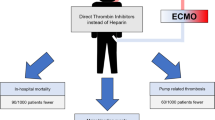
Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a vital technique for severe respiratory or heart failure patients. Bleeding and thrombotic events are common during ECMO and negatively impact patient outcomes. Unfractionated heparin is the primary anticoagulant, but its adverse effects limit its use, necessitating alternative anticoagulants.
Objective
Review available alternative anticoagulants for adult ECMO patients. Explore potential novel anticoagulants for future ECMO use. Aim to reduce complications (bleeding and thrombosis) and improve safety and efficacy for critically ill ECMO patients.
Methods
Comprehensive literature review of existing and emerging anticoagulants for ECMO.
Results
Identified a range of alternative anticoagulants beyond unfractionated heparin. Evaluated their potential utility in mitigating ECMO-related complications.
Conclusion
Diverse anticoagulant options are available and under investigation for ECMO. These alternatives may enhance patient safety and outcomes during ECMO support. Further research and clinical studies are warranted to determine their effectiveness and safety profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a mechanical circulatory life support device used in critically ill patients with respiratory or circulatory failure [1]. In recent years, the use of ECMO has gradually increased, the 2019 coronavirus disease (COVID-19) pandemic has drawn special attention to ECMO, and the role of ECMO has been more prominent than ever in the COVID-19 era [2, 3]. ECMO is widely used to treat adult patients with severe cardiorespiratory failure due to COVID-19 [4]. However, the occurrence of bleeding and thrombotic event (BTE) complications during ECMO, which seriously affects the prognosis, urgently needs to be addressed. A national cohort study from France showed that the incidence of bleeding in ECMO-supported COVID-19 patients (29%) was high and strongly associated with in-hospital mortality [5]. In addition, a meta-analysis showed that the incidence of major bleeding events (including major bleeding and intracranial hemorrhage) in ECMO-supported COVID-19 patients was 47.3%, and the incidence of thrombotic events (including circuit thrombosis, ischemic stroke, and pulmonary embolism) was 35.9% [6]. Two other studies from the Extracorporeal Life Support (ELSO) Registry also reported high rates of bleeding and thrombosis in ECMO patients as the leading complications [7, 8].
Any anticoagulation strategy ideally should keep the balance between prevention of thrombosis and risk of bleeding. An appropriate anticoagulation strategy is essential to prevent thrombosis and minimize the risk of bleeding.
Antithrombin (AT) is an endogenous inhibitor of various coagulation factors [9]. UFH exerts its anticoagulant effect by interacting with AT. The combination of UFH and AT accelerates the formation of complexes between AT and thrombin, thereby inactivating thrombin, which in turn leads to the inactivation of various coagulation factors in the coagulation cascade (Fig. 1) [10]. Unfractionated heparin (UFH) is still the anticoagulant of choice for ECMO because of its rapid onset of action, low price, and ease of neutralization by protamine [11]. However, the use of heparin is subject to many limitations, including heparin-induced thrombocytopenia (HIT), heparin resistance, and the tendency of heparin to bind to various plasma proteins resulting in unpredictable anticoagulant responses [12]. Dose adjustment of heparin is largely dependent on anticoagulation monitoring, which is also controversial. Moreover, the need for AT supplementation during heparin infusion is also a challenge. A national multicenter retrospective study [13] from France showed that HIT caused by using heparin as an anticoagulant under VA-ECMO therapy in adults is a rare complication with a prevalence of approximately 0.36 and an associated mortality rate 33%. Although HIT is relatively rare, it can be a life-threatening complication. Patients with the recent pandemic of COVID-19 infection often have hypercoagulable blood and are prone to heparin resistance [14, 15]. There is an urgent need for safe and effective heparin replacement anticoagulants in this population. Using alternative anticoagulants during ECMO is necessary for serious adverse effects caused by heparin and in specific populations. This article describes the currently available alternative anticoagulants for use during ECMO support, including low molecular weight heparin (LMWHs), direct anticoagulants (DTIs), Factor Xa inhibitors, regional citrate, the broad-spectrum anticoagulant Nafamostat Mesilate (NM), antiplatelet agents, and some new target anticoagulants that may be used in the future.
LMWHs
Low molecular weight heparin (LMWHs) is a type of heparin prepared by depolymerization of UFH, and its molecular weight is only one-third of that of heparin [16]. LMWHs have fewer adverse effects, produce predictable anticoagulation, and are administered subcutaneously at a fixed dose without repeated continuous anticoagulation monitoring [17]. However, there is no evidence that the benefits of LMWHs mentioned above are equally applicable in ECMO patients. LMWHs include enoxaparin, dalteparin, tinzaparin, and nadroparin. Compared with other LMWHs, enoxaparin’s excellent pharmacological and chemical properties, including longer elimination half-life, superior bioavailability, and better anticoagulant effect, make it more widely used in clinical practice [17].
There are limited data on the use of enoxaparin in ECMO patients. One observational study suggests that the use of LMWHs for prophylactic anticoagulation in VV-ECMO patients is feasible [18]. A retrospective study that included 102 patients on perioperative ECMO support for lung transplantation showed no difference in serious bleeding events between the two groups when comparing enoxaparin and UFH for systemic anticoagulation. However, patients on enoxaparin had a lower risk of thromboembolic events [19]. Another study reported systemic standardized anticoagulation with enoxaparin in 62 of 98 patients treated with ECMO for respiratory failure due to COVID-19 and heparin in the other 36 patients. The results showed that the probability of thromboembolic and bleeding events was less in the enoxaparin group than in the UFH group. It is suggested that subcutaneous enoxaparin may be a feasible anticoagulation strategy for COVID-19 patients requiring ECMO support [20]. Alessandro et al. concluded, based on their own center’s experience, that the use of subcutaneous injection of enoxaparin three times daily, in conjunction with anti-factor Xa (anti-Xa) monitoring in ECMO patients, is feasible and safe in the COVID-19 patient population requiring VV-ECMO [21]. However, the half-life of enoxaparin is 4.5 h, and the dosage of three times a day is easy to accumulate in the body and increase the risk of bleeding. In addition, most patients receiving ECMO therapy may have liver and kidney damage, and enoxaparin is easy to accumulate in patients with renal insufficiency, and additional monitoring may be required. The need for dose adjustment and the usefulness of anti-Xa monitoring in patients with renal insufficiency remain controversial [22]. The above limits the application of LMWHs on ECMO. Moreover, due to the relatively small sample size of studies using enoxaparin in ECMO and the single-center and retrospective design of the available studies, further prospective randomized controlled trials (RCT) are needed to determine its safety and efficacy during anticoagulation in ECMO.
