Abstract
Purpose
Intracellular exposure of tacrolimus (TAC) may be a better marker of therapeutic effect than whole blood exposure. We aimed to evaluate the influence of genetic polymorphism on the pharmacokinetics of TAC in peripheral blood mononuclear cells (PBMCs) and develop limited sampling strategy (LSS) models to estimate the area under the curve (AUC0–12h) in the PBMC of Chinese renal transplant patients.
Methods
Ten blood samples of each of the 23 renal transplant patients were collected 0–12h after 14 (10–18) days of TAC administration. PBMCs were separated and quantified. The TAC level in PBMCs was determined, and pharmacokinetic parameters were estimated by noncompartmental study. The AUC0–12h of TAC in whole blood was estimated by Bayesian approach based on a population pharmacokinetic model established in 65 renal transplant patients. The influence of CYP3A5 and ABCB1 genotypes on exposure was estimated. By applying multiple stepwise linear regression analysis, LSS equations for TAC AUC0–12h in the PMBC of renal transplant patients were established, and the bias and precision of various equations were identified and compared.
Results
We found a modest correlation between TAC exposure in whole blood and PBMC (r2 = 0.5260). Patients with the CYP3A5 6986GG genotype had a higher AUC0–12h in PBMCs than those with the 6986 AA or GA genotype (P = 0.026). Conversely, patients with the ABCB1 3435TT genotype had a higher AUC0–12h in PBMC than those with the 3435 CC and CT genotypes (P = 0.046). LSS models with 1–4 blood time points were established (r2 = 0.570–0.989). The best model for predicting TAC AUC0–12h was C2–C4–C6–C10 (r2 = 0.989). The model with C0.5–C6 (r2 = 0.849) can be used for outpatients who need monitoring to be performed in a short period.
Conclusions
The CYP3A5 and ABCB1 genotypes impact TAC exposure in PBMCs, which may further alter the effects of TAC. The LSS model consisting of 2–4 time points is an effective approach for estimating full TAC AUC0–12h in Chinese renal transplant patients. This approach may provide convenience and the possibility for clinical monitoring of TAC intracellular exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal transplantation is a standard and effective treatment for end-stage renal disease. Progress in therapeutic immunosuppression achieved over the last decades has dramatically reduced T cell–mediated rejection [1]. Tacrolimus (TAC), a calcineurin inhibitor, has become a common choice for immunosuppression after solid organ transplantation [2]. TAC binds to an immunophilin FK506-binding protein 12 (FKBP-12), which inhibits the activity of calcineurin, and prevents the dephosphorylation of the nuclear factor of activated T cells (NFAT) [3,4,5]. However, considerable variability in the pharmacokinetics (PK) of TAC has been reported, and long-term graft outcomes can be impacted by insufficient immune suppression [6]. Therapeutic drug monitoring (TDM) is an integral part of post-transplant patient care [7]. TAC trough concentration (C0) is a widely used TDM index, which is considered as a proxy for the overall TAC exposure [8]. TDM significantly improves patient survival, yet 8–30% acute rejection rate remains within 2 years post-transplantation [9, 10], suggesting whole blood TAC cannot universally indicate efficacy. TAC exerts its effect mainly within lymphocytes, important components of peripheral blood mononuclear cells (PBMCs). Some recent studies proposed that PKs of TAC in the lymphocyte or PBMC are more relevant than those in whole blood when predicting treatment efficacy [11]. Capron et al. studied the TAC concentrations in the PBMCs of 96 renal transplant recipients and found that there tended to be an association between acute rejection episodes and significantly lower TAC intracellular concentrations [12]. In another study on liver transplant recipients, they also found that patients experiencing clinical rejection 1 week after transplantation had significantly lower TAC PBMC concentrations than patients who did not suffer from a rejection episode [13]. Various factors affecting intracellular TAC disposition have been suggested. The role of drug metabolism enzymes and transporters in lymphocytes on the distribution and disposition of immunosuppressants has been suggested in previous studies [14]. The equilibrium between influx and efflux mechanisms could influence the TAC concentration in PBMCs, impacting its therapeutic effect [15]. CYP3A5 6986GA genetic polymorphism has an impact on the CYP3A5 expression and further influences the TAC dosing regimen [16]. Nevertheless, it remains to be elucidated whether the most relevant SNP in CYP3A5 could impact the TAC intracellular concentration in comparison to whole blood.
