Abstract
Background
Ticagrelor provides more rapid, potent, and consistent anti-platelet efficacy than clopidogrel. This randomized trial aimed to evaluate the anti-inflammation effects of ticagrelor versus clopidogrel on thrombus aspirated from the ST-elevation myocardial infarction (STEMI) patients.
Method
A total of 98 patients with STEMI and intended percutaneous coronary intervention (PCI) were randomly assigned to receive clopidogrel (600-mg loading dose) or ticagrelor (180-mg loading dose), of whom 55 with large thrombus burden underwent thrombus aspiration during PCI. Thrombus specimens were successfully aspirated from 49 patients. Finally, 24 patients in the clopidogrel group and 23 in the ticagrelor group completed the study. Inflammatory cells within thrombi were assessed by hematoxylin–eosin and immunohistochemistry stainings.
Results
Compared with the clopidogrel group, the number of total inflammatory cells per mm2 thrombus area in the ticagrelor group was decreased by 28% (P = 0.009). The numbers of neutrophils and myeloperoxidase-positive cells per mm2 thrombus area in the ticagrelor group were respectively decreased by 35% (P = 0.016) and 28% (P = 0.047), as compared with those in the clopidogrel group. Moreover, ticagrelor treatment reduced the ratio of monocytes number higher than 250 per mm2 thrombus area compared with clopidogrel treatment (4% versus 29%, P = 0.048).
Conclusion
In patients with undergoing PCI for STEMI, the loading dose ticagrelor regimen was associated with a reduction in inflammatory cell infiltration within thrombus compared with the loading dose clopidogrel regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute ST-segment elevation myocardial infarction (STEMI) is caused by the formation of thrombi in the coronary artery [1]. A coronary thrombus consists mainly of platelets, erythrocytes, and fibrin, and often contains inflammatory cells such as neutrophils and monocytes [2]. It has been demonstrated that inflammatory cells augment the growth of thrombus by interacting with platelets and erythrocytes [3]. Additionally, recent studies showed that thrombus inflammatory cell content was negative correlation with the perfusion of infarcted myocardium after primary percutaneous coronary intervention (PCI) in patients with STEMI [4, 5]. The importance of inflammatory cells in both the progression and prognosis of atherothrombotic disease raises the intriguing possibility of clinical benefit from drugs that inhibits the activation of inflammatory cells. The P2Y12 receptor is the target of antiplatelet drugs like the thienopyridine compounds ticlopidine, clopidogrel, and prasugrel or the direct, reversible antagonists ticagrelor and cangrelor [6]. Beyond the platelet physiology and pharmacology, previous studies have revealed the expression and roles of P2Y12 receptor in inflammatory cells including microglial cells, [7] dendritic cells, [8] monocytes, [9] and unspecified leukocytes [10]. Moreover, the anti-inflammation benefits of P2Y12 receptor inhibitors in atherosclerosis, [11] aneurysm, [12] ischemic brain injury, [13] and cardiac fibrosis [14] have also been observed. However, the impacts of P2Y12 receptor inhibition in thrombus inflammation remain to be determined. Previous studies have revealed that ticagrelor provides more rapid, potent, and consistent P2Y12 receptor inhibition than clopidogrel [15, 16]. The aim of this study was to evaluate the efficacy of P2Y12 receptor inhibition with clopidogrel versus ticagrelor on inflammatory cell infiltration in thrombus aspirated from the STEMI patients.
Materials and methods
This clinical trial was a prospective, randomized, parallel design study undertaken between May, 2016, and February, 2017, in the First Affiliated Hospital of Harbin Medical University, Harbin City, China. All protocols were conformed to the Ethical Guidelines of the 1975 Declaration of Helsinki. The study was approved by the ethics committee of the First Affiliated Hospital of Harbin Medical University (201525) and registered at https://clinicaltrials.gov/ (NCT02639143).
Patients were eligible for inclusion in the trial if they had symptoms suggesting acute myocardial ischemia lasting more than 30 min, ST-segment elevation of more than 0.1 mV in two or more leads on the electrocardiogram (ECG), arrival at the hospital within 12 h of the onset of symptoms, and the intention to perform PCI. Exclusion criteria included an increased risk of bleeding or active bleeding, previous antiplatelet or anticoagulation therapy, and hemodynamic or electrical instability. All patients provided written informed consent.
Randomization was done with a 24-h computerized central automated voice response system. A computer-generated random allocation sequence was generated by the statistician for the Data Safety and Monitoring Board. Participants, those giving the interventions, those assessing outcomes, and those analyzing the data were masked to group assignment.