DTIs
DTIs are a relatively new class of short-acting anticoagulants with predictable pharmacokinetics, do not bind other plasma proteins or cells, are unaffected by serum, produce a predictable anticoagulant response, and inhibit clot-bound and circulating thrombin, producing a more potent anticoagulant effect compared to heparin [23]. The synthetic DTIs argatroban, bivalirudin, and lepirudin have been administered to ECMO patients. Bivalirudin and argatroban are currently among the more studied DTIs during ECMO. One study reported the successful treatment of HIT in a 21-month-old child who received lepirudin anticoagulation after cardiac surgery and ECMO [24]. However, lepirudin was announced as no longer being produced in 2012.
Argatroban
Argatroban is a synthetic small-molecule drug derived from L-arginine that specifically blocks the active site of thrombin, thus acting independently of AT. Argatroban is a monovalent competitive inhibitor of thrombin [25]. Argatroban binds to the active catalytic site of thrombin as a non-covalent bond, forming a reversible complex (Fig. 2). Argatroban, as a first-line anticoagulant in HIT patients, is expected to be an alternative therapy to heparin anticoagulation in ECMO-supported patients [26]. A case report reveals two cases of HIT during ECMO support with heparin as an anticoagulant in adults, immediate discontinuation of heparin to enable anticoagulation with argatroban successfully controlled the HIT, and the patients recovered well [27]. The study by Menk et al. showed that major and minor bleeding occurred between the two groups anticoagulated with argatroban or heparin in patients with acute respiratory distress syndrome (ARDS) receiving ECMO or pumpless extracorporeal lung assist (pECLA). The incidence of thromboembolic events was generally low and similar. Argatroban appears to be a viable, effective, and safe anticoagulant in critically ill ARDS patients receiving extracorporeal lung support [28]. This report suggests that argatroban may be an alternative anticoagulant for pregnant women who develop HIT and AT-III deficiency during ECMO. A meta-analysis that included 337 ECMO patients anticoagulated with argatroban showed that the incidence of BTE complications in the argatroban group was similar to that of patients treated with regular heparin (UFH) [29]. In addition, studies have pointed out that in VV-ECMO patients without HIT, argatroban is not inferior to UFH in bleeding and thrombosis, and related complications are similar, but argatroban has less effect on thrombocytopenia during ECMO [30]. In patients requiring ECMO, argatroban appears to be a potential alternative anticoagulant to UFH. Argatroban has a short half-life (Table 1) and is less likely to accumulate but has a prolonged half-life in patients with hepatic impairment, requiring dose reduction in such patients [30].
Considering the previous experience, argatroban appears to be a safe and effective heparin replacement therapy during ECMO support. However, further studies are needed to determine the efficacy and safety of argatroban relative to other available drugs.
Although the direct drug cost of argatroban is higher, it is comparable to UFH after considering HIT testing and transfusion [27, 30]. And for patients with low AT levels, argatroban may be more cost-effective during ECMO treatment without increasing the risk of adverse events [31]. Argatroban is a parenteral direct thrombin inhibitor that requires close monitoring to ensure safety and efficacy. Furthermore, argatroban undergoes rapid hepatic metabolism, making its administration cautious in patients with impaired liver function [32]. Research has indicated that critically ill patients requiring Extracorporeal Life Support (ECLS) may necessitate a reduction in argatroban dosage [33]. Once a severe bleeding event occurs, no specific reversal agent neutralizes it. An ongoing prospective randomized controlled trial of the safety and feasibility of argatroban in patients supported by ECMO has been registered on December 1, 2021, and results from this trial are expected by the end of 2024 (NCT05226442).
Bivalirudin
Bivalirudin is a synthetic peptide that, unlike heparin, directly inhibits thrombin activity by simultaneously binding to the active catalytic site of thrombin and the substrate recognition site (exosite 1). Bivalirudin reaches its peak blood concentration 2 min after sedation (Table 1) [34]. It has a short half-life of 45 min. Thrombin has three structural domains: an active site and two exosites. Exosite 1 acts as a binding region for substrates (e.g., fibrin), and exosite 2 is the binding region for heparin (Fig. 3) [35]. The Caridi-Scheible team used bivalirudin as an anticoagulant in all 32 runs of the VV-ECMO, but rarely had to change the ECMO circuit due to thrombosis; in contrast, the three patients with UFH in the ECMO circuit required at least one circuit change, and one of them died from a bleeding-related complication [36]. A case report reported that a patient on VV ECMO awaiting lung transplantation developed acute HIT and underwent successful lung transplantation after 21 days of ECMO support with a switch to VA-ECMO and low-dose (target ACT of 160-180S) bivalirudin anticoagulation. Based on the above experience, they concluded that bivalirudin is the first-line anticoagulant for patients undergoing lung transplantation who present with acute HIT using ECMO [37].
A meta-analysis including 9 retrospective studies showed that bivalirudin might provide a survival benefit and reduce thrombosis in adult patients treated with ECMO compared with heparin. However, there was no statistically significant difference between the bivalirudin and heparin groups regarding events leading to significant bleeding [38]. And because of the inclusion of retrospective studies with a relatively low level of evidence, multicenter RCT studies are urgently needed to validate the efficacy and safety of bivalirudin as an anticoagulant for ECMO. Sanfilippo et al. based this meta-analysis positively and suggested several studies that the authors omitted to include [39]. However, including the three studies, the authors ignored does not affect the final result. Bivalirudin is promising as an alternative to plain heparin anticoagulation. Moreover, several meta-analyses published in recent years have also shown the safety and feasibility of bivalirudin instead of heparin, including reduced circuit thrombosis and in-hospital mortality. At the same time, the results of these studies were not uniform as to whether they would reduce the risk of bleeding [40,41,42,43,44,45,46,47].
According to published research and the experience of a few centers, bivalirudin is a promising anticoagulant for ECMO, especially in the setting of HIT and heparin resistance. However, it is noteworthy that 20% of bivalirudin is eliminated via renal clearance, and its half-life is prolonged in patients with renal impairment. Consequently, dose adjustments are warranted for patients undergoing concurrent renal replacement therapy (CRRT) during ECMO support [36]. Therefore, the eventual use in anticoagulation practice for heparin replacement therapy in adult ECMO patients urgently requires large multicenter randomized controlled trials in patients receiving ECMO support.
The use of other DTIs (dabigatran, lepirudin, and desirdin) is limited by their potential for serious adverse effects and poor pharmacokinetic profile compared to the newer DTIs. Therefore, they are not usually used as anticoagulants during ECMO support.