The area under the concentration–time curve (AUC) is considered as a good indicator of a drug’s efficacy and toxicity. However, it is not often feasible to perform TDM using a full-dosing interval AUC because of clinical application constraints. The development of a limited sampling strategy (LSS) has provided a feasible alternative method [17]. These may offer a better means of estimating TAC exposure, yielding greater accuracy than C0 measurements, while being less cumbersome than full AUC0–12h measurements [18, 19]. Although many LSSs for estimation of TAC exposure have been reported for the renal transplant recipients, all existing LSSs for TAC used whole blood matrix [17, 19,20,21,22]. To our knowledge, no published LSSs are available for TAC exposure in PBMCs.
Based on the above perspectives, this study aimed to (i) explore TAC PKs in PBMCs and the relationship with those in whole blood, (ii) evaluate the influence of genetic polymorphism of drug metabolism enzymes and transporters on TAC exposure in PBMCs, and (iii) develop LSS models to estimate TAC intracellular exposure.
Methods
Patients
All of the patients recruited in this study underwent renal transplantation between November 2017 and January 2020 at the transplantation center, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China. All of the patients did not receive immunosuppressive regimen taken prior to transplant surgery. Patients with a second transplantation or a significantly abnormal liver function prior to surgery were excluded in the present study. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki. The hospital’s independent ethics committee approved the study protocol. All the patients who participated in the study provided written informed consent.
Immunosuppressive therapy
TAC (Prograf ®, Astellas Ireland Co., Ltd.) was orally administered at a dosage of 0.1 mg kg−1 day−1 twice daily, with subsequent adjustment through regular monitoring of the TAC trough concentration in whole blood to achieve a steady-state C0 of 10–13 ng mL−1, in the first month, and 5–9 ng mL−1 thereafter. In addition to TAC, immunosuppressive therapy was combined with mycophenolic acid (MPA) and steroids. Either mycophenolate mofetil (MMF, Cellcept®, Roche) or enteric-coated mycophenolate sodium (EC-MPS, Myfortic, Novartis Pharma) was administered; the initial dose of MMF and EC-MPS was 1000 mg or 720 mg within 6h before renal transplantation, and thereafter, a maintenance dose of 1000 mg or 720 mg every 12h was administered, adjusted based on the MPA concentration and observed efficacy and toxicity. Methylprednisolone (500 mg, Pfizer) was administered intravenously during surgery, progressively tapered, and maintained for 1 month later at 5–10 mg oral prednisone daily.
The patients’ physiological and pathological characteristics were collected on the day of TAC monitoring. The demographic data, including body weight, age, sex, and biological and clinical data including albumin, total bilirubin, serum creatinine, alanine aminotransferase, and aspartate aminotransferase, were recorded on the day of TAC monitoring. The creatinine clearance (CLcr) of the patients was calculated according to the Cockcroft-Gault equation. The post-operation date was expressed as the period between the operation day and the day of concentration monitoring. Other medicines that may affect the blood MPA level were also recorded. To guarantee the accuracy, all data were double-checked by different researchers independently.
TAC determined in PBMC and whole blood
For 23 patients, 3.5 mL of peripheral venous blood was collected in EDTA tubes at 0, 0.5, 1, 1.5, 2, 4, 6, 8, 10, and 12h after the dose of TAC. The whole blood was used to isolate the PBMC by density gradient centrifugation that was used to obtain complete PKs of TAC in PBMCs. Cells were counted using a hemocytometry method through a microscope. 0.1–2.5 million PBMC samples were obtained and stored under –80 °C. TAC levels were detected within 1 month. The TAC level in PBMCs was standardized to the level of per million cells. An LC–MS/MS assay method established in our lab was used to determine the TAC level in PBMCs [23]. Briefly, PBMC was treated with acetonitrile for protein precipitation and gradient-eluted through an Agilent Eclipse XDB-C18 column (3.5 μm, 2.1 mm × 100 mm). The mobile phase was water and methanol plus 2 mM ammonium formate; flow rate: 0.3 mL min−1; electrospray ionization sources, positive ion mode; m/z transition: TAC, 821.8 → 768.6; and ascomycin, 809.6 → 756.5 (internal standards). Extraction recovery, matrix effects, and mean relative recovery were 81.3–91.7%, 82.0–89.6%, and 100.7–112.1%, respectively. Intra-day and inter-day coefficients of variation were <15%. Samples were stable under brief room temperature storage and long-term –80 °C storage and three freeze–thaw cycles. The whole blood for TAC TDM was collected within 60 min before the next dose under steady state. TAC levels were measured using a chemiluminescent microparticle immunoassay (CMIA) on an ARCHITECT-i2000SR (Abbott Diagnostics, Chicago, IL, USA) [24]. The assay’s functional sensitivity was 2 ng mL−1 for TAC. A total of 792 TAC trough blood concentrations from 65 patients after 3–215 days of therapy with TAC were collected for the developing of the population PK (PPK) model.
