Abstract
Purpose
To summarize the evidence of efficacy and safety of the use of ketamine and esketamine for depression.
Methods
A literature search was performed in Medline, the Cochrane Library, LILACS, and CRD until November 2020. We included systematic reviews with meta-analyses of randomized controlled trials on the use of ketamine and esketamine in adult patients with depression. Two authors independently performed the study selection and data extraction. The AMSTAR-2 tool was used to appraise the quality of included reviews.
Results
A total of 118 records were identified, and 11 studies fully met the eligibility criteria. Compared to control, ketamine improved the clinical response at 40 min to 1 week and clinical remission at 80 min to 72 h, and esketamine improved both outcomes at 2 h to 4 weeks. Ketamine and esketamine also had a beneficial effect on the depression scales score and suicidality. For adverse events, oral ketamine did not show significant change compared to control, while intranasal esketamine showed difference for any events, such as dissociation, dizziness, hypoesthesia, and vertigo. Most reviews were classified as “critically low quality,” and none of them declared the source of funding of the primary studies and assessed the potential impact of risk of bias in primary studies.
Conclusion
Ketamine and esketamine showed a significant antidepressant action within a few hours or days after administration; however, the long-term efficacy and safety are lacking. In addition, the methodological quality of the reviews was usually critically low, which may indicate the need for higher quality evidence in relation to the theme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a complex psychiatric disorder characterized by the presence of depressed mood, anhedonia, loss of interest, low energy, and fatigue for a minimum 2-week period. Other symptoms can be noted, such as insomnia or hyposomnia, diminished ability to concentrate, significant weight alteration, low self-esteem, and suicidal ideation [1]. Its etiology is not yet fully understood, and one of the pathophysiological mechanisms involved is the functional deficiency of the monoamine neurotransmitters serotonin, noradrenaline, and/or dopamine in the brain synapses. However, other multiple interactions with other brain systems are also involved [1, 2].
According to the World Health Organization, more than 300 million people of all ages suffer from depression, being considered a leading cause of disabling worldwide — 7.5% of all years lived with disability [3]. The economic burden of depression was estimated at $210.5 billion in the USA (increase of 21.5% between 2005 and 2010), with practically half of this amount being due to direct medical costs and the other half being attributed to indirect costs related to absenteeism, presentism, and suicide [4]. In addition, depression has an important impact on activities of daily living and quality of life and affects individuals, often in early life and for sustained periods, thereby causing many disease years [5]. Therefore, depression is a public health problem.
Treatment for depression includes the use of antidepressants, electroconvulsive therapy (ECT), and psychosocial interventions. Antidepressant medications, such as the selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, and monoamine oxidase inhibitors, can be used for treatment of mild, moderate, and severe depression [6]. However, current treatments require a considerable time to induce a response or remission of depression. The average time for antidepressant action of the standard antidepressants is 13 days, which can reach 20 days, considering the response criteria. Still, when patients have a clinical response, it is generally considered suboptimal [7].
At the beginning of this century, Berman et al. (2000) reported that ketamine was able to inhibit the N-methyl-d-aspartate (NMDA) receptor (i.e., the main receptor of the glutamatergic system that plays an important role in the antidepressant effect) [8]. In addition, current evidence suggests that ketamine’s acute antidepressant effect requires opioid system activation [9]. In this context, a growing number of clinical trials have shown that subanesthetic doses of esketamine (S-ketamine) and ketamine (RS-ketamine, a racemic mixture of R-ketamine and S-ketamine) have a rapid antidepressant effect [10, 11]. From that, the off-label use of ketamine and esketamine for depression (except for intranasal esketamine approved by FDA in the USA and EMA in Europe) has increased, giving great concern for the patient’s health and the healthcare system, since the efficacy and safety of these drugs are not yet fully established [12, 13].
In order to deliver accurate estimates of key outcomes of ketamine and esketamine in depression, some systematic reviews with meta-analyses have been published recently — considered the gold standard of evidence in health care [14]. In this sense, it is important to understand the diversity present in the extant systematic review literature. Also, the methodological quality of these systematic reviews is unknown, which is an indispensable step before treatment recommendations can be safely translated into clinical practice. Currently, there is no overview on the use of ketamine/esketamine in patients with depression. Therefore, this overview aimed to summarize the evidence of efficacy and safety of ketamine and esketamine for adult patients with depression from systematic reviews with meta-analyses.
Methods
The search strategy, eligibility criteria, and method of analysis for this overview were specified in advance and documented in a protocol available in Appendix 1.
Literature search
A comprehensive literature search was performed in the Medline (via PubMed), Latin American and Caribbean Health Sciences Literature (LILACS), Cochrane Library, and the Centre for Reviews and Dissemination (CRD) databases until November 29, 2020. The search strategy included the use of Medical Subject Headings (MeSH) terms and keywords related to the health condition (depression), intervention (ketamine and esketamine), and the study design (systematic reviews with meta-analysis). The detailed search strategy of all databases is shown in Appendix 2; keywords were searched in any fields unless otherwise specified. Also, we screened the reference lists of the appraised articles to identify any studies that might have been missed.
Study selection
The selection process was performed in three stages: (1) exclusion of repeated records, (2) analysis of the titles and abstracts, and (3) analysis of the full-text articles. The studies were independently selected by two authors (MBV and TML). Any disagreements were resolved by a third author (PMA). When the full-text article could not be obtained, the corresponding authors were contacted via ResearchGate (www.researchgate.net) or e-mail or both.