Factor Xa inhibitors
FXa is located at the intersection of the coagulation cascade pathways (Fig. 4) and plays an important role in the coagulation process. Therefore, inhibitors targeting FXa factor have excellent anticoagulant activity [48]. Direct factor Xa inhibitors have a direct inhibitory effect on factor Xa and are not dependent on AT. Such agents include rivaroxaban, edoxaban, and apixaban (usually administered orally) and danaparoid. Danaparoid is a low molecular weight heparin that exerts its antithrombotic effect mainly through AT-III-mediated inhibition of the Xa factor. One case reported the use of danaparoid for anticoagulation management with ECMO support in a patient with respiratory failure due to pulmonary embolism with high suspicion of HIT, during which no excessive bleeding or thrombosis of the circuit occurred [49]. The long half-life of danaparoid and the lack of an antidote limit its use in ECMO. However, its minimal effect on the fibrinolytic system and its low potential cause bleeding to make it attractive for ECMO anticoagulation. Another case describes a patient with COVID-19 receiving ECMO support who was diagnosed with HIT and was administered rivaroxaban for various reasons without access to alternative heparin drugs such as DTIs, no adverse effects such as thrombotic or bleeding events were observed, and the patient’s prognosis was favorable [50]. No other cases of direct factor Xa inhibitors being used during ECMO support exist. The paucity of enteral administration modalities and studies limits their use in ECMO.
Mechanisms of thrombosis and therapeutic targets of the anticoagulant. The coagulation cascade includes intrinsic (FXII/XIIa/, FXI/XIa, FIX/FIXa, FX/Xa), extrinsic (TF, FVII/VIIa, and FX/Xa), and common pathways (FX/Xa, FII/IIa, and FXIII/XIIIa). The coagulation process supported by ECMO is mainly initiated through the intrinsic pathway (contact pathway) which is initiated. TF indicates tissue factor; TXA2, thromboxane A2. The dashed arrows indicate the sites of action or inhibition of the drugs. Black arrows represent the factors going from their inactive to active states
Fondaparinux is an indirect factor Xa inhibitor. More data on using this class of anticoagulants on ECMO needs to be collected. One study reported a case of a patient who developed HIT during ECMO support after daily subcutaneous administration of fondaparinux without significant adverse effects [51].
Gastrointestinal dysfunction such as intestinal ischemia and impaired abdominal microcirculation are prone to occur during ECMO, which slows down the intestinal passage of FXa inhibitors, and the half-life of FXa inhibitors (Table 2) is longer, and they are prone to accumulation, thereby increasing bleeding risk [52, 53]. Andexanet Alfa, a novel antidote to the anticoagulation effects of Factor Xa inhibitors, was approved by the FDA in 2018 for the reversal of life-threatening and uncontrollable bleeding caused by apixaban and rivaroxaban [54, 55].
Factor Xa inhibitors have significant anticoagulant effects. Although reversal agents are available, they still need to be used with caution in patients at high bleeding risk.
Nafamostat mesilate
Nafamostat Mesilate (NM) is a synthetic, low molecular serine protease inhibitor with a short half-life (5–8 min) metabolized in the liver and blood and excreted via the kidneys and intestines. NM produces adequate anticoagulation by inhibiting thrombin, fibrin, and factors Xa and XIIa and acts independently of AT as a spectral anticoagulant [56, 57]. NM is currently used for anticoagulation in ECMO patients [58, 59]. Dosage and monitoring of some anticoagulants are shown in Table 3.
The effect of NM on bleeding risk is controversial. Some studies have presented different conclusions. In a sizeable ECMO-supported animal model, NM anticoagulation was associated with fewer bleeding complications than UFH [60]. The results of Han et al. [61] also showed a lower rate of bleeding events with NM compared to heparin (p = 0.05), while Lim et al. [62] reported that bleeding complications were significantly higher in patients treated with NM compared to those treated with heparin (p = 0.03), while thromboembolic events were comparable. In contrast, Lee et al. concluded that NM should be considered a safer method of local anticoagulation in VA-ECMO for patients at high risk of bleeding [63].
NM can be used as an alternative to UFH and combined with UFH. Hyperkalemia is a more common problem with NM anticoagulation, especially in patients with renal failure [64]. In addition, some reports suggest that NM causes severe allergic reactions, such as fatal allergic reactions and severe abdominal pain caused by allergy [65]. Although NM has no antidote, its unique pharmacological profile and very short half-life make it attractive in the ECMO population. Moreover, it has been suggested that NM exhibits anti-inflammatory properties during ECMO treatment [66]. NM also has antiviral activity with potential benefits for patients with moderate to severe COVID-19 [66]. Therefore, it is desirable for ECMO-supported COVID-19 patients.
Because the current use of NM as an anticoagulant in ECMO patients is based on retrospective studies and a few case reports, the quality of evidence could be better. There need to be more prospective trials to verify its efficacy and safety in ECMO, so it is impossible to conclusively state whether it improves bleeding and thrombotic complications in ECMO patients. A multicenter randomized controlled clinical trial investigating the efficacy and safety of NM anticoagulation for VV-ECMO is under recruitment (NCT05555641).
Citrate
Topical citrate anticoagulation for hemodialysis was first introduced in 1961 [32]. It is an ideal alternative to heparin for patients at increased risk of bleeding. It can be effectively anticoagulated through the dialysis circuit without affecting the patient’s systemic coagulation [32]. The primary mechanism of citrate anticoagulation is to prevent platelet activation and coagulation reaction through calcium ion chelation, inhibiting coagulation in both the intrinsic and extrinsic coagulation pathways [32].
Patients on ECMO often require continuous renal replacement therapy (CRRT), and CRRT circuits are usually not heparin-coated, increasing the risk of thrombotic events [67]. CRRT usually employs topical anticoagulation with citrate [68]. A retrospective study indicated that, among patients undergoing CRRT during VV-ECMO, the addition of regional citrate anticoagulation (RCA) to the CRRT circuit (RCA + UFH group) resulted in decreased clotting propensity of the CRRT circuit and prolonged circuit lifespan compared to those receiving sole systemic heparin anticoagulation (UFH group). Furthermore, no complications associated with citrate anticoagulation were documented. This finding suggests that regional citrate anticoagulation, employed as an adjunctive anticoagulation approach for the CRRT circuit during the course of ECMO, may potentially constitute a viable, secure, and efficacious technique [68]. Other studies have suggested that the additional immunomodulatory effects of RCA additional immunomodulatory effects may be more beneficial than heparin in this regard [69]. However, citrate use in local ECMO anticoagulation therapy is limited by citrate clearance [31, 32]. The main problem with citrate is that due to the high blood flow, the amount of citrate theoretically needed for regional anticoagulation would overflow the organism. Moreover, rapid changes in blood flow as sometimes occurring on ECMO would make adaptation of citrate dose impossible. Citrate may be used as add on for regional anticoagulation in additional RRT, but NOT for the ECMO circuit itself. A clinical trial is ongoing evaluating the safety and efficacy of using citrate as a regional anticoagulant in ECMO circuits in high-risk infants under 1 year of age (NT00968565).
