Abstract
Background
Acid-suppressive drugs (ASDs) are being used by increasing number of children and young adults. However, evidence for a relationship between ASD use and the risk of fracture in these groups of patients is conflicting. We conducted a meta-analysis to evaluate the risk of fracture in children and young adults exposed to ASDs.
Methods
A literature search was performed using the PUBMED, EMBASE, and Cochrane Library databases from inception to November 2020. Pooled relative risks (RRs) and 95% confidence intervals (CIs) were calculated to determine the relationship of ASD use with fracture risk in children and young adults.
Results
Six studies reporting the outcomes of more than 900,000 children and young adults with ASD use were included in the meta-analysis. The pooled RR for fracture with the use of proton pump inhibitors (PPIs) versus non-use of these medications was 1.17 (95% CI = 1.1–1.25; P < 0.001) in children and 1.2 (95% CI = 0.87–1.65; P = 0.272) in young adults. By contrast, the use of histamine H2-receptor antagonists (H2RAs) was not significantly associated with fracture risk in children (RR, 1.08, 95% CI = 0.99–1.17; P = 0. 083) or young adults (RR, 1.08, 95% CI = 0.82–1.42; P = 0.589). Significant statistical and clinical heterogeneity among studies were determined for the main analysis and most of the subgroup analyses.
Conclusions
Our study provides evidence linking PPI use to an increased risk of fracture in children. Thus, the use of PPIs in these patients should be carefully considered. However, randomized controlled studies are needed to determine causality and the role of unmeasured/residual confounding factors in this association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid-suppressive drugs (ASDs), including proton pump inhibitors (PPIs) and histamine H2-receptor antagonists (H2RAs), are commonly prescribed for treatment of gastric ulcer, gastroesophageal reflux, eosinophilic esophagitis, and Helicobacter pylori infection [1]. The use of ASDs doubled from 2004 to 2008 [2] and tripled from 2002 to 2009 [3]. Also, this increase occurred in infants, children, and young adults, despite concerns regarding the safety of these drugs in such individuals. Several adverse events have been associated with ASDs, such as Clostridium difficile infection [4], pneumonia [5], dementia [6], and hypomagnesemia [7].
The link between ASD use in adults and fracture risk has been examined extensively. A previous meta-analysis [8] of studies on two types of ASDs, PPIs, and H2RAs concluded that PPIs but not ASDs are associated with an increased risk of fracture, irrespective of the dose (high or typical). Recently, Poly et al. [9] published a meta-analysis focusing on hip fracture, and reported a higher risk of fracture in short- and long-term PPI users. Since then, several studies [10,11,12,13,14,15] have investigated the relationship between the use of ASD use and fractures in children and young adults. In a case–control study, Freedberg et al. [10] reported that PPI use in young adults was associated with an increased risk of fracture; however, in another study [11], the use of PPI and H2RA was not associated with an increased risk of fracture in young adults. In several studies, pediatric ASD exposure [12,13,14] was also shown to increase the risk of fracture later in life, but the results were inconsistent in a subgroup analysis based on ASD type. However, a recent large pediatric cohort study [15] found a moderate association between PPIs and any bone fracture. Thus, the relationship between ASD exposure in children and young adults and the risk of fracture is unclear. In this study, we conducted a systematic literature review and meta-analysis to assess this association. However, since the various factors associated with ASD exposure (i.e., duration, age of exposure, and ASD type) may differentially affect the risk of fracture, these must still be analyzed separately.
Methods
This meta-analysis was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [16]. This systematic review was not registered in PROSPERO or INPLASY.
Literature search
We conducted a comprehensive literature search of the Cochrane Library, PubMed, and EMBASE databases (inception to December 2020) using keywords related to ASDs and fracture risk. The search was performed using the terms: “H2 blocker OR histamine-2 receptor antagonists OR H2RA OR cimetidine OR ranitidine OR famotidine OR nizatidine OR proton pump inhibitors OR proton pumps OR omeprazole OR Nexium OR lansoprazole OR rabeprazole OR pantoprazole OR esomeprazole” AND “osteoporosis or osteopenia or fracture or bone health or bone metabolism or bone mineral density” AND “infant OR child OR children OR pediatric OR adolescent OR young adult.” Reference lists of potential articles were manually searched for additional eligible articles.
