Abstract
The pool boiling of R-134a has been experimentally investigated with an addition of nano particles of Aluminum oxide. The experiments were carried out using a cylindrical stainless-steel heater. The roughness of the heater surface was changed. Different concentrations of nano Aluminum oxide particles to the base R-134a were tested. Different heat fluxes as well as different boiling pressures were considered during the experimental tests. The results show that the suspension of Al2O3 nano particles enhances heat transfer coefficient in the nucleate pool boiling zone for concentrations ranging from 0.01 to 0.25% by volume. Higher heat flux and pressure result in enhancements of 37.6, 55.4, 90.2 and 167.7% corresponding to 0.042, 0.84, 1.54 and 2.35 μm surface roughness respectively. The more concentration of Al2O3 nano particles deteriorates the heat transfer coefficient. An empirical correlation was deduced to formulate the relation among heat transfer coefficient, heat flux, pressure, concentration, and surface roughness within a maximum deviation of about ±9%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nucleate boiling exhibits in various engineering fields such as nuclear energy, electric power generation, electronic chips cooling and air conditioning equipment. The solid particles that are having nominal size ranges from 1 to 100 nm are called nano particles. Low-concentration of such particles in a base fluid is called nano fluids. Nano fluids exhibit higher heat transfer coefficient than base fluids, Eastman et al. [1].
The influence of Al2O3 nano particles addition to diluted the binary water-glycerol on nucleate pool boiling had been experimentally measured up to 91 kW/m2 by Sarafraz et al. [2]. The volumetric concentrations of Al2O3 nano particles were 0.5, 1 and 1.5%. The volume fractions of glycerol into pure water were varied from 1 to 5%. The results indicated that the nano particles result in increasing pool boiling heat transfer coefficient. With increasing the concentration of nano particles, the rate of increase of pool boiling heat transfer coefficient increases. Nucleate pool boiling of Al2O3–water and TiO2–water nano fluids had been experimentally measured over three horizontal surfaces (Stainless steel, Brass and Copper) with similar roughness under atmospheric pressure by Sarafraz and Pyghambarzadeh [3]. The results revealed that the presence of nano particles leads to higher increase in pool boiling heat transfer coefficients over Stainless steel and Brass surfaces than the Copper one. An experimental study for pool boiling of water–Al2O3 nano fluid was investigated by Das et al. [4]. The experiments were carried out using a Stainless steel horizontal cylindrical heater having 20 mm diameter. The Al2O3 particles sizes were from 20 to 50 nm and the concentration was changed from 0.1 to 4%. The results were correlated for both smooth and rough heaters. Another experimental study of pool boiling of water–Al2O3 nano fluid on a horizontal tube having small diameter was carried out by Das et al. [5]. The results showed that the pool boiling of narrow tubes has less deterioration than the large ones due to the difference in bubble sliding mechanism. Pool boiling of nano fluid with of Alumina suspended in water was studied experimentally using four concentrations on a flat surface by Bang and Chang [6]. The results showed that Alumina nano fluid has poor heat transfer enhancement compared with pure water. Nucleate pool boiling heat transfer of TiO2–water nano fluid was experimentally conducted by Suriyawong and Wongwises [7] with various concentrations over horizontal Copper and Aluminum circular surfaces with different roughness. An enhancement in heat transfer coefficient up to 27% was observed rather than pure water. Pool boiling heat transfer experiments for Silica–water nano fluid over a NiCr submerged wire were done by Vassallo et al. [8]. The results showed an increase in the critical heat flux for Silica–water nano fluid compared with pure water. The heat transfer of Al2O3–water and CuO–water through a serpentine micro channel was studied by Sivakumar et al. [9]. The results showed an increase in heat transfer coefficient of nano fluids compared with pure water. The effect of pulsation on heat transfer of Al2O3–water under turbulent flow through a spiral coil submerged in an isothermal medium was done experimentally by Doshmanziari et al. [10]. The pulsation increases the heat transfer coefficient up to 23%. An increase in the critical heat flux for nano fluid compared with pure water was also observed. Nucleate pool boiling of TiO2–R-141b was investigated experimentally using a cylindrical Copper tube as a boiling surface by Trisaksri and Wongwises [11]. The results revealed that the heat transfer coefficient decreases with the increase of TiO2 concentrations. An experimental study of pool boiling of Au–R-141b over horizontal plain tubes was done by Liu and Yang [12]. The results showed that the heat transfer coefficient is more than the twice of pure R-141b. Pool boiling of Al2O3–R-141b at different concentrations had tested experimentally over a Copper flat surface at different heat fluxes by Tang et al. [13]. The results indicated an enhancement in heat transfer characteristics with a surfactant of sodium Dodecyl Benzene Sulphonate. The effect of the surfactant additive of three ones; Sodium Dodecyl Sulfate, Cetyl-Trimethyl Ammonium Bromide and Sorbian Monooleate on nucleate pool boiling heat transfer of Cu–R-113 was investigated experimentally over a horizontal rough surface at different heat flux and concentrations by Peng et al. [14]. The results showed an enhancement in heat transfer but deterioration was observed at high surfactant concentrations. An experimental work was carried out to investigate the best coolant for heat removal among Alumina, Silica and Zinc oxide as nano particles with water as a base fluid by Sayahi and Bahrami [15]. It was found that; Zinc oxide in water is the best one for heat removal. Pool boiling heat transfer for the reduced Graphene oxide with water was investigated experimentally by Kamatchi et al. [16]. An enhancement up to 245% was observed. Pool boiling heat transfer of functionalized Carbon nano tube and non-functionalized Carbon nano tube was experimentally tested at high-heat flux up to the critical value by Sarafraz et al. [17]. It was found that; functionalized Carbon nano tube considerably enhances the heat transfer coefficient.