Antiplatelet agents
In recent years, researchers have continuously explored and optimized therapeutic strategies for hemostatic balance in the extracorporeal circuit. Inhibition of platelet aggregation has been proposed as an additional anticoagulant in addition to anticoagulation. The use of additional anticoagulants minimizes costs without increasing adverse effects.
Cangrelor
Cangrelor is an ATP analogue that inhibits ADP-induced platelet aggregation and is a platelet P2Y12 receptor inhibitor [70]. It acts as an anticoagulant by reversibly inhibiting platelet action. Platelet function returns to normal within 1 h, and its half-life is extremely short, ranging from 3 to 6 min [71, 72]. It is rapidly depleted after discontinuation of infusion and does not require antidotes to reverse. Anticoagulation in ECMO supported patients who require dual antiplatelet therapy (DAPT) is challenging. Anticoagulation with DAPT + mechanical circulatory support (MCS) may increase the risk of bleeding [73]. Data on cangrelor use on ECMO are limited, with only sporadic reports. A single-center retrospective study was designed to describe the outcomes associated with the use of cangrelor during VA-ECMO [73]. This study reported 10 bleeding events and 1 deep vein thrombosis in 13 patients treated with cangrelor 0.75 μg/kg/min who required ECMO support for concomitant cardiogenic shock after percutaneous coronary intervention (PCI).
Katz et al. reported on the experience with cangrelor in 17 patients with cardiogenic shock requiring VA ECMO and Impella support after coronary stenting [74]. Nine patients were treated with VA-ECMO, and 5 were treated with VA-ECMO combined with Impella. All patients received triple bolus therapy with aspirin, heparin, and cangrelor, and the results showed that bleeding events occurred in 6 of the 9 patients, and no patients experienced thrombosis. They concluded that cangrelor below 0.75 μg/kg/min might be beneficial. Another single-center trial also reported that low-dose cangrelor combined with standard-strength bivalirudin anticoagulation was feasible for patients undergoing PCI during VA-ECMO-supported acute coronary syndrome (ACS)-associated cardiogenic shock(CS)/cardiac arrest (CA) [75].
Cangrelor has been used successfully to prevent thrombosis in VA ECMO patients [76]. However, the risk of bleeding is higher with the recent use of cangrelor on ECMO, and low-dose cangrelor may be an effective anticoagulation strategy. Therefore, the risk-to-benefit ratio should be thoroughly evaluated for ECMO therapy.
Prostaglandin E1 (alprostadil; PGE1)
Prostaglandin E1 (PGE1) inhibits platelet aggregation, inhibits ADP-mediated platelet activation, and reduces inflammation [77,78,79]. Low-dose UFH and PGE1 anticoagulation can increase the biocompatibility of in vitro systems and enhance the efficacy of artificial organs without increasing the risk of adverse reactions [80]. A prospective RCT showed that PGE1 as add-on therapy to heparin is safe. They divided VV-ECMO patients into two groups, 24 in each group. One group of patients was anticoagulated with 5 ng/kg/min PGE1 plus low-dose heparin, and the other group used heparin alone for standardized anticoagulation. Both groups included 24 patients. While not reducing the rate of daily packed red blood cell transfusions during ECMO support, the incidence of BTE was reduced, and circuit life was longer in VV-ECMO patients receiving PGE1 [80]. The results need to be verified by a larger sample size and a multi-center prospective trial.
Possible future use of anticoagulants
Developing anticoagulants with low bleeding risk has been challenging, but recent studies suggest that drugs targeting the contact system may be a good option (Fig. 4). Medications that target the coagulation factors of the contact system (intrinsic pathway), including XII, XI, and IX, may provide a safer anticoagulation modality.
FXII is critical in thrombosis but plays a minimal role in hemostasis, and inhibition of FXIIa reduces inflammation [81]. The humanized antibody 3F7 (anti-CD73 monoclonal antibody), developed against FXII factors, selectively blocks the activation of FXIIa coagulation factors with high affinity within a certain range [82]. In a preclinical study, it was shown in an ECMO-supported rabbit model that infusion of 3F7 provided long-period thromboprotection as well as heparin and did not increase the duration of bleeding or blood loss at the site of injury compared with the heparin group [83]. A humanized FXII mouse model generated with human F12 gene (knock-in) mice showed that 3F7 reduced or avoided thrombosis in FXII mice without affecting hemostasis [84]. Targeting factor XII provides safe anticoagulation.
FXIa is also a critical factor in thrombosis and plays a minor role in hemostasis. Drugs developed for FXIa have shown potent antithrombotic effects in animal studies without an increased risk of bleeding [85]. Drugs developed for FXIa include Abelacimab, AB023, and Milvexian. Abelacimab, an FXIa factor inhibitor, is an anti-FXIa antibody that may not cause bleeding while resisting thrombosis [86]. Abelacimab binds FXI with high affinity, thereby preventing its activation by XIIa [87]. It was safe and well tolerated in healthy subjects and patients with atrial fibrillation. Data from PK and PD support its use in clinical development [88]. Abelacimab is validated in a phase III study in patients with cancer-related thrombosis. A clinical trial of milvexian, a small molecule FXIa inhibitor, demonstrated its safe tolerability and favorable pharmacokinetic profile suitable for further clinical development [89]. AB023 has also shown good safety tolerability in a small sample of phase II clinical trials in patients with end-stage renal disease (ESED) receiving heparin-free chronic hemodialysis [90]. Ongoing or future phase 3 clinical trials will help determine the rationale for FXIa inhibitors in patients at higher risk for bleeding or thrombosis. In the ECMO setting, FXIIa is the primary activator of FXIa [91]. Thromboprophylaxis in the ECMO circuit may be an indication for FXI(a) inhibitors.
One study designed a fusion protein Infestin-PN2KPI(IP) by linking the FXIIa inhibitor infestin 4 to the FXIa inhibitor PN2KPI. IP inhibits thrombosis without risk of bleeding and shows sound anticoagulant effects [92].
The research and development of drugs targeting the FIX(a) system has declined in recent years compared to drugs targeting FXII and FXI. FIX(a) is also crucial in coagulation. It contains the rate-limiting steps for thrombin generation and thrombosis, and the available findings suggest that targeting the FIX(a) system to achieve safe anticoagulation is also possible [93].