Genotyping analysis
Genomic DNA was extracted from 200 μL whole blood sample using a genomic DNA purification kit (TIANGEN BIOTECH Co. LTD., Beijing, China) according to the manufacturer’s standard protocol. DNA samples were stored under 4 °C and detected within 1 month. Genotypes of CYP3A5 (rs776746) and ABCB1 (rs1128503, rs2032582, and rs1045642) were determined using a PCR-sequencing method [25]. Briefly, 50 μL reaction mixture contained 15 ~ 50 ng genomic DNA, 1 U of Taq DNA polymerase, 1 × buffer, 0.2 mmol L−1 of dNTP mixture, 1.5 mmol L−1 MgCl2, and 0.5 μmol L−1 of each primer. The reaction was set to occur as follows: 7 min at 94 °C; followed by 30 cycles of 94 °C for 30s, 60 °C for 30s, and 72 °C for 30s; and a final extension at 72 °C for 7 min. The sequences of primers were as follows: CYP3A5*3: 3A5P1 (5′GCC CTT GCA GCA TTT AGT CCT T3′) and 3A5P2 (5′ CCT GCC TTC AAT TTT TCA CTG 3′); ABCB1 1236: 1236P1 (5′ AAT GTT CAC TTC AGT TAC CCA TCT CG 3′) and 1236P2 (5′ AAT GAT TTC CCG TAG AAA CCT TAC 3′); ABCB1 2677: 2677P1 (5′ TAC CCA TCA TTG CAA TAG CAG 3′) and 2677P2 (5′ GAA AAA GAT TGC TTT GAG GAA TGG 3′); and ABCB1 3435: 3435P1 (5′ TGC TGG TCC TGA AGT TGA TCT GTG AAC 3′) and 3435P2 (5′ GGC CAG AGA GGC TGC CAC AT 3′). The product was purified and sequenced on an automated genetic analyzer (ABI PRISM 3730 Sequence Detection System; Applied Biosystems).
Noncompartmental PK study of TAC in PBMCs
The PKs of TAC in PBMCs were estimated using WinNonlin 5.01 (Pharsight). The maximum concentration (Cmax) and time to attain Cmax (Tmax) were obtained directly from the data. The elimination rate constant (Ke) was estimated using least-square regression analysis from the log-transformed concentration–time curve terminal phase with 3–4 time points. AUC0–12h was estimated using the trapezoidal method.
Estimation of the AUC of TAC in whole blood based on the Bayesian assay
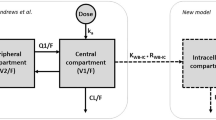
PPK modeling was performed with NONMEM (Version 6, GloboMax, Hanover, MD). Log-transformed concentration data were used to ensure the random effects are sufficiently distributed around zero. The first-order conditional estimation method (FOCE) was applied for the modeling. Model selection was based on the objective function value (OFV), parameter estimates, and standard errors. OFV is proportional to −2 log likelihood of the relevant model, and a lower value indicates a better model. The distribution of empirical Bayes estimates was also an important factor for model selection. The primary PK parameters were clearance (CL/F) and volume of distribution (Vd/F). Ka was fixed based on previously reported literature values.
The inter-individual variability of the parameters was assessed using an exponential function:
where Pi is the individual value, TV(Pi) is the population value for the parameters described in the equation, and ηi is the random deviation of Pi from TV(Pi). The values of ηi were assumed to be independently normally distributed with a mean of 0 and a variance of ω2.
As the concentration data were log transformed, an additive model was used for residual error analysis: lnCobs = lnCpred + ε, where Cobs is the observed concentration, Cpred is the predicted concentration, and ε is a residual error with a mean of 0 and a variance of σ2.
The patients’ physiological and pathological characteristics and genetic polymorphisms were evaluated as the possible covariates of the TAC PPK model. Forward inclusion and backward elimination techniques were used for the final regression model. Each candidate covariate was screened in turn by adding into the base model. Weighted residuals and the change in the OFV were noted in the model-building process. Changes in the OFV approximate the χ2 distribution with the degrees of freedom (df) equal to the number of covariates introduced. A covariate was considered statistically significant when the OFV decreased by 6.63 or greater (P < 0.01, df = 1) when added to the base model (forward inclusion). The full model included all covariates that show significant decrease in OFV. Hence, each covariate remaining in the model was removed in turn by fixing its value as zero. This procedure was repeated until the value of the objective function failed to increase by more than the critical value of 7.88 (P < 0.005, df = 1) (backward elimination). Individual PPK parameters, arithmetic means, and standard deviations were calculated using the NONMEM Bayesian estimates from the POSTHOC output.