From previous review, it is clear in general that the addition of nano particles has an enhancement in pool boiling heat transfer characteristics. Thus; and as a consequence, the present work directs towards measuring of enhancement in pool boiling heat transfer characteristics of a nano fluid formed of Al2O3 nano particles suspended in a base R-134a at different heat flux, different operation pressure, different roughness of the heating surface and different concentration.
2 Experimental test rig and measuring techniques
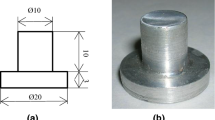
A schematic diagram of the experimental test rig is shown in Fig. 1. It consists mainly of two parts: an evaporator and a condenser. The evaporator is a high Carbon Steel vertical cylinder with a bore of 176 mm, a height of 350 mm and a thickness of 12 mm. A horizontal Stainless steel (316 Alloy) heater having an internal resistance cartridge generates different heat flux according to an adjustable electric power supply, Fig. 2a. Four grooves were cut with 90° apart as shown in Fig. 2b. Four K-type thermocouples were fixed beneath the boiling surface via the grooves. Two thermocouples were used to measure the vapor temperature inside the evaporator cylinder. Different surface roughness of the heater surface was formed using emery papers with rotating the heater at 1200 rpm as provided by Jabardo et al. [18]. The roughness (Ra) is 2.35, 1.54, and 0.82 μm. For mirror surface, fine successive polishing processes were done; using an emery paper of 300 grits, another emery paper of 2500 grits, finer grades of emery cloth on a buffing machine with Alcohol and hot air cleaning to produce the mirror surface. The roughness (Ra) of mirror surface is 0.042 μm, as provided by Mitutoy [19]. The roughness parameters for the tested heating surfaces are given in Table 1. Nano fluid has some requirements; even suspension, stable suspension, durable suspension, low agglomeration of particles, and no chemical change in the suspension as reported by Xuan and Li [20]. In the present study; Al2O3 was used as nano particles with average diameter 40 nm according to the manufacturer (M K Impex Corp.—Canada). R-134a was used as a base fluid. The photograph of Al2O3 nano particles obtained from the transmission electron microscope (TEM) is shown in Fig. 3. Nano fluid was prepared by addition Al2O3 to R-134a with a vibration for 3.5 h to stabilize the dispersion of the nano particles. Al2O3 concentrations are; 0.01, 0.05, 0.1, 0.25 and 0.5% by volume. The properties of Al2O3 and R-134a during nano fluid preparation are given in Table 2. The thermal conductivity of Al2O3–R-134a nano fluid can be calculated using the formula provided by Hamilton and Crosser [21] as follows:
The specific heat and the viscosity of Al2O3–R-134a nano fluid can be calculated using the formula provided by Mahbubul et al. [22] as follows:
The surface tension of Al2O3–R-134a nano fluid can be calculated using the formula provided by Subramani and Prakash [23] as follows:
The surface tension decreases slightly with the increase of concentration and it reduces the vapor bubble radius which enhances the boiling as provided by Raveshi et al. [24].
The thermal conductivity, heat capacity, viscosity and surface tension of Al2O3–R-134a nano fluid are given in Table 3.