The findings suggest that coagulation factors targeting the contact pathway may provide safe and effective anticoagulation.
ECMO without anticoagulation
Ceasing continuous systemic anticoagulation during ECMO support may reduce the bleeding risks in high-risk populations with conditions such as trauma and disseminated intravascular coagulation (DIC). Currently, there is limited research concerning anticoagulation-free periods during ECMO. A meta-analysis encompassing 201 adult patients undergoing ECMO without continuous systemic anticoagulation revealed comparable circuit and patient thrombosis rates between those without continuous systemic anticoagulation and those receiving such treatment. However, drawing conclusions about bleeding outcomes remains challenging [94]. Given significant heterogeneity among the included studies and inherent biases, and in the absence of robust large-scale prospective clinical investigations, omitting routine anticoagulation is not recommended. Nonetheless, omission of anticoagulation might present an attractive option for certain high-risk bleeding patients undergoing ECMO.
Summary and outlook
Bleeding and thrombotic events during ECMO support remain significant causes of poor patient prognosis. Although UFH remains the primary anticoagulant in ECMO therapy, there is an urgent need for alternative anticoagulants in this population with HIT and heparin resistance. Our article gives a more comprehensive overview of current or future drugs available for ECMO anticoagulation to provide safer and more effective anticoagulation options for critically ill patients, thereby improving their prognosis. The drugs currently available for UFH replacement therapy for ECMO patients have limitations, all carry a risk of bleeding, and there are limited data on their use on ECMO. There is relatively more experience with using DTIs in ECMO, but whether they have better safety and efficacy than heparin remains to be demonstrated in large RCTs. Direct or indirect factor Xa inhibitors are also a new class of anticoagulants, and there are few studies on their use in the ECMO circuit, and much research is still needed. In order to improve the prognosis of critically ill patients supported by ECMO, the search for anticoagulants with low bleeding risk is crucial. It is critical that further clinical research be conducted on alternative anticoagulants in adult patients in order for management to be maximized without placing the ECMO patients at unnecessary risk. Several novel target agents are undergoing relevant clinical trials due to their superior pharmacological properties. It should be noted that each anticoagulant and anticoagulation strategy that replaces standardized anticoagulation with heparin requires RCTs to determine their safety and efficacy for application in clinical practice. Expect more anticoagulants with increased efficacy while reducing side effects, appropriate antidotes, predictable pharmacokinetics, and superior pharmacological properties to complement and optimize anticoagulation strategies in anticipation of maximum improvement in patient clinical outcomes.
Availability of data and materials
All data generated or analyzed during this study are included in the published studies.
References
Lequier l, Annich G, Al-Ibrahim O, Bembea M, Brodie D (2014) Brogan T.ELSO Anticoagulation guidelines. Ann Arbor MI: Extracorporeal Life Support Organization
Kowalewski M, Fina D, Słomka A et al (2020) COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care 24(1):205. https://doi.org/10.1186/s13054-020-02925-3
Supady A, Combes A, Barbaro RP et al (2022) Respiratory indications for ECMO: focus on COVID-19. Intensive Care Med 48(10):1326–1337. https://doi.org/10.1007/s00134-022-06815-w
Badulak J, Antonini MV, Stead CM et al (2021) Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J 67(5):485–495. https://doi.org/10.1097/MAT.0000000000001422
Mansour A, Flecher E, Schmidt M et al (2022) Bleeding and thrombotic events in patients with severe COVID-19 supported with extracorporeal membrane oxygenation: a nationwide cohort study. Intensive Care Med 48(8):1039–1052. https://doi.org/10.1007/s00134-022-06794-y
Jin Y, Zhang Y, Liu J, Zhou Z (2023) Thrombosis and bleeding in patients with COVID-19 requiring extracorporeal membrane oxygenation: a systematic review and meta-analysis. Res Pract Thromb Haemost 7(2):100103. https://doi.org/10.1016/j.rpth.2023.100103
Nunez JI, Gosling AF, O’Gara B et al (2022) Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intens Care Med 48:213–224. https://doi.org/10.1007/s00134-021-06593-x
Chung M, Cabezas FR, Nunez JI et al (2020) Hemocompatibility related adverse events and survival on venoarterial extracorporeal life support: an ELSO registry analysis. JACC Heart Fail 8:892–902. https://doi.org/10.1016/j.jchf.2020.09.004
Panigada M, Cucino A, Spinelli E et al (2020) A randomized controlled trial of antithrombin supplementation during extracorporeal membrane oxygenation. Crit Care Med 48(11):1636–1644. https://doi.org/10.1097/CCM.0000000000004590
Piacente C, Martucci G, Miceli V et al (2020) A narrative review of antithrombin use during veno-venous extracorporeal membrane oxygenation in adults: rationale, current use, effects on anticoagulation, and outcomes. Perfusion 35(6):452–464. https://doi.org/10.1177/0267659120913803
Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P (2013) Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 14(2):e77–e84. https://doi.org/10.1097/PCC.0b013e31827127e4
Rivosecchi RM, Arakelians AR, Ryan J et al (2021) Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: heparin versus bivalirudin. Crit Care Med 49(7):1129–1136. https://doi.org/10.1097/CCM.0000000000004944
Kimmoun A, Oulehri W, Sonneville R et al (2018) Prevalence and outcome of heparin-induced thrombocytopenia diagnosed under veno-arterial extracorporeal membrane oxygenation: a retrospective nationwide study. Intens Care Med 44:1460–1469. https://doi.org/10.1007/s00134-018-5346-y
Singhania N, Bnsal S, Nimmatoori DP et al (2020) Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs 20:393–403. https://doi.org/10.1007/s40256-020-00431-z