Based on a Bayesian approach, the POSTHOC subroutine of NONMEM without an estimation step (MAXEVAL = 0) was used to estimate the individual CL/F of the other 23 patients whose PBMCs were extracted. The PPK parameters, IIV, and residual variability were set as the final model’s data. TAC whole blood AUC between 0 and 12h was calculated based upon the individual dose and CL/F as follows:
LSS model establishment and verification
SPSS 25.0 and SAS 9.1.3 software for Windows were used for statistical analysis. The results are expressed as the mean ± standard deviation (SD); P < 0.05 was considered to indicate statistical significance. Stepwise multiple linear regression analysis (MRA) was employed to determine the model equation used to predict the AUC0–12h for PBMC TAC. At different time points, the PBMC TAC concentrations served as independent variables for MRA, while AUC0–12h served as the dependent variable. The model equation was determined, and the AUC0–12h for TAC was calculated according to the following equation:
where b is the intercept, Mi is the partial correlation coefficient, and Cti is the PBMC TAC concentration at sampling time ti. The prediction error (PE) and absolute prediction error (APE) were calculated using the following equations:
where AUC(0–12)Obs and AUC(0–12)Est are the observed and estimated AUC values, respectively. The mean prediction error (MPE) and mean absolute prediction error (MAPE) were used to compare the performance among the various models. The acceptable criteria for MPE and MAPE are <15% [26]. A Bland–Altman plot was used to evaluate the agreement between the estimated and observed PBMC AUC0–12h, and the difference is expressed as the mean ± 1.96 SD.
Results
Patient characteristics
A total of 88 renal transplant recipients were included in the study. The conventional TDM data of 65 patients were obtained 3–215 days after TAC therapy; the data were used for PPK model construction. PBMCs were collected from the other 23 patients after about 2 weeks (10–18 days) of therapy with TAC. The demographic and clinical characteristics of these patients are summarized in Table 1.
The TAC PPK model in whole blood
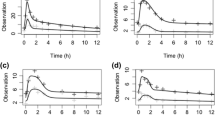
The one-compartment open model with first-order elimination was applied. As there was insufficient available information from the absorption phase, Ka was fixed at 3.86h−1 [27]. The base model estimates a mean value of 31.4 L/h for CL/F and 2300 L of Vd/F. After the forward inclusion and backward elimination step, the CYP3A5 genotype and hematocrit reduced the OFV by > 7.88 (P < 0.005) when tested as covariates of CL/F individually against the base model. After all the covariates were included in the model, the inter-individual variability of CL/F decreased from 44.9 to 23.6%. The results of the model-building process are shown in Table 2. Figure 1 shows goodness-of-fit plots for the final PPK model. A bootstrap test was used for model validation. Most of the 1000 bootstrap runs were successful (Table 3).
The goodness-of-fit of the final population pharmacokinetic model of tacrolimus in Chinese renal transplant patients. (A) The population predicted concentration (PRED) vs. the measured concentration (CONC); (B) the individual predicted concentration (IPRE) vs. CONC; (C) the weighted residual error (WRES) vs. PRED; (D) WRES vs. time
TAC PKs in PBMC and impact of genotypes
The PK parameters of TAC in whole blood and PBMCs in 23 renal transplant recipients are shown in Table 4. We found that the C0 of TAC in PBMCs was 1.28 ± 0.88 ng/million PBMCs. The AUC0–12h of TAC in PBMCs was 23.8 ± 14.1 ng⋅h/million PBMC. TAC AUC0–12h in PBMCs was correlated well with TAC AUC0–12h in whole blood (r2 = 0.5260, P < 0.0001). However, the correlation between TAC C0 in PBMCs and whole blood was poor (r2 = 0.1677, P = 0.0523) (Fig. 2).
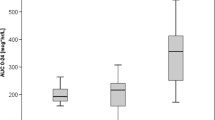
A significant trend to lower TAC AUC0–12h was observed in PBMCs for recipients with a graft expresser of CYP3A5 (carrier of at least one CYP3A5 6986A allele) (P = 0.026). Although there was no significant association between ABCB1 1236C > T or 2677G > T/A polymorphism and TAC PKs in PBMCs, patients with the ABCB1 3435TT genotype seemed to have higher AUC0–12h in PBMCs (P = 0.046) (Fig. 3).