Two opposed glass windows facing each other for visual observations were welded to the evaporator wall. The pressure throughout the experiment was measured by a pressure gauge at the top of the evaporator tank. A condenser condenses the vapor and the condensate liquid returns to the evaporator vessel for the re-evaporation once again. The condensation of the refrigerant takes place by cold water at an approximately constant temperature. Two thermocouples were used to measure the temperature of the condensed refrigerant coming from the condenser coil to the evaporator. Another two ones measure the inlet and outlet temperatures of the cooling water. A guard room consists of a thermostat, a cooling coil, an air electric heater and a fan was used to avoid any heat exchange between the evaporator and the environment. One thermocouple was fixed inside the guard room to check an approximately constant temperature inside it. A digital temperature indicator (Manufacturer: BK PRECISION, Model: 710, K-type) with 0.1 °C resolution was used to record the temperature. In the steady state; one can find the heat removal by the condenser equals the heat addition by the heater. The power consumed by the heater was calculated by measuring both the current and the voltage by an ammeter of 0.01 A resolution and a voltmeter of 0.1 V resolution. The water flow rate was measured using by a rotameter having a range from 0.2 to 4.9 L/min with a repeatability error of about 0.5%.
In a typical experiment; first of all; a vacuum pump was used to evacuate the atmospheric air from the evaporator and its attachments. Nano fluid was charged and heated to a certain saturation temperature and pressure using a higher value of heat flux. The saturation pressure and temperature were kept constant by varying water flow rate and heat flux. The experiments were performed at four saturation pressures; 4, 8, 12 and 16 bars that corresponding to saturation temperatures of R-134a 8.9, 31.3, 46.3 and 57.9 °C respectively. The experimental data were recorded at the steady state which its criteria are variations in the saturation temperature of ±0.1 °C. At the end of experiments, the heater was cleaned by a water jet to remove the sticking particles. The summary of the experimental program was provided in Table 4.
3 Experimental data reduction
Experimental investigations were carried out to observe the boiling characteristics using a cylindrical heater. Heat flux, q can be calculated as follows:
The surface temperature, T s can be calculated as follows:
The boiling heat transfer coefficient, h can be calculated as follows:
The ratio between the heat removal rate by the condenser and the heat addition rate by the evaporator, R can be calculated as follows:
0.921 ≤ R ≤ 0.962. During the experimental program.
3.1 Uncertainty analysis
The uncertainty in heat transfer coefficient is as follows as provided by Holman [25]:
The recorded experimental data from one experiment are:
Therefore;
Thus;
Thus; the uncertainty percentage in calculating heat transfer coefficient from measurements is ±0.812%.
4 Results and discussions
The addition of Al2O3 nano particles increases heat transfer coefficient as shown in Fig. 4. This is due to the increase in the thermal conductivity of the nano fluid. The suspended nano particles decrease the surface tension of the fluid significantly which decreases the radius of bubbles and as a consequence more active nucleation sites on the heating surface occurs. Therefore, the boiling heat transfer coefficient enhances. As the nano particles concentration increases, the heat transfer coefficient of nano fluids decreases. This deterioration is due to the deposition of nano particles on the heating surface which covers the surface and the heating surface becomes locally isolated and subsequently the rate of heat transfer from the surface toward the nano fluid decreases significantly.
The influence of surface roughness on the pool boiling heat transfer coefficient at a concentration 0.25 vol% alumina is shown in Fig. 5. The results show that the heat transfer coefficient increases by increasing the surface roughness. This result agrees the results of Narayan [26], where; the increase in the surface particle interaction parameter; which is the ratio between the average surface roughnesses to the average particle diameter, generates several smaller active cavities and consequently an enhancement in heat transfer coefficient has been achieved.
The enhancement ratio is defined as follows:
where h is the heat transfer coefficient of nano fluid and h pure is the one for pure R-134a at the same conditions. Based on the measured data, the enhancement ratio reaches up to 150% as shown in Fig. 6.
An empirical correlation was deduced to formulate the relation among h and the parameters (p, q, ϕ and Ra) for pool boiling of R134a-Al2O3 using EUREQA FORMLIZE software within a maximum deviation of about ±9% as follows:
The calculated heat transfer coefficient from the deduced correlation versus the experimental one was illustrated in Fig. 7.
5 Comparison among present work and previous ones
The current experimental results for pure R-134a were validated by comparing the results by the following correlation which had been deduced by Cooper [27] at the same operating conditions;
Referring to Fig. 8, one can find a fair validation has been established.