Maier CL, Truong AD, Auld SC et al (2020) COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 395:1758–1759. https://doi.org/10.1016/S0140-6736(20)31209-5
Fareed J, Walenga JM, Williamson K, Emanuele RM, Kumar A, Hoppensteadt DA (1985) Studies on the antithrombotic effects and pharmacokinetics of heparin fractions and fragments. Semin Thromb Hemost 11(1):56–74. https://doi.org/10.1055/s-2007-1004360
Fareed J, Hoppensteadt D, Walenga J et al (2003) Pharmacodynamic and pharmacokinetic properties of enoxaparin: implications for clinical practice. Clin Pharmacokinet 42(12):1043–1057. https://doi.org/10.2165/00003088-200342120-00003
Krueger K, Schmutz A, Zieger B, Kalbhenn J (2017) Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs 41(2):186–192. https://doi.org/10.1111/aor.12737
Gratz J, Pausch A, Schaden E et al (2020) Low molecular weight heparin versus unfractioned heparin for anticoagulation during perioperative extracorporeal membrane oxygenation: a single center experience in 102 lung transplant patients. Artif Organs 44(6):638–646. https://doi.org/10.1111/aor.13642
Wiegele M, Laxar D, Schaden E et al (2022) Subcutaneous enoxaparin for systemic anticoagulation of COVID-19 patients during extracorporeal life support. Front Med (Lausanne) 9:879425. Published 2022 Jul 11. https://doi.org/10.3389/fmed.2022.879425
Circelli A, Bissoni L, Scognamiglio G et al (2023) Anticoagulation on extracorporeal support: an alternative strategy. ASAIO J 69(3):e131. https://doi.org/10.1097/MAT.0000000000001786
Jaspers TCC, Keyany A, Maat B, Meijer K, van den Bemt PMLA, Khorsand N (2022) Therapeutically dosed low molecular weight heparins in renal impairment: a nationwide survey. Eur J Clin Pharmacol 78(9):1469–1479. https://doi.org/10.1007/s00228-022-03344-9
Lequier L, Annich G, Al-Ibrahim O et al (2014) ELSO anticoagulation guidelines. Ann Arbor MI:ELSO 1–17
Knoderer CA, Knoderer HM, Turrentine MW, Kumar M (2006) Lepirudin anticoagulation for heparin-induced thrombocytopenia after cardiac surgery in a pediatric patient. Pharmacotherapy 26(5):709–712. https://doi.org/10.1592/phco.26.5.709
Fitzgerald D, Murphy N (1996) Argatroban: a synthetic thrombin inhibitor of low relative molecular mass. Coron Artery Dis 7(6):455–458
Marchetti M, Barelli S, Gleich T et al (2022) Managing argatroban in heparin-induced thrombocytopenia: a retrospective analysis of 729 treatment days in 32 patients with confirmed heparin-induced thrombocytopenia. Br J Haematol 197(6):766. https://doi.org/10.1111/bjh.18120
Rougé A, Pelen F, Durand M et al (2017) Argatroban for an alternative anticoagulant in HIT during ECMO. J Intens Care 5(1):1–5. https://doi.org/10.1186/s40560-017-0235-y
Menk M, Briem P, Weiss B et al (2017) Efficacy and safety of argatroban in patients with acute respiratory distress syndrome and extracorporeal lung support. Ann Intensive Care 7(1):82. https://doi.org/10.1186/s13613-017-0302-5
Geli J, Capoccia M, Maybauer DM et al (2022) Argatroban anticoagulation for adult extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med 37(4):459–471. https://doi.org/10.1177/0885066621993739
Fisser C, Winkler M, Malfertheiner MV et al (2021) Argatroban versus heparin in patients without heparin-induced thrombocytopenia during venovenous extracorporeal membrane oxygenation: a propensity-score matched study. Crit Care 25:1–10. https://doi.org/10.1186/s13054-021-03581-x
Cho AE, Jerguson K, Peterson J et al (2021) Cost-effectiveness of argatroban versus heparin anticoagulation in adult extracorporeal membrane oxygenation patients. Hosp Pharm 56(4):276–281. https://doi.org/10.1177/0018578719890091
Rajsic S, Breitkopf R, Jadzic D et al (2022) Anticoagulation strategies during extracorporeal membrane oxygenation: a narrative review. J Clin Med 11(17):5147. https://doi.org/10.3390/jcm11175147
Dingman JS, Smith ZR, Coba VE, Peters MA, To L (2020) Argatroban dosing requirements in extracorporeal life support and other critically ill populations. Thromb Res 189:69–76. https://doi.org/10.1016/j.thromres.2020.02.021
Gladwell TD (2002) Bivalirudin: a direct thrombin inhibitor. Clin Therap 24(1):38–58. https://doi.org/10.1016/s0149-2918(02)85004-4
Kam PCA, Kaur N, Thong CL (2005) Direct thrombin inhibitors: pharmacology and clinical relevance. Anaesthesia 60(6):565–574. https://doi.org/10.1111/j.1365-2044.2005.04192.x
Caridi-Scheible M, Nichols K, Gallagher J (2021) Bivalirudin in venovenous extracorporeal membrane oxygenation: moving forward in the real world. Crit Care Med 49(7):1208–1210. https://doi.org/10.1097/CCM.0000000000004964
Koster A, Niedermeyer J, Gummert J, Renner A (2017) Low dose bivalirudin anticoagulation for lung transplantation with extracorporeal membrane oxygenation in a patient with acute heparin-induced thrombocytopenia. Eur J Cardiothorac Surg 51(5):1009–1011
Li DH, Sun MW, Zhang JC, Zhang C, Deng L, Jiang H (2022) Is bivalirudin an alternative anticoagulant for extracorporeal membrane oxygenation (ECMO) patients? A systematic review and meta-analysis. Thromb Res 210:53–62. https://doi.org/10.1016/j.thromres.2021.12.024
Sanfilippo F, La Via L, Murabito P, Pappalardo F, Astuto M (2022) More evidence available for the use of Bivalirudin in patients supported by extracorporeal membrane oxygenation. Thromb Res 211:148–149. https://doi.org/10.1016/j.thromres.2022.02.007
Sanfilippo F, Asmussen S, Maybauer DM et al (2017) Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med 32(5):312–319. https://doi.org/10.1177/0885066616656333
Li MJ, Shi JY, Zhang JH (2022) Bivalirudin vs. heparin in paediatric and adult patients on extracorporeal membrane oxygenation: a meta-analysis. Br J Clin Pharmacol 88(6):2605–2616. https://doi.org/10.1111/bcp.15251
Liu L, Liu F, Tan J, Zhao L (2022) Bivalirudin versus heparin in adult and pediatric patients with extracorporeal membrane oxygenation therapy: a systematic review and meta-analysis. Pharmacol Res 177:106089. https://doi.org/10.1016/j.phrs.2022.106089
Ma M, Liang S, Zhu J et al (2022) The efficacy and safety of bivalirudin versus heparin in the anticoagulation therapy of extracorporeal membrane oxygenation: a systematic review and meta-analysis. Front Pharmacol 13:771563. Published 2022 Apr 14. https://doi.org/10.3389/fphar.2022.771563