To be included in the present overview, the articles had to meet the following criteria: (1) be a systematic review with pairwise meta-analysis of randomized controlled trials (RCT); (2) be published in English, Spanish, or Portuguese; (3) have evaluated the use of ketamine or esketamine or both (monotherapy or associated with other drugs, any route of administration and frequency of use) in comparison with placebo or other drugs; (4) report any efficacy and safety outcomes; and (5) in adults with major depressive disorder or bipolar disorder. Articles were excluded if they were (1) narrative reviews; (2) systematic reviews without meta-analysis; (3) meta-analyses not from systematic reviews; (4) network meta-analysis; (5) systematic reviews including concomitant use of ECT and ketamine or esketamine as intervention; (6) systematic reviews with meta-analysis including another target population, intervention, or primary study design; (7) systematic reviews that did not have the full-text article available.
Data extraction
Data extraction was using a spreadsheet preformatted in Microsoft Excel® by two independent researchers (MBV and TML), and any disagreement was resolved by a third author (PMA). The following information was collected: author(s), year of publication, literature search period, databases used in literature search, target population, intervention (dose and route of administration), comparators, outcome measures, number of RCTs and patients included in the meta-analysis, statistical model for meta-analysis, pooled effect size, heterogeneity, publication bias, quality of evidence by the GRADE approach [15], and funding source.
Quality assessment
The methodological quality of included systematic reviews was assessed using the AMSTAR-2 (Assessment of Multiple Systematic Reviews) tool [16]. The AMSTAR-2 is a 16-item questionnaire, with the majority of questions being judged as “yes,” “partial yes,” or “no.” The overall rating was based on weaknesses in critical domains (items: 2, 4, 7, 9, 11, 13, and 15) as following: “high,” no or one non-critical weakness; “moderate,” more than one non-critical weakness but no critical flaws; “low,” one critical flaw with or without non-critical weaknesses; and “critically low,” more than one critical flaw with or without non-critical weaknesses. One investigator (TML) conducted the evaluation of the studies, and a second one (PMA) verified this evaluation.
Data synthesis
The characteristics of systematic reviews and their methodological quality were descriptively summarized using systematically structured tables. The estimates of effect size from meta-analyses (and their 95% confidence intervals [95% CI]) were expressed as mean difference (MD), standardized mean difference (SMD), relative risk (RR), and odds ratio (OR), depending on what the authors had reported.
Results
Search results
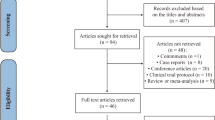
The electronic search identified 118 potentially relevant records. After removing duplicates and screening titles and abstracts, 21 studies were selected for full-text reading. Of these, 11 systematic reviews with pairwise meta-analysis on use of ketamine or esketamine or both for treatment depression fully met the eligibility criteria and were included in the present overview [17,18,19,20,21,22,23,24,25,26,27]. All included systematic reviews were found for full-text examination. A flowchart of the literature search is shown in Fig. 1. The excluded studies and the reasons for their exclusion are detailed in Appendix 3.
Characteristics of systematic reviews
Characteristics of the 11 reviews included in this overview are shown in Table 1. All included systematic reviews were published in English between 2015 and 2020. Most reviews included primary studies evaluating patients with major depression disorder or bipolar disorder [18, 20,21,22, 24, 26]. Two reviews included patients with bipolar depression [19, 23], and three reviews included patients with major depression disorder [17, 25, 27].
Almost all reviews involved ketamine as monotherapy in the intervention arm [17,18,19,20,21,22,23,24,25]. One review involved ketamine or esketamine [26], and another review involved only esketamine in the intervention arm [27]. Seven reviews included studies whose comparator was placebo or active-control [18, 20,21,22, 24,25,26], and four reviews included studies that used placebo as comparator [17, 19, 23, 27].
Nine reviews assessed clinical remission or clinical response or both [17, 19,20,21,22,23,24,25, 27], and all of them assessed the severity of depressive symptoms through validity scales, except the review by Fornaro et al. (2020) [23]. Most reviews used the Montgomery-Åsberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HDRS/HAM-D) [17, 18, 20, 22, 24,25,26,27], two reviews used the Brief Psychiatric Rating Scale (BPRS) and Clinician-Administered Dissociative States Scale (CADSS) [20, 21], two reviews used MADRS [19, 27], and one review used 9-Item Patient Health Questionnaire (PHQ-9) [27]. Six reviews assessed other outcomes, such as suicidality [22, 26], acceptability [19, 23, 27], disability [27], and adverse events [25, 27].
Three reviews did not report a source of support [18, 21, 22], four received research funding from institute organization [17, 19, 26, 27], and four declared no support from any organization [20, 23,24,25].
Results on clinical response
Ketamine produced a significant clinical response compared to placebo at 40 min, 80 min, 2 h, and 4 h after intervention [21]. Seven reviews showed a significant effect of ketamine in the clinical response at 24 h compared to placebo or active-control or both [17, 19,20,21,22,23,24]. However, very-low-dose ketamine for patients with major depression disorder did not show significant difference in the clinical response at the same time compared to placebo and active-control [22]. In addition, one review reported that ketamine was significantly better than placebo and active-control in the clinical response at 48 h [21].
Regarding clinical response at 72 h, ketamine produced a significant clinical response compared to placebo or active-control or both in four reviews [17, 20,21,22]. On the other hand, one study did not show a significant effect of ketamine in the clinical response at the same time compared to placebo for patients with bipolar disorder (very low-quality evidence) [19]. In addition, very-low-dose ketamine for patients with major depression disorder was not significantly better than placebo and active-control in the clinical response at 72 h [22].
Four reviews showed significant effect of ketamine in the clinical response at 1 week compared to placebo or active-control or both [20,21,22, 24]. However, it is important to note that there was no significant difference between ketamine and placebo at the same time in patients with bipolar depression [22]. One review showed a tendency to significant difference between ketamine and placebo in the clinical response at 2 weeks [21]. In addition, one systematic review showed a significant effect of ketamine in the clinical response overall compared to placebo and active-control [24]. The only review that evaluated oral ketamine did not present a significant result [25].