Study selection
Two of the authors independently evaluated the eligibility of all relevant articles found in the databases based on the selection criteria. Full texts were retrieved after reading the titles and abstracts. Any discrepancies were resolved by discussion with a third author. Peer-reviewed studies were included if they met the following (PICO) criteria. Types of studies: randomized controlled trials (RCTs), cohort, nested case control, and case–control studies; types of participants: children and young adults (aged < 29 years at the time of fracture); types of interventions: the experimental intervention was ASD use (PPIs or H2RAs; at least one prescription) before the development of fracture, with either a no-ASD treatment or placebo control group; and types of outcome measures: a list of fracture outcomes reported in the studies, including the adjusted odds ratios (ORs), relative risks (RRs), and 95% confidence interval (CIs) or other appropriate data, was compiled to estimate risk.
Data extraction and quality assessment
The following data were extracted: first author, publication year, study design, study location, age, ascertainment of ASD exposure, assessment of fracture, number of participants, statistical adjustments, and study quality. The most adjusted effect-size estimate was included in the analysis if more than one estimate was presented. We assessed the methodologic quality of the included studies using the Newcastle–Ottawa Scale (NOS) as recommended by the Cochrane Collaboration [17]. A score of > 7 points was suggestive of a high-quality study.
Main and subgroup analyses
The association between the use of ASDs and risk of fracture was investigated in adjusted analyses. A sensitivity analysis was conducted to investigate the influence of single studies on the overall risk estimate, by omitting the studies on an individual basis. In addition, subgroup analyses were performed, based on study quality (high vs. low), the number of variables adjusted for (≥ 5 vs. < 5), the type of agent (PPI vs. H2RA), fracture outcome (upper limb vs. lower limb vs. other fracture), sex (male vs. female), and exposure during 1 year of life. Subgroup analyses based on age range were also conducted between children (aged < 18 years) and young adults (aged 18–29 years).
Statistical analysis
Based on the assumption that the RRs approximated the ORs, study estimates were combined irrespective of which measure of association was reported, because the incidence of the outcomes of interest was low in all populations. A random-effects model was used to pool the obtained effect estimates, accounting for between-study variance. The heterogeneity of the results across studies was determined using Higgins’ I2. An I2 value at 0–25%, 25–50%, 50–75%, and more than 75% represents very low, low, medium, and high heterogeneity, respectively [18]. Splitting one study to several estimates leads to substantially more weight being assigned to that study in the meta-analysis, especially in a random-effects model. Therefore, we used a fixed-effects model to produce a pooled RR if more than three estimates from one study were provided, and this pooled RR was included in the meta-analysis [19]. Publication bias was not assessed because the meta-analysis included fewer than 10 studies [20]. Stata SE software (version 10.0; Stata Corp, College Station, TX) was used to perform the statistical analysis.
Results
Search results
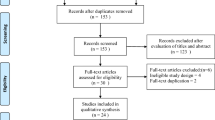
Searches of three databases yielded 597 potentially eligible articles. Duplicate (n = 565) and ineligible articles were excluded after reading the titles and abstracts; 32 citations were left for full-text screening. Finally, six studies [10,11,12,13,14,15] were included in the meta-analysis. Figure 1 shows the number of articles excluded at each stage of the eligibility assessment, together with the reasons for exclusion.
Characteristics of the included studies
The characteristics of the included studies are listed in Table 1. Five [11,12,13,14,15] were cohort studies and one [10] was a nested case–control study. The publication year ranged from 2015 to 2020, and the patient cohort included in the studies was as early as 1994. The sample size of the included studies varied from 65,432 to 2,490,526; the pooled total was 4,690,968. Among the six studies, two [14, 15] evaluated PPI only; four [10,11,12,13] evaluated PPI and H2RA separately. Four studies [12,13,14,15] evaluated fracture risk in children, one in young adults [11], and the remaining one [10] in children and young adults separately. All six studies involved Western populations (three [12,13,14] in the USA, one [11] in Israel, and two [10, 15] in Europe). The extent of adjustment for potential clinical risk factors varied considerably among the studies, and four [11, 12, 14, 15] studies adjusted for more than five important potential confounders.
Based on the methodological quality assessment scores, four studies were of high quality; the breakdown of the scores is provided in Tables S1 and S2 (see Supplemental Content).
Meta-analysis
PPI use and fracture risk
All analyses results are shown in Table 2. According to our meta-analysis of six studies featuring 12 estimates, exposure to PPIs was associated with an increased risk of fracture in children and young adults (RR, 1.2, 95% CI = 1.18–1.29; P < 0.001). High heterogeneity was observed among the studies (I2 = 66.8%). Sensitivity analysis revealed no substantial change in the pooled risk estimates upon exclusion of any single study; the pooled RRs for fracture ranged from 1.18 to 1.22.