Patients who meet the inclusion criteria and none of the exclusion criteria will be consented. Initial background assessments include demographics, cardiovascular risk factors, relevant medical and surgical histories, clinical characteristics, and laboratory data. Immediately after randomization and before coronary angiography, patients randomly allocated clopidogrel received a 600-mg loading dose followed by 75 mg once daily. Those allocated ticagrelor received a 180-mg loading dose followed by 90 mg twice daily. All patients received a 300-mg loading dose of aspirin followed by 100 mg daily during the treatment period. During PCI, thrombus aspiration was performed in the infarct-related artery with large thrombus burden (Thrombolysis In Myocardial Infarction thrombus score ≥ 3; or “cut-off” occlusion pattern and/or vessel diameter ≥ 3.5 mm) [17]. The infarct-related artery was defined as a major coronary artery perfusing an area compatible with the distribution of ST-segment elevation in the ECG.
Immunohistochemical analysis
Aspirated thrombi were immediately washed with saline, fixed in 10% formalin, and embedded in paraffin. The paraffin embedded samples were serially sectioned to produce 5-μm-thick sections. Every first and second section was stained with hematoxylin–eosin; the other sections were used for immunohistochemical staining of neutrophils (mouse monoclonal anti-CD11b, 1:500, Abcam, Hong Kong), monocytes (mouse monoclonal anti-CD14, 1:500, Abcam, Hong Kong), and myeloperoxidase (MPO) (mouse monoclonal anti-MPO, 1:500, Abcam, Hong Kong) [5]. The numbers of total inflammatory cells, neutrophils, MPO-positive cells, and monocytes per mm2 thrombus area were calculated in eight random 600 × microscopic images. A pathologist performed the histopathological analyses while blinded to the groups.
High-sensitivity C-reactive protein analysis
Blood samples were drawn before and 24 h after PCI to measure serum high-sensitivity C-reactive protein (hs-CRP) level. The content of CRP in plasma was detected as per the manufacturer’s instructions of the human high sensitivity CRP ELISA kit (Sigma, USA).
Power analysis
To estimate the group size, an observational pilot study of 22 patients treated with ticagrelor (n = 11) or clopidogrel (n = 11) was performed prior to the present study. This pilot study revealed that the patients treated with ticagrelor showed a significant improvement on the total inflammatory cells per mm2 thrombus (836.60 ± 350.80 versus 1670.00 ± 720.00, P < 0.03). With α = 0.10, two-tailed and a power of 85%, we needed 10 patients each group. Considering a compliance rate of 90%, we asked 22 patients to take part in our pilot study.
Statistical analysis
Continuous variables were described as mean ± standard deviation if it follows normal distribution, otherwise, as median and interquantile; categorical variables were represented as count and percentage. A Shapiro–Wilk test was first used to test the normality of the data. Differences in continuous variables between groups were analyzed by t tests, and differences in categorical variables by χ2 tests. A value of P < 0.05 was considered as statistically significant. Statistical evaluation was performed using Statistical Analysis System software (version 9.2, SAS institute, Cary, NC, USA).
Results
Patient characteristics
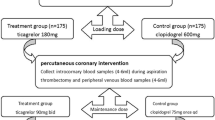
A total of 98 patients with STEMI were enrolled and randomly assigned treatment in this trial, of whom 55 underwent thrombus aspiration during PCI. Thrombus specimens were successfully aspirated from 49 patients. However, 2 specimens in ticagrelor group, despite serial sectioning, contained only minimal fragments of thrombus insufficient for histopathological analysis. Finally, 24 patients in the clopidogrel group and 23 in the ticagrelor group completed the study (Fig. 1). Baseline characteristics of the randomized patients are shown in Table 1. There were no differences in demographic characteristics of STEMI patients with between both groups. Time from the onset of chest pain to randomization was 205.42 ± 135.23 min in the clopidogrel group and 241.30 ± 139.98 min in the ticagrelor group. Time from randomization to thrombus aspiration was 78.79 ± 39.95 min in the clopidogrel group and 70.22 ± 28.60 min in the ticagrelor group (Table 1). Baseline characteristics were well matched between the two groups (Table 1).