Finally, esketamine was statistically superior compared to placebo in the clinical response at 2 h, 1 week, 4 weeks, by 2 to 28 days and by 8 to 28 days (high-quality evidence) [27]. The results on clinical response are displayed in Table 2.
Results on clinical remission
Ketamine produced a significant clinical remission of symptoms compared to placebo at 80 min, 2 h, and 4 h after intervention [21]. Regarding the clinical remission at 24 h, four reviews showed significant effect of ketamine compared to placebo or active-control or both [20,21,22, 24]. On the other hand, two reviews involving a smaller number of patients did not report significant differences in the clinical remission of symptoms between groups at the same time [17, 19]. In addition, ketamine was significantly better than placebo and active-control in the clinical remission at 48 h [21].
Data on clinical remission of symptoms at 72 h was presented by five reviews. Ketamine produced a significant clinical remission compared to placebo or active-control or both in most of them [17, 20,21,22]. However, very-low-dose ketamine for patients with major depression disorder did not show significant difference in the clinical remission at the same time compared to placebo and active-control [22].
Two reviews showed significant effect of ketamine in the clinical remission of symptoms at 1 week compared to placebo and active-control [20, 22], and two reviews did not present a significant difference between groups [21, 24]. One review assessed the clinical remission of symptoms at 2 weeks and did not show a significant difference between ketamine and placebo [21]. In addition, two reviews did not show a significant difference between ketamine versus placebo and active-control in the clinical remission overall [24, 25].
Finally, esketamine was statistically superior compared to placebo in the clinical remission at 2 h, 4 h, 24 h, 1 week, 4 weeks, and by 8 to 28 days (high-quality evidence) [27]. The results on clinical remission are displayed in Table 3.
Results on depression scales
Four reviews showed significant beneficial effects of ketamine in the HAM-D/HDRS and MADRS scores at 24 h compared to placebo or active-control or both [17, 18, 20, 22]. However, one systematic review did not show significant effect in the same time compared to placebo and active-control [24]. Two reviews showed a beneficial effect at 72 h [17, 22]. However, Xu et al. (2016) reported that very-low-dose ketamine did not improve the HAM-D/HDRS and MADRS scores at the same time compared to placebo and active control [22].
Three reviews showed data of HAM-D/HDRS and MADRS scores at 1 week [18, 22, 24]. All of them showed significant beneficial effects of ketamine compared to placebo or active-control or both. However, Lee et al. (2015) did not show significant difference between ketamine and placebo for patients with bipolar depression [18], and Xu et al. (2016) did not show significant difference between ketamine (normal or very-low-dose) compared to placebo and active-control [22]. In addition, Nuñez et al. (2020) showed a significant effect of oral ketamine compared to placebo or active-control or both at 2 and 3 weeks [25], and two studies showed superior effect of ketamine compared to placebo and active-control in the overall scores [24, 25].
Regarding the MADRS score, one review showed that ketamine produced a significant beneficial effect compared to placebo at 24 h and 72 h [19]. However, the superior result was not observed at 1 and 2 weeks (very low-quality evidence) [19]. In addition, Zheng et al. (2020) showed a significant beneficial effect of esketamine compared to placebo (high-quality evidence) [27].
Finally, two reviews assessed the use of ketamine through BPRS and CADSS score [20, 21] and one assessed the use of esketamine through PHQ-9 score (high-quality evidence) [27]. All reviews showed the intervention arm was significantly better than placebo or active-control or both to improve these scores. The results on depression scales are shown in Table 4.
Results on suicidality, acceptability, disability, and adverse events
One review presented the results of the suicidal ideation at 4 h, 24 to 72 h, 2 to 4 weeks, and more than 4 weeks, observing a significant difference between ketamine or esketamine or both compared to placebo and active control at 4 h and 24 to 72 h (low-quality evidence) [26]. Xu et al. (2016) presented a significant difference between ketamine versus placebo and active control at 24 and 72 h. However, this result was not observed at 1 week [22].
Three reviews did not show a significant difference between ketamine or esketamine versus placebo in the acceptability of the treatment (total dropout and dropout due to lack of efficacy) [19, 23, 27]. In contrast, Zheng et al. (2020) showed a significant difference between esketamine and placebo on acceptability (dropout due to adverse events) (high-quality evidence) [27]. In addition, only one review evaluated disability and showed a significant difference between esketamine and placebo (high-quality evidence) [27].
In terms of adverse events, one systematic review on oral ketamine reported this outcome and did not show a significant difference between ketamine versus placebo and active-control [25]. Finally, Zheng et al. (2020) showed significant difference between esketamine and placebo in the dissociation, dissociative disorder, dizziness postural, feeling abnormal, feeling drunk, hypoesthesia, oral hypoesthesia, lethargy, nausea, paresthesia, sedation, somnolence, throat irritation, vertigo, vision blurred, and vomiting (high-quality evidence) [27]. The results on suicidality, acceptability, disability, and adverse events are displayed in Table 5.
Methodological quality of systematic reviews
Methodological quality of 11 systematic reviews based on the AMSTAR-2 tool is shown in Table 6. Three reviews presented “low quality” [17, 19, 24] while eight reviews presented “critically low quality” [18, 20,21,22,23, 25,26,27].
All reviews included the components of PICO (Population, Intervention, Control, and Outcomes) in their research questions. Moreover, only three reviews did not perform study selection and data extraction in duplicate [20, 21, 25], two reviews did not provide a satisfactory explanation for any heterogeneity observed in their results [21, 25], and one review did not use a satisfactory technique for assessing the risk of bias (RoB) in primary studies, did not use appropriate methods for statistical combination of their results, and did not report any potential sources of conflict of interest [21].