In a subgroup analysis by age range, a significant association was observed in children (RR, 1.17, 95% CI = 1.1–1.25; P < 0.001; I2 = 55.4%) (Fig. 2A). However, no increased risk of fracture was detected in young adults (RR, 1.2, 95% CI = 0.87–1.65; P = 0.272; I2 = 46.3%) (Fig. 2B).
In a subgroup analysis by type of study quality, a significant association was observed in high quality studies (RR, 1.18, 95% CI = 1.1–1.28; P < 0.001; I2 = 62.7%). A non-significant effect toward an increased risk of fracture was detected in low quality studies (RR, 1.21, 95% CI = 1–1.46; P = 0.051; I2 = 62.7%).
Regarding the number of adjustment variables revealed a significantly increased fracture risk in those adjusting for at least 5 variables (RR, 1.32, % CI = 1.11–1.57; P = 0.002; I2 = 80.6%) and for fewer than 5 variables (RR, 1.16, 95% CI = 1.08–1.23; P < 0.001; I2 = 37.6%).
Subgroup analyses by sex showed no significant association between PPI use and hip fracture risk in male (RR, 1, 95% CI = 0.75–1.34; P = 0.989; I2 = 39%). In contrast, we found significant association in female (RR, 1.13, 95% CI = 1.07–1.2; P < 0.001; I2 = 0%).
When we grouped studies by fracture outcome, we found a significant positive association between PPI use and upper limb fracture risk (RR, 1.08, 95% CI = 1.04–1.13; P < 0.001; I2 = 0%) and lower limb fracture risk (RR, 1.24, 95% CI = 1.08–1.42; P = 0.002; I2 = 0%), whereas there was no significant association between PPI use and the risk of other fractures (RR, 1.38, 95% CI = 0.98–1.96; P = 0.069; I2 = 82.7%).
When we limited our analysis to studies reporting PPI exposure during 1 year of life, a significant positive association between PPI use and subsequent fracture risk was observed (RR, 1.34, 95% CI = 1.08–1.65; P = 0.008; I2 = 68.6%).
H2RA use and fracture risk
According to our meta-analysis of four studies featuring 8 estimates, exposure to H2RAs was associated with an increased risk of fracture in children and young adults (RR, 1.09, 95% CI = 1–1.18; P < 0.038). High heterogeneity was observed among the studies (I2 = 58.8%). Sensitivity analysis revealed no substantial change in the pooled risk estimates upon exclusion of any single study; the pooled RRs for fracture ranged from 1 to 1.17.
In a subgroup analysis by age range, no increased risk of fracture was detected in children (RR, 1.08, 95% CI = 0.99–1.17; P = 0.083; I2 = 61.5%) (Fig. 3A) or young adults (RR, 1.08, 95% CI = 0.82–1.42; P = 0.589; I2 = 49%) (Fig. 3B).
In a subgroup analysis by type of study quality, a significant association was observed in low quality studies (RR, 1.19, 95% CI = 1–1.41; P = 0.047; I2 = 42.9%). A non-significant effect toward an increased risk of fracture was detected in high quality studies (RR, 1.05, 95% CI = 0.99–1.1; P = 0.089; I2 = 16.1%).
Regarding the number of adjustment variables revealed a significantly increased fracture risk in those adjusting for fewer than 5 variables (RR, 1.2, % CI = 1.09–1.33; P < 0.001; I2 = 34.3%). In studies adjusting for at least 5 variables, there was no association between H2RA use and fracture risk (RR, 1.03, 95% CI = 0.98–1.08; P = 0.222; I2 = 0%).
When we limited our analysis to studies reporting H2RA exposure during 1 year of life, a non-significant effect toward an increased risk of fracture was detected between H2RA use and subsequent fracture risk was observed (RR, 1.06, 95% CI = 0.99–1.13; P = 0.071; I2 = 35.2%).
Discussion
Compared to non-use, PPI use in children, but not young adults, was associated with an increase in the risk of fracture, according to the pooled estimate. By contrast, there was no evidence that H2RA use was significantly associated with an increased risk of fracture in children or young adults. However, confidence in the results was reduced by the considerable heterogeneity and small number of studies analyzed.
The biological mechanisms underlying the relationship between ASD exposure and the subsequent risk of fracture are unclear. ASD may interfere with calcium absorption in the small intestine by increasing the pH [21]. Calcium solubility is believed to be important for its absorption and an acidic gastric environment is necessary for the release of ionized calcium from insoluble calcium salts [22]. High-dose PPIs blocked calcium absorption and thereby decreased bone mineral density in rat [23]. Furthermore, insufficient calcium absorption might lead to compensatory secondary hyperparathyroidism [24], increasing the rate of osteoclastic bone resorption and decreasing bone mineral density. In addition, PPIs are reported to impair osteoclast function and enhance their apoptosis by inhibiting osteoclastic vacuolar H + /K + ATPase activity [25]. Under such conditions, old bone is not replaced.
