbergensis. J Gen Microbiol 92:76–80
Mochaba F, O’Connor-Cox ESC, Axcell BC (1996a) Effects of yeast quality on the accumulation and release of metal causing beer instability. J Am Soc Brew Chem 54:164–171
Mochaba F, O’Connor-Cox ESC, Axcell BC (1996b) Metal ion concentration and release by a brewing yeast: characterization and implications. J Am Soc Brew Chem 54:155–163
Pankiewicz U, Jamroz J (2010) Effect of pulsed electric fields upon accumulation of magnesium in Saccharomyces cerevisiae. Eur Food Res Technol 231:663–668
Pankiewicz U, Jamroz J (2011) Effect of pulsed electric fields upon accumulation of zinc in Saccharomyces cerevisiae. J Microbiol Biotechnol 21:646–651
Pankiewicz U, Jamroz J (2013) Application of pulsed electric field for enrichment of Saccharomyces cerevisiae cells with calcium ions. Ital J Food Sci 4:394–402
Pankiewicz U, Sujka M, Włodarczyk-Stasiak M, Mazurek A, Jamroz J (2014) Effect of pulse electric fields (PEF) on accumulation of magnesium and zinc ions in Saccharomyces cerevisiae cells. Food Chem 157:125–131
Pasternakiewicz A, Tuszyński T (1997) Effect of calcium, magnesium, cobalt (II), and zinc cations on the Saccharomyces cerevisiae growth. Pol J Food Nutr Sci 4:61–70
Shao Z, Sun F (2007) Intracellular sequestration of manganese and phosphorus in a metal-resistant fungus Cladosporium cladosporioides from deep-sea sediment. Extremophiles 11:435–443
Stehlik-Tomas V, Zetic VG, Stanzer D, Grba S, Vahcic N (2004) Zinc, copper and manganese enrichment in yeast Saccharomyces cerevisiae. Food Technol Biotechnol 42:115–120
Torregrosa F, Esteve MD, Frigola A, Cortes C (2006) Ascorbic acid stability during refrigerated storage of orange-carrot juice treated by high pulsed electric field and comparison with pasteurized juice. J Food Eng 73:339–345
Tuszyński T, Pasternakiewicz A (2000) Bioaccumulation of metal ions by yeast cells of Saccharomyces cerevisiae. Pol J Food Nutr Sci 4:31–39
Vinopal S, Ruml T, Kotrba P (2007) Biosorption of Cd2+ and Zn2+ by cell surface-engineered Saccharomyces cerevisiae. Int Biodeter Biodegr 60:96–102
Walker GM, Maynard AI (1996) Magnesium-limited growth of Saccharomyces cerevisiae. Enzym Microbiol Technol 18:455–459
Williams RJP, Frausto da Silva JJR (2000) The distribution of elements in cells. Coord Chem Rev 200–202:247–348
Yamanaka M, Hara K, Kudo J (2005) Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering Transmission Electron Microscopy and proteomic analysis. Appl Environ Microbiol 71:7589–7593
Yaseen M, Pedley K, Howell S (1982) Regulation of insulin secretion from islets of langerhans rendered permeable by electric discharge. Biochem J 206:81–87
Zimmermann U (1986) Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol 105:175–256
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pankiewicz, U., Sujka, M. & Jamroz, J. Bioaccumulation of the Selected Metal Ions in Saccharomyces cerevisiae Cells Under Treatment of the Culture with Pulsed Electric Field (PEF). J Membrane Biol 248, 943–949 (2015). https://doi.org/10.1007/s00232-015-9844-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-015-9844-3