In contrast, no review reported on the sources of funding for the studies included in their review and assessed the potential impact of risk of bias in individual studies on their results. Five reviews did not report an “a priori” design and indicated the existence of a protocol [18, 20,21,22, 27]. Furthermore, only five reviews provided a list of excluded studies and justify their exclusions [17, 19, 20, 23, 24], four of the reviews conducted an adequate investigation of publication bias and discuss its likely impact on the results of the review [18, 20, 24, 26], and two of them considered the risk of bias in individual studies when interpreting/discussing their results [17, 19].
Overlap of primary studies across the systematic reviews
In this overview, there are two pairs of systematic reviews on ketamine that included the same RCT (Appendix 4). McGirr et al. [20] (critically low-methodological quality) and Newport et al. [21] (critically low-methodological quality) included the same seven RCT; however, in common comparisons on clinical response and remission at 24 h, 72 h, and 1 week as well as BPRS score and CADSS score, they did not use the same RCT or considered different sample sizes. Other than that, they performed other comparisons that did not overlap, either by outcome, time measured, or subgroup analyses. Moreover, McCloud et al. [19] (low-methodological quality) and Fornaro et al. [23] (critically low-methodological quality) included the same two RCT, performed meta-analyses for response rate at 24 h and drop-out rate with both RCT, and used the same statistical model for meta-analyses (random effects model); however, the number of events they reported for the outcomes was different and, therefore, they showed slightly distinct results. In both cases, it was not possible to identify whether there was an error in data extraction by the review authors or whether data were extracted from different sources for the same primary study (e.g., different reports, unpublished data). There was no overlap of RCT across the systematic reviews that evaluated esketamine (Appendix 5).
Discussion
Main findings
To the best of our knowledge, this is the first overview of systematic reviews with meta-analyses evaluating efficacy and safety of ketamine and esketamine in adult patients with depression. Ketamine showed a significantly greater clinical response compared to control in most results between 24 h and 1 week post-intervention, except for very low doses for patients with major depression disorder at 24 h and 72 h, for patients with bipolar disorder at 72 h and 1 week, and for oral ketamine. Esketamine was statistically superior compared to placebo in the clinical response at all results evaluated between 2 h and 4 weeks.
For clinical remission, ketamine was significantly superior compared to control in most results between 80 min and 72 h post-intervention. The findings were inconsistent from 1 week after the intervention and not significant for oral ketamine. In addition, esketamine showed significant beneficial effects compared to placebo in the clinical remission at all results evaluated between 2 h and 4 weeks. When compared to control, ketamine showed significant reduction on scores of BPRS, CADSS, MADRS until 72 h, and in most results between at 24 h and 3 weeks post-intervention for the HAM-D/HDRS and MADRS depression scales. Esketamine was significantly better than placebo to improve the PHQ-9 score.
A recent systematic review with network meta-analysis compared the efficacy of 21 antidepressants for the acute treatment of adults with major depressive disorder [28]. The analyses were performed about 8 weeks post-intervention and the pooled effect size for clinical response, and clinical remission for antidepressants was frequently smaller than reported by the systematic reviews on the efficacy of ketamine and esketamine included in this overview. Therefore, ketamine and its isomer produce a rapid, powerful, and persistent action in adult patients with depression. Despite ketamine and esketamine presenting a faster onset of action and more likely to sustain it having clear therapeutic advantages [11, 29, 30], it is important to note that little is known about their long-term efficacy.
The effects of ketamine and esketamine on suicidal ideation were apparent up to 72 h post-intervention, but not at longer time points compared to control. According to the recent literature, there is no scientific evidence to support the use of suicide risk assessment tools to predict suicidal acts. However, they can complement the clinical assessment and be the starting point of the suicide prevention process [31]. Considering that patients using antidepressants have a higher risk of suicide in the first week of treatment compared to subsequent weeks, the use of medications with antidepressant effects within hours or a few days might have a positive impact on the patient's prognosis [32, 33].
Finally, only two systematic reviews with meta-analysis reported adverse events. Compared to control, the use of oral ketamine did not cause more adverse events, while intranasal esketamine showed significantly more dropouts due to adverse events and any events, such as dissociative disorder, dizziness, oral hypoesthesia, and vertigo. The main justification for not pooling data on ketamine used in different routes of administration is that many RCTs did not present data on adverse events; then, acute (up to 2 weeks) and long-term adverse events are lacking. In the absence of data, it would be imprudent to assume that there are no serious safety concerns [12]. Even because a systematic review (including different types of study designs) showed a qualitative summary of the adverse events from the use of ketamine. According to Short et al. (2018), acute adverse events associated with ketamine are common and include mainly psychiatric, psychotomimetic, cardiovascular, and neurological changes. Moreover, these authors suggest a selective reporting bias with limited assessment of long-term safety [34].
Methodological quality of systematic reviews
All systematic reviews included in this overview were classified as “low quality” or “critically low quality” according to the AMSTAR-2 critical appraisal criteria. This result is consistent with overviews that also used the AMSTAR-2 instrument to assess the methodological quality of systematic reviews on various treatments for depression [35, 36].
About the items of the AMSTAR-2 tool, all reviews did not report on the sources of funding for the studies included in their study (item 10). This finding is very worrying, since studies that receive industry funding can favor sponsored products, and they are less likely to be published compared to financially independent studies [37,38,39]. The failure to evaluate this item seems to be common in systematic reviews on treatments for mental disorders [35, 36, 40, 41].