Limited sampling strategy
Seventeen LSS models with 1–4 time points were selected (Table 5). Models with a single time-point TAC concentration were not satisfactory (r2 = 0.570–0.787). The correlation between C0 and AUC0–12h was poor (r2 = 0.570), with a substantial prediction error among various samples (MPE = 13.4 ± 47.6%, MAPE = 37.0 ± 31.8%). A single time point is not sufficient to estimate the AUC0–12h of TAC in PBMCs. Models containing two- to four-time-point TAC concentrations provided better results, especially for three- and four-time points. For two-time-point TAC concentrations, the best equation was model 10 with C0.5–C6 (r2 = 0.849). For three- and four-time-point TAC concentrations, models 14 and 17 were the best, with C2–C4–C6 (r2 = 0.974) and C2–C4–C6–C10 (r2 = 0.989), respectively. Figure 4 and Fig. 5 provide the correlation and Bland–Altman analysis of the estimated and measured AUC0–12h.
The obtained LSS models were validated through bootstrap analysis. From the original dataset, 1000 replicated datasets were generated and used to evaluate the different models’ stability and accuracy of the parameter estimates (Table 6). The mean values and 95% CI were comparable to the original dataset’s parameter estimates. These data indicated that the selected models’ accuracy and robustness were acceptable.
Discussion
In this study, TAC PKs in PBMCs and the relationship with those in whole blood were evaluated. The influence of genetic polymorphism of CYP3A5 and ABCB1 on TAC exposure in PBMCs in Chinese renal transplant patients was investigated. In addition, we developed LSS models for predicting TAC AUC0–12h in PBMC using the MRA method in Chinese renal transplant recipients for the first time.
As TAC is strongly lipophilic and widely distributed in erythrocytes, whole blood is most frequently sampled to monitor TAC [28]. Recent studies have suggested that the unbound fraction of TAC is pharmacologically active and used as the TDM index [29]. As TAC targets T lymphocytes, essential components of PBMC, some recent studies suggested TAC levels in PBMCs can be used as a new TDM index. The results suggested PKs in lymphocytes or PBMCs have more significant relationship with the efficacy and toxicity in some patients. It is obvious that drug measurement in PBMC is more technically challenging than routine whole blood TDM, which requires complex pretreatment and a highly sensitive determination method. There are a few studies on the relationship between intracellular and whole blood TAC exposure; the results are conflicting. Han et al. [30] found that the relationship of whole blood and PBMC concentrations seemed to be linear (r2 = 0.67, P < 0.0001). Conversely, Lemaitre’s [31] preliminary study on 40 renal transplant patients showed lack of correlation between TAC whole blood trough concentrations and TAC trough concentrations in PBMCs (r2 = 0.259; P = 0.183). In our study, we found the correlation between C0 in PBMC and whole blood was not significant (r2 = 0.1677, P = 0.0523). However, there was good correlation between TAC AUC0–12h in PBMCs and whole blood which was more significant (r2 = 0.5260, P < 0.0001). However, some studies had shown that the decrease in the TAC intercellular exposure would be largely non-proportional to the decrease in the TAC whole blood exposure during the early period [30]. TAC intercellular exposure had a larger impact on the activation of T cells than the exposure in whole blood. Therefore, monitoring the TAC in whole blood and TAC in PBMCs together in the early period might be valuable in preventing rejection during the tapering period compared with monitoring whole blood TAC concentrations alone, particularly when tacrolimus must be tapered.
The association between the CYP3A5 genotype and TAC concentration and dose requirement has been observed among solid organ transplantations [16, 32, 33]. Patients with CYP3A5 6986GG genotype require lower TAC doses to maintain the desired therapeutic range than those with CYP3A5 6986GA and CYP3A5 6986AA alleles. Previous reports also suggested that CYP3A5 is weakly expressed in lymphocytes [30, 32], which may also impact the disposition of TAC in lymphocytes. In our study, we established a PPK model based on conventional TDM data in whole blood and found that the CYP3A5 genotype significantly affects CL/F. The inter-individual variation decreased significantly after the CYP3A5 genotype was included as a covariate. More importantly, we found that the CYP3A5 genotype significantly influences intracellular PK exposure. As there was only one patient with the CYP3A5 6896AA genotype, we integrated the AA and GA genotypes in one group. Intracellular TAC AUC0–12h was significantly affected by the CYP3A5 genotype (P = 0.026). TAC is also a substrate of P-glycoprotein (P-gp), which is coded by adenosine triphosphate-binding cassette subfamily B member 1 (ABCB1) [34]. There is much data regarding the genetic polymorphism of ABCB1 on the PKs of TAC, but data in this regard are not consistent [35,36,37,38]. The influence of the ABCB1 genotype on TAC disposition in lymphocytes was also suggested. Capron et al. determined the TAC PBMC concentrations and ABCB1 genotypes in 96 renal transplant recipients 7 days after transplantation. They found that ABCB1 genotyping might help better individualize TAC immunosuppressive therapy in renal transplantation [12]. The ABCB1 genotypes impact on the TAC concentration in the target cells (lymphocytes) or grafts could have significant clinical consequences. This finding was also suggested in a study by Capron et al. on liver transplant patients [13]. A link has been shown between the intra-lymphocyte production of interleukin-2 (IL-2) and the ABCB1 C3435T genotypes. In 3435TT homozygous cells with a less active efflux pump, TAC has a better pharmacodynamic effect [15, 39]. This information suggests that monitoring the TAC intracellular exposure can help prevent rejection during the tapering period compared with monitoring the whole blood alone in some situations. Our study obtained similar results. We found that PBMC AUC0–12h in patients with ABCB1 3435 CC/CT was about 72.6% of those in TT patients. 3435TT carriers had less expression of ABCB1 and a lower activity of P-gp, which may further decrease the efflux of TAC and higher intracellular exposure.