Although these modifying effects are biologically plausible, the included studies reported conflicting results, and there was significant heterogeneity in our meta-analysis. This could be partly explained by differences in study quality, sex, fracture outcome, and age range, although other possible sources of variability could not be thoroughly explored due to the small number of included studies. The presence of clinical heterogeneity would be expected to lead to some degree of heterogeneity in the statistical results.
Children, particularly very young ones, are more vulnerable to drug toxicity because of their physiological immaturity and a slower rate of drug clearance and drug metabolism [26]. Therefore, children and young adults may be at higher risk of fracture for physiological reasons. In our analyses stratified by age, PPI use was associated with an increased fracture risk among children, whereas there was no significant association between PPI or H2RA use and the risk of fracture in young adults. The highest risk (RR = 1.34) of fracture was seen in children with PPI exposure during the first year of life. This implies that age may modulate the effect of ASD on fracture risk. However, our results are limited by the small sample size. Further investigation is required to confirm the link and identify its underlying mechanism.
The various types of ASDs may have different effects on the risk of fracture. PPIs inhibit acid production by about 90%, compared to 60–70%, for H2RAs, suggesting that PPIs result in a greater reduction in calcium absorption. A previous meta-analysis [8] of 11 studies demonstrated a modest association between PPI use and risk of fracture in adults, but no association for H2RA use. Although our overall analysis showed associations of both PPI and H2RA use with fracture risk in children and young adults, subgroup analyses by age suggested a link only between PPI exposure and fracture risk in children. In the only included study [15] that directly compared PPI and H2RA use, there was no difference in fracture risk. However, the authors noted that the absence of a significant association should be interpreted with caution due to residual confounding factors and limited statistical power of the study.
Third, ASDs increase fracture risk by reducing calcium absorption, suggesting an effect of ASD exposure duration on fracture risk. A previous meta-analysis [27] showed that shorter duration of PPI use was associated with an increased risk of hip fracture, whereas there was no significant increase in the risk of hip fracture in long-term PPI users. If such an association exists, it may be attributable to a mechanism other than calcium absorption. The cut-off of cumulative duration was inconsistent among the included studies, which hindered subgroup analysis based on treatment duration. Freedberg et al. [10] found a dose–response effect with increased total exposure to PPIs in young adults, but not in children. In contrast, Wang et al. [15] demonstrated higher risk with a longer cumulative duration of PPI use in children. However, there has been no direct comparison between short- and long-term uses. Additional research on the effect of ASD treatment duration on the risk of fracture in children and young adults is needed before definite conclusions can be reached.
Residual confounders may have been a source of heterogeneity in our study. Interestingly, subgroup analyses based on the number of variables adjusted for showed lower risks of fracture in association with both PPI and H2RA use when the data were adjusted for at least five variables. This finding is consistent with the associations seen in the subgroup analysis based on study quality.
Finally, the different degrees of acid-suppression by ASDs may modulate the risk of fracture. Fleishman et al. [14] reported no correlation between fracture risk and individual PPIs (but data not shown in their manuscript), whereas Wang et al. [15] concluded that only omeprazole was associated with an increased risk of any fracture. We were unable to analyze drug-related differences because only the study of Wang et al. provided data on individual PPIs. Further studies are needed on the association between individual ASDs and fracture risk in children and young adults.
To our knowledge, this systematic review and meta-analysis is the first to evaluate the effect of ASD use on fracture risk in children and young adults. The main strength of this meta-analysis lies in stratification of the data. Nevertheless, this work had several limitations. First, the number of included studies was small and the types of analyses differed. Thus, the subgroup analysis was limited by the sample size and further investigation is needed. Second, as in all meta-analyses of observational studies, uncontrolled confounding variables may have affected the results. Third, there were insufficient data on the cumulative dose of ASD used in the included studies; therefore, we could not determine whether this parameter is associated with fracture risk. Fourth, publication bias was not evaluated, due to the limited number of studies; tests for publication bias typically have low power when there are few studies.
In conclusion, this systematic review and meta-analysis suggested an association between PPI use and the risk of fracture in children. To confirm our results, randomized controlled studies are needed.