In this overview, no systematic review assessed how their results varied in relation to the inclusion or exclusion of individual studies with a high risk of bias (item 12). A justification of the authors of the reviews was the small number of RCTs included in the combined effect estimates that made this analysis unfeasible. Non-adherence to this item was less frequent in the literature on systematic reviews of treatments for mental disorders [35, 36, 41].
The development of a research protocol prior to conducting a review is considered a critical item by the AMSTAR-2 (item 2). Nevertheless, only a few reviews have fully adhered to this item, which is like the findings of other overviews on treatments for mental disorders [35, 36, 40, 41]. Adherence to a well-developed protocol promotes transparency of the review process and can help avoid the biased post hoc decisions, for example selective outcome reporting [42].
Opportunities for future research
This overview revealed that there is room for improvement in the future studies. Though the number of RCT evaluating ketamine and its esketamine isomer in depression has grown in recent years, the evidence summarized is from 20 RCTs including ketamine (Appendix 4) and four RCTs including esketamine (Appendix 5). From that, it was noted that more evidence is needed on the effects of these drugs on a treatment period longer than 2–4 weeks (i.e., able to elucidate long-term efficacy and safety), head-to-head trials (directly comparing ketamine and esketamine or using active-control with antidepressant drugs), and also on the use of different doses and routes of administration of both drugs (ketamine was frequently administered by intravenous route and esketamine by intranasal route).
In addition, future systematic reviews with meta-analyses on this theme should be appropriately designed and conducted, primarily in reporting an explicit statement that the methods were established prior to conducting the review and justifying any significant deviations from the protocol, reporting on the sources of funding for the RCT included in the systematic review, assessing the potential impact of risk of bias in RCT on the results of the meta-analysis; accounting for risk of bias in RCT when interpreting/discussing the results of the review, and conducting an adequate investigation of publication bias and discuss its likely impact on the results of the review.
Strengths and limitations of the overview
To our knowledge, this is the first overview to summarize evidence on the use of ketamine and esketamine in adult patients with depression. In addition, the study assessed the methodological quality of systematic reviews using the validated AMSTAR-2 tool. However, this study also presents some limitations. Only systematic reviews with meta-analysis were included in this overview. Reviews including simultaneous use of ECT and ketamine or esketamine were excluded, and this may have excluded important reviews on the theme and decreased the number of reviews to compose the new evidence generated from this overview. We did not conduct searches for unpublished reviews from thesis repositories and conference proceedings, or ongoing reviews. In addition, the quality of evidence for the outcomes was extracted based on the assessment of this parameter by the review authors, and not all reviews performed this analysis. Finally, we do not provide the prediction interval, which would be helpful to assess whether the between-study variation was clinically significant.
Conclusion
The findings of this overview showed a significant superiority of ketamine and esketamine in most results for clinical response, clinical remission, depression scales scores, and suicidal ideation compared to control. No systematic review performed a meta-analysis for adverse events of ketamine (except for oral ketamine and esketamine). It is very important to note that the data came from the first 2 weeks of treatment with ketamine and 4 weeks for esketamine, and the long-term efficacy and safety are lacking. In addition, most reviews showed a critically low methodological quality, which limits the reliability of the evidence. Thus, it is necessary to carry out more primary studies, and future systematic reviews should follow the quality assessment tools so that best evidence can be used in the decision-making for the use of ketamine and its isomer in adult patients with depression.
References
Brigitta B (2002) Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci 4(1):7–20
Hasler G (2010) Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 9(3):155–161
World Health Organization (2017) Depression and other common mental disorders: global health estimates. World Health Organization CC BY-NC-SA 3.0 IGO
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015) The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76:155–162
Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N (2013) Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 11:129
Gautam S, Jain A, Gautam M, Vahia VN, Grover S (2017) Clinical practice guidelines for the management of depression. Indian J Psychiatry 59(Suppl 1):S34–S50
Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA Jr (2010) The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals (Basel) 3(1):19–41
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF (2018) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175(12):1205–1215
Wilkinson ST, Sanacora G (2017) Considerations on the off-label use of ketamine as a treatment for mood disorders. JAMA 318(9):793–794
Sanders B, Brula AQ (2021) Intranasal esketamine: from origins to future implications in treatment-resistant depression. J Psychiatr Res 137:29–35
Loo C (2018) Can we confidently use ketamine as a clinical treatment for depression? Lancet Psychiatry 5(1):11–12
López-Díaz Á, Murillo-Izquierdo M, Moreno-Mellado E (2019) Off-label use of ketamine for treatment-resistant depression in clinical practice: European perspective. Br J Psychiatry 215(2):447–448
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) (2020) Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. http://www.training.cochrane.org/handbook
Schünemann H, Brozek J, Guyatt G, Oxman A, editors (The GRADE Working Group) (2013) GRADE handbook for grading quality of evidence and strength of recommendations. Available from: http://www.guidelinedevelopment.org/handbook
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008
Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A (2015) Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 9:CD011612
Lee EE, Della Selva MP, Liu A, Himelhoch S (2015) Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry 37(2):178–184
McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, Brett D, Amit BH, McShane R, Hamadi L, Hawton K, Cipriani A (2015) Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev 9:CD011611
McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW (2015) A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45(4):693–704
Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172(10):950–966
Xu Y, Hackett M, Carter G, Loo C, Gálvez V, Glozier N, Glue P, Lapidus K, McGirr A, Somogyi AA, Mitchell PB, Rodgers A (2016) Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol 19(4):pyv124