Wieruszewski PM, Macielak SA, Nei SD et al (2023) Heparin versus bivalirudin for anticoagulation in adult extracorporeal membrane oxygenation: a systematic review and meta-analysis. ASAIO J 69(2):137–144. https://doi.org/10.1097/MAT.0000000000001808
M’Pembele R, Roth S, Metzger A et al (2022) Evaluation of clinical outcomes in patients treated with heparin or direct thrombin inhibitors during extracorporeal membrane oxygenation: a systematic review and meta-analysis. Thromb J 20(1):42. Published 2022 Jul 28. https://doi.org/10.1186/s12959-022-00401-2
Gu J, Yu H, Lin D (2023) Superiority of bivalirudin over heparin anticoagulation therapy for extracorporeal membrane oxygenation? Too early to draw conclusions. Heliyon 9(2). https://doi.org/10.1016/j.heliyon.2023.e13530
Burstein B, Wieruszewski PM, Zhao YJ, Smischney N (2019) Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med 8:87–98. https://doi.org/10.5492/wjccm.v8.i6.87
Zheng W, Dai X, Xu B, Tian W, Shi J (2023) Discovery and development of Factor Xa inhibitors (2015–2022). Front Pharmacol 14:1105880. Published 2023 Feb 21. https://doi.org/10.3389/fphar.2023.1105880
Wilde MI, Markham A (1997) Danaparoid: A review of its pharmacology and clinical use in the management of heparin-induced thrombocytopenia. Drugs 54:903–924. https://doi.org/10.2165/00003495-199754060-00008
Phan XT, Nguyen TH, Tran TT et al (2020) Suspected heparin-induced thrombocytopenia in a COVID-19 patient on extracorporeal membrane oxygenation support: a case report. Thromb J 18(1):1–5. https://doi.org/10.1186/s12959-020-00252-9
Parlar AI, Sayar U, Cevirme D, Yuruk MA, Mataraci I (2014) Successful use of fondaparinux in a patient with heparin-induced thrombocytopenia while on extracorporeal membrane oxygenation after mitral valve redo surgery. Int J Artif Organs 37(4):344–347. https://doi.org/10.5301/ijao.5000302
Lu GY, Xu H, Li JH, Chen JK, Ning YG (2023) Safety and outcome of early enteral nutrition in patients receiving extracorporeal membrane oxygenation. Clin Nutr 42(9):1711–1714. https://doi.org/10.1016/j.clnu.2023.07.021
Davis RC 2nd, Durham LA 3rd, Kiraly L, Patel JJ (2021) Safety, tolerability, and outcomes of enteral nutrition in extracorporeal membrane oxygenation. Nutr Clin Pract 36(1):98–104. https://doi.org/10.1002/ncp.10591SundaramS,GikakisN,HackCE,etal
Milling TJ Jr, Middeldorp S, Xu L et al (2023) Final study report of Andexanet Alfa for major bleeding with factor Xa inhibitors. Circulation 147(13):1026–1038. https://doi.org/10.1161/CIRCULATIONAHA.121.057844
Rogers KC, Finks SW (2019) A new option for reversing the anticoagulant effect of factor Xa inhibitors: Andexanet Alfa (ANDEXXA). Am J Med 132(1):38–41. https://doi.org/10.1016/j.amjmed.2018.06.028
Sundaram S, Gikakis N, Hack CE et al (1996) Nafamostat mesilate, a broad spectrum protease inhibitor, modulates platelet, neutrophil and contact activation in simulated extracorporeal circulation. Thromb Haemost 75:76–82
Sanfilippo F, CurròJ M, La Via L et al (2022) Use of nafamostat mesilate for anticoagulation during extracorporeal membrane oxygenation: a systematic review. Artif Organs 46(12):2371–2381. https://doi.org/10.1111/aor.14276
Han SJ, Kim HS, Kim KI et al (2011) Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation. J Korean Med Sci 26(7):945–950. https://doi.org/10.3346/jkms.2011.26.7.945
Lang Y, Zheng Y, Qi B et al (2022) Anticoagulation with nafamostat mesilate during extracorporeal life support. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2022.07.022
Han SJ, Han W, Song HJ et al (2018) Validation of nafamostat mesilate as an anticoagulant in extracorporeal membrane oxygenation: a large-animal experiment. Korean J Thorac Cardiovasc Surg 51:114–121. https://doi.org/10.5090/kjtcs.2018.51.2.114
Han W, San Bok J, Cho HJ et al (2019) Single-center experience of extracorporeal membrane oxygenation mainly anticoagulated with nafamostat mesilate. J Thorac Dis 11:2861–2867. https://doi.org/10.21037/jtd.2019.06.30
Lim JY, Kim JB, Choo SJ, Chung CH, Lee JW, Jung SH (2016) Anticoagulation during extracorporeal membrane oxygenation; nafamostat mesilate versus heparin. Ann Thorac Surg 102(2):534–539. https://doi.org/10.1016/j.athoracsur.2016.01.044
Lee JH, Park JH, Jang JH et al (2022) The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation. Acute Crit Care 37(2):177–184. https://doi.org/10.4266/acc.2021.01312
Lang Y, Zheng Y, Qi B et al (2022) Anticoagulation with nafamostat mesilate during extracorporeal life support. Intern J Cardiol. https://doi.org/10.1016/j.ijcard.2022.07.022
Hernández-Mitre MP, Tong SYC, Denholm JT et al (2022) Nafamostat mesylate for treatment of COVID-19 in hospitalised patients: a structured, narrative review. Clin Pharmacokinet 61(10):1331–1343. https://doi.org/10.1007/s40262-022-01170-x
Clark JA, Schulman G, Golper TA (2008) Safety and efficacy of regional citrate anticoagulation during 8-hour sustained low-efficiency dialysis. Clin J Am Soc Nephrol 3(3):736–742. https://doi.org/10.2215/CJN.03460807
Shum HP, Kwan AMC, Chan KC et al (2014) The use of regional citrate anticoagulation continuous venovenous hemofiltration in extracorporeal membrane oxygenation. ASAIO J 60(4):413–418. https://doi.org/10.1097/MAT.0000000000000085
Giani M, Scaravilli V, Stefanini F et al (2020) Continuous renal replacement therapy in venovenous extracorporeal membrane oxygenation: a retrospective study on regional citrate anticoagulation. ASAIO J 66:332–338. https://doi.org/10.1097/MAT.0000000000001003
Tiranathanagul K, Jearnsujitwimol O, Susantitaphong P et al (2011) Regional citrate anticoagulation reduces polymorphonuclear cell degranulation in critically ill patients treated with continuous venovenous hemofiltration. Ther Apher Dial 15:556–564. https://doi.org/10.1111/j.1744-9987.2011.00996.x
Akers WS, Oh JJ, Oestreich JH et al (2010) Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol 50:27–35. https://doi.org/10.1177/0091270009344986
De Luca L, Steg PG, Bhatt DL et al (2021) Cangrelor: clinical data, contemporary use, and future perspectives. J Am Heart Assoc 10:e022125. https://doi.org/10.1161/JAHA.121.022125