The heat transfer coefficient reaches twice that of pure R-141b by addition of nano Au particles as provided by Liu and Yang [12]. The effect of the addition of carbon nano tubes (CNTs) on boiling heat transfer of refrigerant R-22 and water was investigated by Park and Jung [28]. Test results showed that CNTs increase heat transfer coefficients at low heat fluxes. Also, the addition of (CNTs) to R-123 and R-134a shows an increase in heat transfer coefficient at low heat fluxes as reported by Park and Jung [29]. Referring to Fig. 9, about 48% enhancement was observed due to the addition of nono-Al2O3 in the present work rather than those from the addition of nano CNCs either to R-22 or R-141b.
6 Conclusions
The effect of addition of nano particles of Aluminum oxide to a base R-134a on pool boiling heat transfer at different concentrations had been experimentally investigated using a horizontal stainless steel cylindrical heater having different surface roughness. The tests were carried out at different operating pressure and different heat fluxes. The results can be systemized as follows:
The heat transfer coefficient can be doubled with the increase of the nano particles concentration up to 0.25%. But it comes down with exceed of nano particles concentration.
An enhancement in heat transfer coefficient up to 37.6, 55.4, 90.2, 167.7% corresponding to 0.042, 0.84, 1.54, and 2.35 μm surface roughness, respectively, were recorded.
An empirical correlation was deduced to describe the variation of heat transfer coefficient with heat flux, pressure, surface roughness and nano particles concentrations within a deviation of ±9%.
In a comparison with previous works, about 48% enhancement was observed due to the addition of nono-Al2O3 to R-134a in the present work rather than those from the addition of nano CNCs either to R-22 or R-141b.
Abbreviations
- C :
-
Specific heat [J/(kg K)]
- d :
-
Diameter of the heater (m)
- h :
-
Heat transfer coefficient [W/(m2 K)]
- i :
-
Electric current (A)
- k :
-
Thermal conductivity [W/(m K)]
- l :
-
Length of the heater (m)
- M :
-
Molecular mass (kg/k mol)
- \( m^{.} \) :
-
Mass flow rate (kg/s)
- p :
-
Pressure (Pa)
- n :
-
Shape factor, n = 3/τ
- q :
-
Heat flux (W/m2)
- R :
-
Heat ratio
- Ra :
-
Arithmetical mean of roughness profile (μm)
- Rp :
-
Maximum roughness peak height (μm)
- Rq :
-
Root mean square roughness average (μm)
- Rv :
-
Maximum roughness valley depth (μm)
- Rz :
-
Irregularities roughness height of ten points (μm)
- r :
-
Radius (m)
- ρ :
-
Density (kg/m3)
- σ :
-
Surface tension (N/m)
- T :
-
Temperature (K)
- τ :
-
Sphericity, τ = 1 for sphere, τ = 0.5 for cylinder
- v :
-
Voltage (V)
- ϕ :
-
Concentration by volume
- ξ :
-
Enhancement ratio
- avg :
-
Average
- bf :
-
Base fluid
- cr :
-
Critical
- i :
-
Inner
- o :
-
Outer
- mr :
-
Mirror
- nf :
-
Nano fluid
- np :
-
Nano particles
- p :
-
Pressure
- s :
-
Surface
- sat :
-
Saturation
- w :
-
Water
References
Eastman JA, Choi SUS, Li S, Yu W, Thomson LJ (2001) Anomalously increased effective thermal conductivities of ethylene glycol-based nano fluids containing copper nano particles. Appl Phys Lett 78(6):718–720
Sarafraz MM, Peyghambarzadeh SM, Fazel SAA, Vaeli N (2013) Nucleate pool boiling heat transfer of binary nano mixtures under atmospheric pressure around a smooth horizontal cylinder, periodica polytechnica. Chem Eng 57(1–2):71–77
Sarafraz MM, Pyghambarzadeh SM (2012) Nucleate pool boiling heat transfer to Al2O3–water and TiO2–water nano fluids on horizontal smooth tubes with dissimilar homogeneous materials. Chem Biochem Eng Q 26(3):199–206
Das SK, Putra N, Roetzel W (2003) Pool boiling characteristics of nano fluids. Int J Heat Mass Transf 46:851–862
Das SK, Putra N, Roetzel W (2003) Pool boiling of nano fluids on horizontal narrow tubes. Int J Multiph Flow 29:1237–1247
Bang IC, Chang SH (2005) Boiling heat transfer performance and phenomena of Al2O3–water nano fluids from a plain surface in a pool. Int J Heat Mass Transf 48:2407–2419