Because collecting multiple time points over a dosing interval is inconvenient for routine clinical monitoring of the AUC of TAC in PBMC, LSS models may be valuable. Two primary methods have been used to develop LSS models: MRA and maximum a posteriori, which is also known as Bayesian analysis [40]. LSS models obtained using MAP are based on an a priori PPK model and, in combination with demographic, pathophysiological, and limited concentration–time data from the individual patients, are used to predict individualized parameters, including AUC. On the other hand, MRA is simple and easy to use, and its accuracy relies on appropriate choices and the number of sampling times. Our study attempted to find clinically applicable LSS equations using MRA to estimate intracellular AUC0–12h of TAC. To the best of our knowledge, this is the first study using the LSS model of PBMC TAC in Chinese renal transplant recipients. Monitoring C0 in whole blood is currently a routine clinical practice. However, we found a moderate correlation between C0 and AUC0–12h (r2 = 0.570) with suboptimal imprecision in PBMC. Different LSS models with four or fewer samples were tested. We found models 6h (r2 = 0.787), 0.5–6h (r2 = 0.849), 2–4–6h (r2 = 0.974), and 2–4–6–10h (r2 = 0.989) were the best for the 1-, 2-, 3-, and 4-time-point models. C4 and C6 are crucial for the prediction. The model Eqs. 14 (C2–C4–C6) and 17 (C2–C4–C6–C10) were superior to other models with 3- or 4-time points. The MPE and MAPE values of model Eqs. 14 and 17 were −0.00 ± 10.24%, 0.00 ± 8.24%, and 7.82 ± 6.38% and 6.08 ± 5.40%, respectively. As the models with C2–C4–C6–C10have better prediction performance, it is valuable for inpatients whose dosage regulation needs the accurate estimation of AUC0–12h of TAC in the early postoperative period. We found there was good correlation between C6 AUC (r2 = 0.787). However, the MAPE of C6 was 21.3 ± 13.6%, which is higher than 15%. The results suggested that the precision of prediction did not meet the criteria. C0.5–C6 also had relatively good precision and low bias (MAPE < 10.6%; 76.5% of its predictions have a PE value in the range of ± 15%). This approach facilitates clinical follow-up and is probably more suitable for outpatients who require sample collection in a relatively short period.
A few limitations exist with this report. First, as a limited number of patients with rich-time PBMC levels were included, the LSS study dataset was not divided into index and validation groups; instead, a Bootstrap method was used to validate the equations obtained. Second, the AUC0–12h in whole blood was estimated using a Bayesian approach, and the PPK model used was based on sparse samples. The CL/F and AUC0–12h obtained may have bias and further impact the correlation between PBMC and whole blood. Third, as the number of patients is limited, the relationship between TAC concentration of PBMCs and the effects or side effects was not proved. Besides, statistical analysis of combined CYP3A5 and ABCB1 genotype on TAC PBMC pharmacokinetics was not performed. More patients and longer (2 years) follow-up of effects and adverse effects of TAC therapy for patients are needed in our further study.
Conclusions
In summary, we found there is a significant impact of CYP3A5 (6986G > A) or ABCB1(3435C > T) genetic polymorphism on TAC PBMC AUC0–12h. In addition, clinically reliable LSS equations for estimating the intracellular AUC of TAC were established, predicting TAC intracellular exposure. The results should be further confirmed in larger cohorts to tailor the immunosuppressive therapy.