Vaduganathan M, Qamar A, Badreldin HA et al (2017) Cangrelor use in cardiogenic shock: a single-center real-world experience. JACC Cardiovasc Interv 10(16):1712–1714. https://doi.org/10.1016/j.jcin.2017.07.009
Ciolek AM, Ma KL, Garan AR et al (2020) Use of cangrelor during venoarterial extracorporeal membrane oxygenation following percutaneous coronary intervention. Artif Organs 44:339–340. https://doi.org/10.1111/aor.13563
Katz A, Lewis TC, Arnouk S et al (2021) Clinical use of cangrelor after percutaneous coronary intervention in patients requiring mechanical circulatory support. Ann Pharmacother 55(10):1215–1222. https://doi.org/10.1177/1060028021994621
Baldetti L, Nardelli P, Ajello S et al (2022) Antithrombotic therapy with cangrelor and bivalirudin in venoarterial extracorporeal membrane oxygenation patients undergoing percutaneous coronary intervention: a single-center experience [published online ahead of print, 2022 Dec 12]. ASAIO J. https://doi.org/10.1097/MAT.0000000000001871
Patel JS, Kooda K, Igneri LA (2022) A narrative review of the impact of extracorporeal membrane oxygenation on the pharmacokinetics and pharmacodynamics of critical care therapies [published online ahead of print,2022 Oct 15]. Ann Pharmacother 10600280221126438. https://doi.org/10.1177/10600280221126438
Kreutz RP, Nystrom P, Kreutz Y et al (2013) Inhibition of platelet aggregation by prostaglandin E1 (PGE1) in diabetic patients during therapy with clopidogrel and aspirin. Platelets 24(2):145–150. https://doi.org/10.3109/09537104.2012.661107
Hulshof AM, Vries M, Verhezen P et al (2021) The influence of prostaglandin E1 and use of inhibitor percentage on the correlation between the multiplate and verify now in patients on dual antiplatelet therapy. Platelets 32(4):463–468. https://doi.org/10.1080/09537104.2020.1754378
Goerge T, Ho-Tin-Noe B, Carbo C et al (2008) Inflammation induces hemorrhage in thrombocytopenia. Blood 111:4958–4964. https://doi.org/10.1182/blood-2007-11-123620
Buchtele N, Schörgenhofer C, Schwameis M et al (2022) Add-on prostaglandin E1 in venovenous extracorporeal membrane oxygenation: a randomized, double-blind, placebo-controlled pilot trial. Am J Respir Crit Care Med 206(2):170–177. https://doi.org/10.1164/rccm.202110-2359OC
Kenne E, Nickel KF, Long AT et al (2015) Factor XII: a novel target for safe prevention of thrombosis and inflammation. J Intern Med 278(6):571–585. https://doi.org/10.1111/joim.12430
Kenne E, Renné T (2014) Factor XII: a drug target for safe interference with thrombosis and inflammation. Drug Discov Today 19(9):1459–1464. https://doi.org/10.1016/j.drudis.2014.06.024
Larsson M, Rayzman V, Nolte MW et al (2014) A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med 6(222):222ra17. https://doi.org/10.1126/scitranslmed.3006804
Beck S, Stegner D, Loroch S et al (2021) Generation of a humanized FXII knock-in mouse—a powerful model system to test novel antithrombotic agents. J Thromb Haemost 19(11):2835–2840. https://doi.org/10.1111/jth.15488
Kluge KE, Seljeflot I, Arnesen H, Jensen T, Halvorsen S, Helseth R (2022) Coagulation factors XI and XII as possible targets for anticoagulant therapy. Thromb Res 214:53–62. https://doi.org/10.1016/j.thromres.2022.04.013
Poenou G, Dumitru Dumitru T, Lafaie L, Mismetti V, Heestermans M, Bertoletti L (2022) Factor XI inhibition for the prevention of venous thromboembolism: an update on current evidence and future perspectives. Vasc Health Risk Manag 18:359–373. https://doi.org/10.2147/VHRM.S331614
Koch AW, Schiering N, Melkko S et al (2019) MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood 133(13):1507–1516. https://doi.org/10.1182/blood-2018-10-880849
Yi BA, Freedholm D, Widener N et al (2022) Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa. J Thromb Haemost 20(2):307–315. https://doi.org/10.1111/jth.15577
Perera V, Wang Z, Luettgen J et al (2022) First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci. 15(2):330–342. https://doi.org/10.1111/cts.13148. (published correction appears in Clin Transl Sci. 2022 Apr;15(4):1085)
Lorentz CU, Tucker EI, Verbout NG et al (2021) The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood 138(22):2173–2184. https://doi.org/10.1182/blood.2021011725
Greco A, Laudani C, Spagnolo M et al (2023) Pharmacology and clinical development of factor XI inhibitors. Circulation 147(11):897–913. https://doi.org/10.1161/CIRCULATIONAHA.122.062353
Jiang S, Jia Z, Zheng Y et al (2022) Bifunctional fusion protein targeting both FXIIa and FXIa displays potent anticoagulation effects. Life Sci 309:121021. https://doi.org/10.1016/j.lfs.2022.121021
Afosah DK, Ofori E, Mottamal M et al (2022) Factor IX(a) inhibitors: an updated patent review (2003-present). Expert Opin Ther Pat 32(4):381–400. https://doi.org/10.1080/13543776.2022.2026926
Olson SR, Murphree CR, Zonies D et al (2021) Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J 67(3):290–296. https://doi.org/10.1097/MAT.0000000000001230
Acknowledgements
All authors are grateful to our institution for purchasing some quality journal literature that we can access for free and learn a lot from.
Author information
Authors and Affiliations
Contributions
XLQ and LH conducted the literature search and source document search and wrote the paper. WZS was responsible for reviewing the data and drawing the figures for this article. TS was responsible for the design and full text review of this study; WQ was responsible for the full text review. XLQ and TS were responsible for revising the manuscript, and all authors read and approved the final version. XLQ and LH were responsible for downloading and processing the data in this paper and writing the paper. WZS was responsible for reviewing the data in this paper and drawing the graphs and tables. TS was responsible for the design of this study and reviewing the full text; WQ was responsible for reviewing the full text. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, S., Xu, L., Li, H. et al. Anticoagulants in adult extracorporeal membrane oxygenation: alternatives to standardized anticoagulation with unfractionated heparin. Eur J Clin Pharmacol 79, 1583–1594 (2023). https://doi.org/10.1007/s00228-023-03568-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03568-3