Fornaro M, Carvalho AF, Fusco A, Anastasia A, Solmi M, Berk M, Sim K, Vieta E, de Bartolomeis A (2020) The concept and management of acute episodes of treatment-resistant bipolar disorder: a systematic review and exploratory meta-analysis of randomized controlled trials. J Affect Disord 276:970–983
Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, Beauchamp S (2020) A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 - January 2019. J Affect Disord 277:831–841
Nuñez NA, Joseph B, Pahwa M, Seshadri A, Prokop LJ, Kung S, Schak KM, Vande Voort JL, Frye MA, Singh B (2020) An update on the efficacy and tolerability of oral ketamine for major depression: a systematic review and meta-analysis. Psychopharmacol Bull 50(4):137–163
Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, Cipriani A, Hawton K (2020) Ketamine for suicidal ideation in adults with psychiatric disorders: a systematic review and meta-analysis of treatment trials. Aust N Z J Psychiatry 54(1):29–45
Zheng W, Cai DB, Xiang YQ, Zheng W, Jiang WL, Sim K, Ungvari GS, Huang X, Huang XX, Ning YP, Xiang YT (2020) Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 265:63–70
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391(10128):1357–1366
Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA Jr (2008) Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry 69(6):946–958
Ionescu DF, Rosenbaum JF, Alpert JE (2015) Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci 17(2):111–126
Runeson B, Odeberg J, Pettersson A, Edbom T, Jildevik Adamsson I, Waern M (2017) Instruments for the assessment of suicide risk: a systematic review evaluating the certainty of the evidence. PLoS One 12(7):e0180292
Simon GE, Savarino J, Operskalski B, Wang PS (2006) Suicide risk during antidepressant treatment. Am J Psychiatry 163(1):41–47
Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, Pough KA, Prince TA, Ramsey NS, Savsani KH, Scandlen L, Cavaretta MJ, Raffa RB (2017) What is the mechanism of Ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther 42(2):147–154
Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5(1):65–78
Li M, Niu J, Yan P, Yao L, He W, Wang M, Li H, Cao L, Li X, Shi X, Liu X, Yang K (2020) The effectiveness and safety of acupuncture for depression: an overview of meta-analyses. Complement Ther Med. 50, 102202.
Matthias K, Rissling O, Pieper D, Morche J, Nocon M, Jacobs A, Wegewitz U, Schirm J, Lorenz RC (2020) The methodological quality of systematic reviews on the treatment of adult major depression needs improvement according to AMSTAR 2: a cross-sectional study. Heliyon. 6(9):e04776
Lexchin J, Bero LA, Djulbegovic B, Clark O (2003) Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 326(7400):1167–1170
DeAngelis CD, Fontanarosa PB (2008) Impugning the integrity of medical science: the adverse effects of industry influence. JAMA 299(15):1833–1835
Flaherty DK (2013) Ghost- and guest-authored pharmaceutical industry-sponsored studies: abuse of academic integrity, the peer review system, and public trust. Ann Pharmacother 47(7–8):1081–1083
Chung VCH, Wu XY, Feng Y, Ho RST, Wong SYS, Threapleton D (2018) Methodological quality of systematic reviews on treatments for depression: a cross-sectional study. Epidemiol Psychiatr Sci 27(6):619–627
Mangolini VI, Andrade LH, Lotufo-Neto F, Wang YP (2019) Treatment of anxiety disorders in clinical practice: a critical overview of recent systematic evidence. Clinics. 74:e1316
Stewart L, Moher D, Shekelle P (2012) Why prospective registration of systematic reviews makes sense. Syst Rev 1:7
Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G (2011) The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res 191(2):122–7
Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L (2016) Intravenous esketamine in adult treatmentresistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80:424–431
Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76:970–976
Zarate CA Jr, Singh JB, Carlson PJ et al (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–64
Diazgranados N, Ibrahim L, Brutsche NE et al (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67(8):793–802
Zarate CA Jr, Brutsche NE, Ibrahim L et al (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71(11):939–46
Murrough JW, Iosifescu DV, Chang LC et al (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170(10):1134–42
Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett 34(4):287–93
Lai R, Katalinic N, Glue P et al (2014) Pilot dose–response trial of i.v. ketamine in treatment-resistant depression. World J Biol Psychiatry 15(7):579–584
Hu YD, Xiang YT, Fang JX et al (2016) Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med 46(3):623–635
Loo CK, Gálvez V, O’Keefe E et al (2016) Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134(1):48–56
Jafarinia M, Afarideh M, Tafakhori A et al (2016) Efficacy and safety of oral ketamine versus diclofenac to alleviate mild to moderate depression in chronic pain patients: a double-blind, randomized, controlled trial. J Affect Disord 204:1–8
George D, Gálvez V, Martin D et al (2017) Pilot Randomized Controlled Trial of Titrated Subcutaneous Ketamine in Older Patients with Treatment-Resistant Depression. Am J Geriatr Psychiatry 25(11):1199–1209
Grunebaum MF, Ellis SP, Keilp JG et al (2017) Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord 19(3):176–183
Su TP, Chen MH, Li CT et al (2017) Dose-Related Effects of Adjunctive Ketamine in Taiwanese Patients with Treatment-Resistant Depression. Neuropsychopharmacology 42(13):2482–2492
Arabzadeh S, Hakkikazazi E, Shahmansouri N et al (2018) Does oral administration of ketamine accelerate response to treatment in major depressive disorder? Results of a double-blind controlled trial. J Affect Disord 235:236–241
Chen MH, Li CT, Lin WC et al (2018) Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. J Affect Disord 241:1–7
Grunebaum MF, Galfalvy HC, Choo TH et al (2018) Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 175(4):327–335
Domany Y, Bleich-Cohen M, Tarrasch R et al (2019) Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry 214(1):20–26
Ionescu DF, Bentley KH, Eikermann M et al (2019) Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord 243:516–524
Fava M, Freeman MP, Flynn M et al (2020) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 25(7):1592–1603
Canuso CM, Singh JB, Fedgchin M et al (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175(7):620–630
Daly EJ, Singh JB, Fedgchin M et al (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75(2):139–148
Fedgchin M, Trivedi M, Daly EJ et al (2019) Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 22(10):616–630
Popova V, Daly EJ, Trivedi M et al (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 176(6):428–438. Erratum in: Am J Psychiatry. 2019;176(8):669
Author information
Authors and Affiliations
Contributions
PMA and TML contributed to the development of the review protocol. MBV, TML, and PMA conducted selected articles, data extraction, and quality assessment of included reviews. PMA and TML contributed to drafting and critical revisions of the manuscript. MBV critically reviewed the manuscript. All the authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1. Protocol of overview
-
1.