References
Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M et al (2015) Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol 26(7):1711–1720. https://doi.org/10.1681/ASN.2014060588
Bittersohl H, Schniedewind B, Christians U, Luppa PB (2018) A simple and highly sensitive on-line column extraction liquid chromatography-tandem mass spectrometry method for the determination of protein-unbound tacrolimus in human plasma samples. J Chromatogr A 1547:45–52. https://doi.org/10.1016/j.chroma.2018.03.010
Schreiber SL, Crabtree GR (1992) The mechanism of action of cyclosporin A and FK506. Immunol Today 13(4):136–142. https://doi.org/10.1016/0167-5699(92)90111-j
Spencer CM, Goa KL, Tacrolimus GJC (1997) An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 54(6):925–975. https://doi.org/10.2165/00003495-199754060-00009
Thomson AW, Bonham CA, Zeevi A (1995) Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit 17(6):584–591. https://doi.org/10.1097/00007691-199512000-00007
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V et al (1995) Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 29(6):404–430. https://doi.org/10.2165/00003088-199529060-00003
Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A et al (2009) Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit 31(2):139–152. https://doi.org/10.1097/FTD.0b013e318198d092
Marquet P, Albano L, Woillard JB, Rostaing L, Kamar N, Sakarovitch C et al (2018) Comparative clinical trial of the variability factors of the exposure indices used for the drug monitoring of two tacrolimus formulations in kidney transplant recipients. Pharmacol Res 129:84–94. https://doi.org/10.1016/j.phrs.2017.12.005
Bamoulid J, Staeck O, Halleck F, Dürr M, Paliege A, Lachmann N et al (2015) Advances in pharmacotherapy to treat kidney transplant rejection. Expert Opin Pharmacother 16:1627–1648
Boudjema K, Camus C, Saliba F, Calmus Y, Salamé E, Pageaux G et al (2011) Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant 11:965–976
Bahmany S, de Wit LEA, Hesselink DA, van Gelder T, Shuker NM, Baan C et al (2019) Highly sensitive and rapid determination of tacrolimus in peripheral blood mononuclear cells by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 33(1):e4416. https://doi.org/10.1002/bmc.4416
Capron A, Mourad M, De Meyer M, De Pauw L, Eddour DC, Latinne D et al (2010) CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics 11(5):703–714. https://doi.org/10.2217/pgs.10.43
Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P (2012) Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study. Transpl Int 25(1):41–47. https://doi.org/10.1111/j.1432-2277.2011.01365.x
Christians U, Strom T, Zhang YL, Steudel W, Schmitz V, Trump S et al (2006) Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit 28(1):39–44. https://doi.org/10.1097/01.ftd.0000183385.27394.e7
Tron C, Lemaitre F, Verstuyft C, Petitcollin A, Verdier MC, Bellissant E (2019) Pharmacogenetics of membrane transporters of tacrolimus in solid organ transplantation. Clin Pharmacokinet 58(5):593–613. https://doi.org/10.1007/s40262-018-0717-7
Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W et al (2015) Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther 98(1):19–24. https://doi.org/10.1002/cpt.113
Mendonza AE, Zahir H, Gohh RY, Akhlaghi F (2007) Tacrolimus in diabetic kidney transplant recipients: pharmacokinetics and application of a limited sampling strategy. Ther Drug Monit 29(4):391–398. https://doi.org/10.1097/FTD.0b013e31811f319b
Ting LS, Villeneuve E, Ensom MH (2006) Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit 28(3):419–430. https://doi.org/10.1097/01.ftd.0000211810.19935.44
Op den Buijsch RA, van de Plas A, Stolk LM, Christiaans MH, van Hooff JP, Undre NA et al (2007) Evaluation of limited sampling strategies for tacrolimus. Eur J Clin Pharmacol 63(11):1039–1044. https://doi.org/10.1007/s00228-007-0354-9
Wong KM, Shek CC, Chau KF, Li CS (2000) Abbreviated tacrolimus area-under-the-curve monitoring for renal transplant recipients. Am J Kidney Dis 35(4):660–666. https://doi.org/10.1016/s0272-6386(00)70013-8
Chen YH, Zheng KL, Chen LZ, Dai YP, Fei JG, Qiu J et al (2005) Clinical pharmacokinetics of tacrolimus after the first oral administration in combination with mycophenolate mofetil and prednisone in Chinese renal transplant recipients. Transplant Proc 37(10):4246–4250. https://doi.org/10.1016/j.transproceed.2005.11.055
Cheung CY, van der Heijden J, Hoogtanders K, Christiaans M, Liu YL, Chan YH et al (2008) Dried blood spot measurement: application in tacrolimus monitoring using limited sampling strategy and abbreviated AUC estimation. Transpl Int 21(2):140–145. https://doi.org/10.1111/j.1432-2277.2007.00584.x