Detection of inflammatory cell infiltration of thrombus aspirated and hs-CRP
The primary endpoint of the number of total inflammatory cells per mm2 thrombus area was 28% less in the ticagrelor group than in the clopidogrel group (1173.00 ± 561.00 versus 1639.00 ± 607.00, P = 0.009, Fig. 2A and B). The numbers of neutrophils and MPO-positive cells per mm2 thrombus area in the ticagrelor group were respectively decreased by 35% (209.30 ± 139.90 versus 321.20 ± 165.80, P = 0.016, Fig. 3A and B) and 28% (238.90 ± 140.00 versus 332.60 ± 172.60, P = 0.047, Fig. 3C and D), as compared with those in the clopidogrel group. There was no difference in the number of monocytes per mm2 thrombus area between the clopidogrel and ticagrelor groups (30.36 ± 41.84 versus 56.48 ± 80.83, P = 0.184, Fig. 3E and F). However, ticagrelor treatment reduced the ratio of monocytes number higher than 250 per mm2 thrombus area compared with clopidogrel treatment (4% versus 29%, P = 0.048, Fig. 3G). The serum concentration of hs-CRP did not differ between the clopidogrel and ticagrelor groups neither before (6.23 ± 8.86 versus 6.49 ± 8.91, P = 0.94) nor 24 h after PCI (18.11 ± 21.90 versus 13.69 ± 15.14, P = 0.49) (Fig. 4).
Histological analysis of inflammatory cells within thrombi. A Representative histologic transverse sections stained with hematoxylin and eosin illustrate the presence of inflammatory cells within thrombi. Scar bar: 0.1 mm. B The numbers of inflammatory cells per mm2 thrombus area were calculated (clopidogrel group: n = 24; ticagrelor group: n = 23). Data are presented as mean ± standard deviation and analyzed by t tests
Immunohistochemical analysis of neutrophils, MPO-positive cells, and monocytes within thrombi. A Representative immunohistochemical transverse sections stained with CD11b illustrate the presence of neutrophils within thrombi. Scar bar: 0.1 mm. B The numbers of neutrophils per mm2 thrombus area were calculated (clopidogrel group: n = 24; ticagrelor group: n = 23). Data are presented as mean ± standard deviation and analyzed by t tests. C Representative immunohistochemical transverse sections stained with MPO illustrate the presence of MPO-positive cells within thrombi. Scar bar: 0.1 mm. D The numbers of MPO-positive cells per mm2 thrombus area were calculated (clopidogrel group: n = 24; ticagrelor group: n = 23). Data are presented as mean ± standard deviation and analyzed by t tests. E Representative immunohistochemical transverse sections stained with CD14 illustrate the presence of monocytes within thrombi. Scar bar: 0.1 mm. F The numbers of CD14-positive cells per mm2 thrombus area were calculated (clopidogrel group: n = 24; ticagrelor group: n = 23). Data are presented as mean ± standard deviation and analyzed by t tests. G The ratio of CD14-positive cells number higher than 250 per mm2 thrombus area were calculated (clopidogrel group: n = 24; ticagrelor group: n = 23). Data were analyzed by χ2 tests
Discussion
In this study, the major finding is that ticagrelor treatment provided greater attenuation of thrombus inflammatory cell infiltration than clopidogrel treatment in STEMI patients.The P2 purinergic receptors consist of two families: the ionotropic receptors (P2X) contain channels that permit ion flow, whereas the metabotropic receptors (P2Y) are G-protein coupled second messenger systems [18]. In inflammatory cells, P2Y receptors are involved in pro-inflammatory responses, [19] migration [20], and phagocytosis [21]. A substantial body of literature [9, 22] has demonstrated that the P2Y12 receptor plays a pivotal role in inflammatory cell migration when activated by its ligands, ATP and ADP. Webster et al. reported that P2Y12 deficiency or inhibition by clopidogrel attenuated microglia migration in response to injured neurons [22]. Kronlage et al. demonstrated that monocytes from mice deficient in P2Y12 exhibited impaired chemotaxis [9]. In agreement with these reports, we showed in this study that pharmacological P2Y12 receptor inhibition by clopidogrel and ticagrelor was protective against inflammatory cell infiltration in thrombus.