References
Malfertheiner P, Fass R, Quigley EM, Modlin IM, Malagelada JR, Moss SF, Holtmann G, Goh KL, Katelaris P, Stanghellini V, Talley NJ, Tytgat GN, Wright NA (2006) Review article: from gastrin to gastro-oesophageal reflux disease–a century of acid suppression. Aliment Pharmacol Ther 23(6):683–690
Uzoigwe OF (2016) Proton pump inhibitors and fracture: do they do what it says on the tin? Osteoporos Int 27(4):1671–1672
Illueca M, Alemayehu B, Shoetan N, Yang H (2014) Proton pump inhibitor prescribing patterns in newborns and infants. J Pediatr Pharmacol Ther 19(4):283–287
Dial S, Delaney JA, Barkun AN, Suissa S (2005) Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294(23):2989–2995
Arai N, Nakamizo T, Ihara H, Koide T, Nakamura A, Tabuse M, Miyazaki H (2017) Histamine H2-blocker and proton pump inhibitor use and the risk of pneumonia in acute stroke: a retrospective analysis on susceptible patients. PLoS One 12(1):e0169300
Chen LY, Lin HJ, Wu WT, Chen YC, Chen CL, Kao J, You SL, Chou YC, Sun CA (2020) Clinical use of acid suppressants and risk of dementia in the elderly: a pharmaco-epidemiological cohort study. Int J Environ Res Public Health 17(21):8271
Srinutta T, Chewcharat A, Takkavatakarn K, Praditpornsilpa K, Eiam-Ong S, Jaber BL, Susantitaphong P (2019) proton pump inhibitors and hypomagnesemia: a meta-analysis of observational studies. Medicine (Baltimore) 98(44):e17788
Eom CS, Park SM, Myung SK, Yun JM, Ahn JS (2011) Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med 9(3):257–267
Poly TN, Islam MM, Yang HC, Wu CC, Li YJ (2019) Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int 30(1):103–114
Freedberg DE, Haynes K, Denburg MR, Zemel BS, Leonard MB, Abrams JA, Yang YX (2015) Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporos Int 26(10):2501–2507
Fedida B, Schermann H, Ankory R, Rotman D, Shichman I, Yoffe V, Shlaifer A, Luger E (2019) Fracture risk of young adults receiving proton-pump inhibitors and H2-receptor antagonists. Int J Clin Pract 73(5):e13339
Malchodi L, Wagner K, Susi A, Gorman G, Hisle-Gorman E (2019) Early acid suppression therapy exposure and fracture in young children. Pediatrics 144(1)
Wagner K, Wagner S, Susi A, Gorman G, Hisle-Gorman E (2019) Prematurity does not increase early childhood fracture risk. J Pediatr 207:148–153
Fleishman N, Richardson T, Attard T (2020) The clinical characteristics of fractures in pediatric patients exposed to proton pump inhibitors. J Pediatr Gastroenterol Nutr 70(6):815–819
Wang YH, Wintzell V, Ludvigsson JF, Svanstrom H, Pasternak B (2020) Association between proton pump inhibitor use and risk of fracture in children. JAMA Pediatr 174(6):543–551
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Higgins JP (2014) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane collaboration. Available at: www.cochrane-handbook.org. 6 Dec 2014
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333(7568):597–600
Bo-Linn GW, Davis GR, Buddrus DJ, Morawski SG, Santa Ana C, Fordtran JS (1984) An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest 73(3):640–647
Wood RJ, Serfaty-Lacrosniere C (1992) Gastric acidity, atrophic gastritis, and calcium absorption. Nutr Rev 50(2):33–40
Chonan O, Takahashi R, Yasui H, Watanuki M (1998) Effect of L-lactic acid on calcium absorption in rats fed omeprazole. J Nutr Sci Vitaminol (Tokyo) 44(3):473–481
Zhang Y, Lai WP, Wu CF, Favus MJ, Leung PC, Wong MS (2007) Ovariectomy worsens secondary hyperparathyroidism in mature rats during low-Ca diet. Am J Physiol Endocrinol Metab 292(3):E723–E731
Richards JB, Goltzman D (2008) Proton pump inhibitors: balancing the benefits and potential fracture risks. CMAJ 179(4):306–307
Ward RM, Kearns GL (2013) Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs 15(2):119–131
Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K (2011) Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 106(7):1209–1218
Author information
Authors and Affiliations
Contributions
JY and DNB conceived the study and revised the manuscript critically for important intellectual content. TJZ and JY made substantial contributions to its design, acquisition, analysis, and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval was required for this review as all data were already published in peer-reviewed journals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, J., Zhou, Tj., yang, J. et al. Use of acid-suppressive drugs and risk of fracture in children and young adults: a meta-analysis of observational studies. Eur J Clin Pharmacol 78, 365–373 (2022). https://doi.org/10.1007/s00228-021-03245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03245-3