Suriyawong A, Wongwises S (2010) Nucleate pool boiling heat transfer characteristics of TiO2–water nano fluids at very low concentrations. Exp Therm Fluid Sci 34:992–999
Vassallo P, Kumar R, D’Amico S (2004) Pool boiling heat transfer experiments in silica–water nano fluids. Int J Heat Mass Transf 47:407–411
Sivakumar A, Alagumurthi N, Senthilvelan T (2016) Experimental investigation of forced convective heat transfer performance in nano fluids of Al2O3/water and CuO/water in a serpentine shaped micro channel heat sink. Heat Mass Transf 52:1265–1274
Doshmanziari FI, Zohir AE, Kharvani HR, Vahid DJ, Kadivar MR (2016) Characteristics of heat transfer and flow of Al2O3/water nano fluid in a spiral-coil tube for turbulent pulsating flow. Heat Mass Transf 52:1305–1320
Trisaksri V, Wongwises S (2009) Nucleate pool boiling heat transfer of TiO2–R141b nano fluids. Int J Heat Mass Transf 52:1582–1588
Liu DW, Yang CY Effect of nano particles on pool boiling heat transfer of refrigerant 141b, ICNMM2007-30221, Puebla, Mexico
Tang X, Zhao YH, Diao YH (2014) Experimental investigation of the nucleate pool boiling heat transfer characteristics of d-Al2O3–R141b nano fluids on a horizontal plate. Exp Therm Fluid Sci 52:88–96
Peng H, Ding G, Hu H (2011) Effect of surfactant additives on nucleate pool boiling heat transfer of refrigerant-based nano fluid. Exp Therm Fluid Sci 35:960–970
Sayahi T, Bahrami M (2016) Investigation on the effect of type and size of nano particles and surfactant on pool boiling heat transfer of nano fluids. Tran ASME 13:031502-2
Kamatchi R, Venkatachalapathy S, Nithya C, Experimental investigation and mechanism of critical heat flux enhancement in pool boiling heat transfer with nano fluids. Heat Mass Transf doi:10.1007/s00231-015-1749-2
Sarafraz MM, Hormozi F, Silakhori M, Peyghambarzadeh SM (2016) On the fouling formation of functionalized and non-functionalized carbon nano tube nano fluids under pool boiling condition. Appl Therm Eng 95:433–444
Jabardo JMS, Ribatski G, Stelute E (2009) Roughness and surface material effects on nucleate boiling heat transfer from cylindrical surfaces to refrigerants R-134a and R-123. Exp Therm Fluid Sci 33:579–590
Mitutoy, Portable surface roughness tester SURFTEST SJ-210 Series, Bulletin No. 2140
Xuan Y, Li Q (2000) Heat transfer enhancement of nano fluids. Int J Heat Fluid Flow 21:58–64
Hamilton RL, Crosser OK (1962) Thermal conductivity of heterogeneous two component systems. Ind Eng Chem Fundam 1(3):187–191
Mahbubul IM, Saadah A, Saidur R, Khairul MA, Kamyar A (2015) Thermal performance analysis of Al2O3/R-134a nano refrigerant. Int J Heat Mass Transf 85:1034–1040
Subramani N, Prakash MJ (2011) Experimental studies on a vapour compression system using nano refrigerants. Int J Eng Sci Technol 3(9):95–102
Raveshi MR, Keshavarz A, Mojarrad MS, Amiri S (2013) Experimental investigation of pool boiling heat transfer enhancement of alumina–water–ethylene glycol nano fluids. Exp Therm Fluid Sci 44:805–814
Holman JP (2001) Experimental methods for engineers, 7th edn. McGraw-Hill, New York
Narayan GP, Anoop K, Das SK (2007) Mechanism of enhancement/deterioration of boiling heat transfer using stable nano particle suspensions over vertical tubes. J Appl Phys 102:074317
Cooper MG (1984) Saturation nucleate pool boiling—a simple correlation. In: 1st UK Natl. Heat Transfer Conf. (I. Chem. E. symposium series no. (86) vol 2, pp 785–793
Park KJ, Jung D (2007) Enhancement of nucleate boiling heat transfer using carbon nano tubes. Int J Heat Mass Transf 50:4499–4502
Park KJ, Jung D (2007) Boiling heat transfer enhancement with carbon nano tubes for refrigerants used in building air-conditioning. Energy Build 39:1061–1064
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eid, E.I., Khalaf-Allah, R.A., Taher, S.H. et al. An experimental investigation of the effect of the addition of nano Aluminum oxide on pool boiling of refrigerant 134A. Heat Mass Transfer 53, 2597–2607 (2017). https://doi.org/10.1007/s00231-017-2010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-017-2010-y