However, it is still possible that the effect of clopidogrel and ticagrelor seen on thrombus inflammation could be due to its direct effect on platelets. Platelets are an important source of pro-inflammatory mediators and cytokines that can recruit inflammatory cells such as neutrophils and monocytes [23]. In addition, activated platelets have been shown to stimulate platelet-leukocyte interactions, which are involved in the pathogenesis of several inflammatory diseases. Xiao et al. showed that in patients with NSTEMI, platelet-monocyte and platelet-neutrophil conjugates in whole blood were significantly reduced by clopidogrel treatment [24]. In a murine model of abdominal aortic aneurysms, Liu et al. reported that inhibition of platelet substantially decreased monocytes recruitment into aneurysm tissue [12]. Moreover, activation of P2Y12 receptor has been shown to be involved in the formation of platelet-leukocyte aggregates at the site of the growing thrombus [25]. These studies suggest that platelet P2Y12 receptor inhibition likely contributes to clopidogrel and ticagrelor-induced attenuation of inflammatory cell infiltration in thrombus by interrupting platelet-monocyte and platelet-neutrophil conjugates.
Infiltration of inflammatory cells contributes to the evolution of thrombus formation and growth. It has been reported that activated neutrophils and monocytes induce alterations of erythrocyte properties, which result in hyperaggregability, and increase the expression of tissue factor activity, leading to a thrombotic state [26, 27]. Furthermore, inflammatory cell infiltration could lead to poor procedural outcome during PCI in patients with STEMI. Arakawa et al. demonstrated that high neutrophil density in an aspirated thrombus is associated with impaired coronary microcirculation and left ventricular dysfunction [28]. Additionally, Yunoki et al. showed that the thrombi aspirated in patients with distal embolization contained more myeloperoxidase-positive cells (neutrophils and monocytes) than thrombi aspirated in patients without distal embolization [4, 5].
Ticagrelor is a direct-acting inhibitor of P2Y12 receptor, providing more rapid, potent, and consistent P2Y12 receptor inhibition than clopidogrel [15]. This support our results that the thrombus inflammatory cell infiltration in patients receiving ticagrelor treatment was less than that in patients receiving clopidogrel treatment. Such anti-inflammation effects may, at least in part, explain the previous findings in the PLATO trial that ticagrelor treatment provided greater mortality benefit than clopidogrel treatment in STEMI patients [29].
Routine thrombus aspiration is not recommended by current guidelines, but in cases of large thrombus burden after opening the vessel with a guide wire or a balloon, thrombus aspiration may be considered [30]. In this case, thrombus aspiration was performed in 55 of 98 patients with large thrombus burden in the infarct-related artery. At the time of patient enrollment (2016–2017), international guidelines recommended prasugrel or ticagrelor rather than clopidogrel as first-line antiplatelet strategy for STEMI patients. However, guidelines of China recommended either clopidogrel or ticagrelor as first-line antiplatelet strategy for STEMI patients. In accordance with this, patients with STEMI were randomly assigned to receive clopidogrel or ticagrelor.
Study limitations
Despite the encouraging findings, our study has several limitations. First, it involved a relatively small number of patients and was conducted at a single center. Second, the small sample size of thrombus was insufficient for further western blotting and real-time PCR analysis. Third, the level of inflammatory factors in both patients was not determined. The dropout rate in both groups after randomization was high. Therefore, further larger-scale studies are needed to validate the clinical effect of ticagrelor in patients with STMEI. Finally, our study was not powered to detect clinical long-term safety differences between the 2 treatment groups.
Conclusions
In conclusion, our findings in this study indicate that the loading dose ticagrelor regimen was associated with a reduction in inflammatory cell infiltration within thrombus compared with the loading dose clopidogrel regimen in patients with undergoing PCI for STEMI.