Review question
To summarize the evidence of efficacy and safety of ketamine for patients with depression from systematic reviews with meta-analyses
-
2.
Searches
A comprehensive literature search will be performed until October 2019 in the Medline (via PubMed), Latin American and Caribbean Health Sciences Literature (LILACS), Cochrane Library, and the Centre for Reviews and Dissemination (CRD) databases. In addition, we will screen the reference lists of the appraised articles to identify any studies which might have been missed. A standardized search strategy will include MeSH terms or text words related to health condition (depression), intervention (ketamine), and study design (systematic review with meta-analysis)
-
3.
Study selection
The selection process will be performed in three steps: (1) exclusion of repeated records, (2) analysis of the titles and abstracts, and (3) analysis of the full-text articles. The studies will be independently selected by two authors. Any disagreements will be resolved by a third author. When the full-text article could not be obtained, the corresponding authors will be contacted via ResearchGate (www.researchgate.net) or e-mail or both
To be included in the overview, the articles must meet the following criteria: (1) be a systematic review with meta-analysis of randomized controlled trials; (2) be published in English, Spanish or Portuguese; (3) have evaluated the use of ketamine (monotherapy or associated with other drugs, any route of administration and frequency of use) compared to placebo or other drugs; (4) report any efficacy and safety outcomes; and (5) in patients with major depressive disorder or bipolar disorder
Articles will be excluded if they were (1) narrative reviews, (2) systematic reviews without meta-analysis; (3) meta-analyses not from systematic reviews; (4) systematic reviews including use concomitant of ECT and ketamine as intervention; (5) systematic reviews with meta-analysis including another target population, intervention, or primary study design; and (6) systematic reviews that did not have the full-text article available
-
4.
Data extraction
Data extraction will be performed by two independent authors, and any disagreement will be resolved by a third researcher. The following information will be collected on a spreadsheet preformatted in Microsoft Excel: author(s), year of publication, literature search, population, intervention, comparators, outcome measures, number of RCTs and patients included in the outcome analysis, pooled effect size, heterogeneity, publication bias, and funding source
-
5.
Quality assessment
The methodological quality of included systematic reviews will be assessed using the AMSTAR-2. The AMSTAR-2 is a 16-item questionnaire, with the majority of questions being judged as “yes,” “partial yes,” or “no.” The items in this tool are as follows: Item 1: Did the research questions and inclusion criteria for the review include the components of PICO? Item 2: Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? Item 3: Did the review authors explain their selection of the study designs for inclusion in the review? Item 4: Did the review authors use a comprehensive literature search strategy? Item 5: Did the review authors perform study selection in duplicate? Item 6: Did the review authors perform data extraction in duplicate? Item 7: Did the review authors provide a list of excluded studies and justify the exclusions? Item 8: Did the review authors describe the included studies in adequate detail? Item 9: Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? Item 10: Did the review authors report on the sources of funding for the studies included in the review? Item 11: If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? Item 12: If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? Item 13: Did the review authors account for RoB in individual studies when interpreting/ discussing the results of the review? Item 14: Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? Item 15: If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? Item 16: Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
The overall confidence in the results of the review will be rated either high, moderate, low, or critically low. One investigator conducted the evaluation of the studies, and a second one verified this evaluation
-
6.
Strategy for data synthesis
The characteristics of systematic reviews and their methodological quality will be descriptively summarized using systematically structured tables. The estimates of effect size from meta-analyses (and their 95% confidence intervals [95% CI]) will be expressed as mean difference (MD), standardized mean difference (SMD), relative risk (RR), and odds ratio (OR), depending on what the authors had reported
-
7.
Funding
None
-
8.
Conflicts of interest
None known
Appendix 2. Search strategy by database
Database | Search strategy |
|---|---|
Medline (via PubMed) | systematic[sb] AND (((ketamine[MeSH Terms]) OR (ketamine) OR (ketalar) OR (ketaset) OR (ketanest) OR (calipsol) OR (kalipsol) OR (calypsol) OR (esketamine) OR (spravato)) AND ((mental disorders[MeSH Terms]) OR (depression[MeSH Terms]) OR (depressive disorder[MeSH Terms]) OR (depressive disorder, major[MeSH Terms]) OR (bipolar disorder[MeSH Terms]) OR (depress*) OR (dysthymi*) OR (“affective disorder*”) OR (“mood disorder*”) OR (unipolar) OR (bipolar))) |
LILACS | ((MH:”depression”) OR (MH:”depressive disorder”) OR (depression) OR (depressive) OR (dysthymia) OR (dysthymic) OR ("affective disorder") OR (“affective disorders”) OR (“mood disorder”) OR (“mood disorders”) OR (unipolar) OR (bipolar)) AND ((MH:ketamine) OR (ketamine) OR (ketalar) OR (ketaset) OR (ketanest) OR (calipsol) OR (kalipsol) OR (calypsol) OR (esketamine) OR (spravato)) AND ((MH:review) OR (MH: “review literature as topic”) OR ((review OR overview) AND systematic) OR “systematic review” OR review OR (meta-analysis OR metanalysis OR metaanalysis)) |
The Cochrane Library | #1 MeSH descriptor: [depression] explode all trees #2 MeSH descriptor: [depressive disorder] explode all trees #3 MeSH descriptor: [depressive disorder, major] explode all trees #4 MeSH descriptor: [bipolar disorder] explode all trees #5 (depression):ti,ab,kw #6 (depressive):ti,ab,kw #7 (dysthymia):ti,ab,kw #8 (dysthymic):ti,ab,kw #9 ("affective disorder"):ti,ab,kw #10 ("affective disorders"):ti,ab,kw #11 ("mood disorder"):ti,ab,kw #12 ("mood disorders"):ti,ab,kw #13 (unipolar):ti,ab,kw #14 (bipolar):ti,ab,kw #15 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16 MeSH descriptor: [Ketamine] explode all tree #17 ketamine:ti,ab,kw #18 Ketalar:ti,ab,kw #19 Ketaset:ti,ab,kw #20 Ketanest:ti,ab,kw #21 Calipsol:ti,ab,kw #22 Kalipsol:ti,ab,kw #23 Calypsol:ti,ab,kw #24 esketamine #25 spravato #26 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #27 (#15 AND #26) |
Center for Reviews and Dissemination (CRD) | (ketamine) OR (esketamine) AND (depression) |
Appendix 3. List of excluded studies
Reason for exclusion | Authors, year | Title | Reference |
|---|---|---|---|
Systematic review that did not conduct a meta-analysis | Serafini et al. (2014) | The role of ketamine in treatment-resistant depression: a systematic review | Curr Neuropharmacol, 2014;12(5):444–61 |
Short et al. (2018) | Side-effects associated with ketamine use in depression: a systematic review | Lancet Psychiatry, 2018;5(1):65–78 | |
Cao et al. (2019) | Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review | Prog Neuropsychopharmacol Biol Psychiatry, 2019;92:109–17 | |
Did not include only RCT in meta-analysis | Caddy et al. (2014)a | Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and metaanalysis of efficacy | Ther Adv Psychopharmacol, 2014;4(2):75–99 |
Fond et al. (2014)a | Ketamine administration in depressive disorders: a systematic review and metaanalysis | Psychopharmacology (Berl), 2014;231(18):3663–76 | |
Tashakkori et al. (2021) | The time course of psychotic symptom side effects of ketamine in the treatment of depressive disorders: a systematic review and meta-analysis | Australas Psychiatry. 2021;29(1):80–87 | |
Yuan et al. (2020) | Application of antidepressants in depression: a systematic review and meta-analysis | J Clin Neurosci. 2020;80:169–181 | |
It involves other causes of suicidal ideation in addition to depression | Wilkinson et al. (2018) | The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis | Am J Psychiatry, 2018; 175(2):150–58 |
Excluded article because the meta-analysis was miscalculated | Parsaik et al. (2015)b | Efficacy of ketamine in bipolar depression: systematic review and meta-analysis | J Psychiatr Pract, 2015; 21(6):427–35 |
Bahjie et al. (2021)c | Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis | J Affect Disord. 2021;278:542–555 |
Appendix 4. Randomized controlled trials included in the systematic reviews with meta-analyses on ketamine for depression
RCT 1 | RCT 2 | RCT 3 | RCT 4 | RCT 5 | RCT 6 | RCT 7 | RCT 8 | RCT 9 | RCT 10 | RCT 11 | RCT 12 | RCT 13 | RCT 14 | RCT 15 | RCT 16 | RCT 17 | RCT 18 | RCT 19 | RCT 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Authors, year | Berman et al. (2000) [8] | Zarate et al. (2006) [46] | Diazgranados et al. (2010) [47] | Zarate et al. (2012) [48] | Murrough et al. (2013) [49] | Sos et al. (2013) [50] | Lai et al. (2014) [51] | Lapidus et al. (2014) [45] | Hu et al. (2016) [52] | Loo et al. (2016) [53] | Jafarinia et al. (2016) [54] | George et al. (2017) [55] | Grunebaum et al. (2017) [56] | Su et al. (2017) [57] | Arabzadeh et al. (2018) [58] | Chen et al. (2018) [59] | Grunebaum et al. (2018) [60] | Domany et al. (2019) [61] | Ionescu et al. (2019) [62] | Fava et al. (2020) [63] |
Caddy et al. (2015) [17] | X | X | X | |||||||||||||||||
Lee et al. (2015) [18] | X | X | X | X | X | |||||||||||||||
McCloud et al. (2015) [19] | X | X | ||||||||||||||||||
McGirr et al. (2015) [20] | X | X | X | X | X | X | X | |||||||||||||
Newport et al. (2015) [21] | X | X | X | X | X | X | X | |||||||||||||
Xu et al. (2016) [22] | X | X | X | X | X | X | X | X | ||||||||||||
Witt et al. (2020) [26] | X | X | X | X | X | X | X | X | X | |||||||||||
Fornaro et al. (2020) [23] | X | X | ||||||||||||||||||
Marcantoni et al. (2020) | X | X | X | X | X | X | X | |||||||||||||
Nuñez et al. (2020) [25] | X | X | X |
Appendix 5. Randomized controlled trials included in the systematic reviews with meta-analyses on esketamine for depression
Rights and permissions
About this article
Cite this article
Lima, T.d., Visacri, M.B. & Aguiar, P.M. Use of ketamine and esketamine for depression: an overview of systematic reviews with meta-analyses. Eur J Clin Pharmacol 78, 311–338 (2022). https://doi.org/10.1007/s00228-021-03216-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03216-8