References
Svilaas T, Vlaar PJ, van der Horst IC et al (2008) Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 358:557–567
Silvain J, Collet JP, Nagaswami C et al (2011) Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 57:1359–1367
De Caterina R, D’Ugo E, Libby P (2016) Inflammation and thrombosis - testing the hypothesis with anti-inflammatory drug trials. Thromb Haemost 116:1012–1021
Yunoki K, Naruko T, Sugioka K et al (2012) Erythrocyte-rich thrombus aspirated from patients with ST-elevation myocardial infarction: association with oxidative stress and its impact on myocardial reperfusion. Eur Heart J 33:1480–1490
Yunoki K, Naruko T, Inoue T et al (2013) Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc Interv 6:377–385
Winter MP, Grove EL, De Caterina R et al (2017) Advocating cardiovascular precision medicine with P2Y12 receptor inhibitors. Eur Heart J Cardiovasc Pharmacother 3:221–234
Lou N, Takano T, Pei Y et al (2016) Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci USA 113:1074–1079
Ben Addi A, Cammarata D, Conley PB et al (2010) Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 185:5900–5906
Kronlage M, Song J, Sorokin L et al (2010) Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal 3:ra55
Diehl P, Olivier C, Halscheid C et al (2010) Clopidogrel affects leukocyte dependent platelet aggregation by P2Y12 expressing leukocytes. Basic Res Cardiol 105:379–387
Li D, Wang Y, Zhang L et al (2012) Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 32:e81-89
Liu O, Jia L, Liu X et al (2012) Clopidogrel, a platelet P2Y12 receptor inhibitor, reduces vascular inflammation and angiotensin II induced-abdominal aortic aneurysm progression. PLoS ONE 7:e51707
Yamauchi K, Imai T, Shimazawa M et al (2017) Effects of ticagrelor in a mouse model of ischemic stroke. Sci Rep 7:12088
Jia LX, Qi GM, Liu O et al (2013) Inhibition of platelet activation by clopidogrel prevents hypertension-induced cardiac inflammation and fibrosis. Cardiovasc Drugs Ther 27:521–530
Dehghani P, Lavoie A, Lavi S et al (2017) Effects of ticagrelor versus clopidogrel on platelet function in fibrinolytic-treated STEMI patients undergoing early PCI. Am Heart J 192:105–112
Storey RF, Angiolillo DJ, Patil SB et al (2010) Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol 56:1456–1462
Napodano M, Dariol G, Al Mamary AH et al (2014) Thrombus burden and myocardial damage during primary percutaneous coronary intervention. Am J Cardiol 113:1449–1456
Hechler B, Gachet C (2015) Purinergic receptors in thrombosis and inflammation. Arterioscler Thromb Vasc Biol 35:2307–2315
Haynes SE, Hollopeter G, Yang G et al (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519
Elliott MR, Chekeni FB, Trampont PC et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286
Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K et al (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446:1091–1095
Webster CM, Hokari M, McManus A et al (2013) Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS ONE 8:e70927
Smyth SS, McEver RP, Weyrich AS et al (2009) Platelet functions beyond hemostasis. J Thromb Haemost 7:1759–1766
Xiao Z, Theroux P (2004) Clopidogrel inhibits platelet-leukocyte interactions and thrombin receptor agonist peptide-induced platelet activation in patients with an acute coronary syndrome. J Am Coll Cardiol 43:1982–1988
Leon C, Alex M, Klocke A et al (2004) Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood 103:594–600
Iba T, Levy JH (2018) Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost 16:231–241
Baskurt OK, Meiselman HJ (1998) Activated polymorphonuclear leukocytes affect red blood cell aggregability. J Leukoc Biol 63:89–93
Arakawa K, Yasuda S, Hao H et al (2009) Significant association between neutrophil aggregation in aspirated thrombus and myocardial damage in patients with ST-segment elevation acute myocardial infarction. Circ J 73:139–144
Steg PG, James S, Harrington RA et al (2010) Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 122:2131–2141
Ibanez B, James S, Agewall S et al (2018) 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39:119–177
Acknowledgements
We are grateful to Dr. Zhong Hua Wang, Dr. Xin Yong Sun, and Dr. Yi Lun Zou in the Department of Cardiology, the First Affiliated Hospital, Harbin Medical University. The authors would like to acknowledge the department of Clinical Pharmacology of the First Affiliated Hospital, Harbin Medical University.
Funding
This study was supported by grants from the Shenzhen Key Medical Discipline Construction Fund (No.szxk003) and Shenzhen People’s Hospital Young College academic backbone to support project. SYJCYJ 202008.
Author information
Authors and Affiliations
Contributions
Jing Shen, Guang Zhong Liu, Guo Dong Wu, Xin Sun, and Ye Tian designed and conducted the experiment; Jing Shen, Guang Zhang Liu, and Guo Dong Wu.analyzed the data and wrote the manuscript. Lijian Sheng, Zhengyu Cao, Shu Yuan Guo, and Shao Hong Dong conducted the experiments. Jing Shen, Guang Zhong Liu, and Xin Sun revised the manuscript; and all authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The study was approved in advance by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University and was conducted in accordance with International Conference on Harmonization Guideline for Good Clinical Practice and Declaration of Helsinki.
Informed consent
All subjects provided written informed consents before their participation.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, J., Liu, G., Wu, G. et al. Ticagrelor versus clopidogrel in reducing inflammatory cell infiltration of thrombus aspirated in patients with ST-elevation myocardial infarction. Eur J Clin Pharmacol 78, 1391–1398 (2022). https://doi.org/10.1007/s00228-022-03348-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03348-5