Chen B, Lu JQ, Shao K, Zhai XH, An HM, Shi HQ et al (2020) Establishment of a liquid chromatography-tandem mass spectrometry method for the determination of immunosuppressant levels in the peripheral blood mononuclear cells of Chinese renal transplant recipients. Ther Drug Monit 42(5):686–694. https://doi.org/10.1097/ftd.0000000000000765
Li ZY, Yan CL, Yan R, Feng ZR (2010) Analytical performance of the Abbott Architect i2000 tacrolimus assay in Chinese patients after renal transplantation. Transplant Proc 42(10):4534–4537. https://doi.org/10.1016/j.transproceed.2010.09.155
Chen B, Fang J, Zhang W, Jin Z, Yu Z, Cai W (2009) Detection of C1236T, G2677T/A, and C3435T polymorphism of MDR1 by amplification refractory mutation system PCR. J Clin Lab Anal 23(2):110–116. https://doi.org/10.1002/jcla.20299
Barraclough KA, Isbel NM, Kirkpatrick CM, Lee KJ, Taylor PJ, Johnson DW et al (2011) Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol 71(2):207–223. https://doi.org/10.1111/j.1365-2125.2010.03815.x
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43(10):623–653. https://doi.org/10.2165/00003088-200443100-00001
Andrews LM, Li Y, De Winter BCM, Shi YY, Baan CC, Van Gelder T et al (2017) Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol 13(12):1225–1236. https://doi.org/10.1080/17425255.2017.1395413
Stienstra NA, Sikma MA, van Dapperen AL, de Lange DW, van Maarseveen EM (2016) Development of a simple and rapid method to measure the free fraction of tacrolimus in plasma using ultrafiltration and LC-MS/MS. Ther Drug Monit 38(6):722–727. https://doi.org/10.1097/ftd.0000000000000351
Han SS, Yang SH, Kim MC, Cho JY, Min SI, Lee JP et al (2016) Monitoring the intracellular tacrolimus concentration in kidney transplant recipients with stable graft function. PLoS ONE 11(4):e0153491. https://doi.org/10.1371/journal.pone.0153491
Lemaitre F, Blanchet B, Latournerie M, Antignac M, Houssel-Debry P, Verdier MC et al (2015) Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clin Biochem 48(6):406–411. https://doi.org/10.1016/j.clinbiochem.2014.12.018
Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW et al (2005) Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem 51(8):1374–1381. https://doi.org/10.1373/clinchem.2005.050047
Larriba J, Imperiali N, Groppa R, Giordani C, Algranatti S, Redal MA (2010) Pharmacogenetics of immunosuppressant polymorphism of CYP3A5 in renal transplant recipients. Transplant Proc 42(1):257–259. https://doi.org/10.1016/j.transproceed.2009.11.028
Farrell RJ, Menconi MJ, Keates AC, Kelly CP (2002) P-glycoprotein-170 inhibition significantly reduces cortisol and ciclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment Pharmacol Ther 16(5):1021–1031. https://doi.org/10.1046/j.1365-2036.2002.01238.x
Picard N, Bergan S, Marquet P, van Gelder T, Wallemacq P, Hesselink DA et al (2016) Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther Drug Monit 38(Suppl 1):S57–S69. https://doi.org/10.1097/ftd.0000000000000255
Staatz CE, Goodman LK, Tett SE (2010) Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet 49(4):207–221. https://doi.org/10.2165/11317550-000000000-00000
Haufroid V (2011) Genetic polymorphisms of ATP-binding cassette transporters ABCB1 and ABCC2 and their impact on drug disposition. Curr Drug Targets 12(5):631–646. https://doi.org/10.2174/138945011795378487
Staatz CE, Goodman LK, Tett SE (2010) Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet 49(3):141–175. https://doi.org/10.2165/11317350-000000000-00000
Vafadari R, Bouamar R, Hesselink DA, Kraaijeveld R, van Schaik RH, Weimar W et al (2013) Genetic polymorphisms in ABCB1 influence the pharmacodynamics of tacrolimus. Ther Drug Monit 35(4):459–465. https://doi.org/10.1097/FTD.0b013e31828c1581
Mahmood I, Miller R (1999) Comparison of the Bayesian approach and a limited sampling model for the estimation of AUC and Cmax: a computer simulation analysis. Int J Clin Pharmacol Ther 37:439–445
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 81973387).
Author information
Authors and Affiliations
Contributions
Bing Chen and Pei-jun Zhou designed the study. Xi-han Wang, Xiao-Hui Zhai, and Bing Chen conducted the genotyping and TAC PK study in the PBMCs of renal transplant patients. Kun Shao and Hui-min An analyzed the clinical data of the patients. Xi-han Wang and Bing Chen analyzed the results and drafted the manuscript. All of the authors read and approved the final manuscript. The authors confirm that the PI for this paper is Bing Chen.
Corresponding authors
Ethics declarations
The study was conducted in accordance with the ethical standards of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Ruijin Hospital, which is affiliated with the Shanhai Jiao-Tong University School of Medicine. All the patients who participated in the study provided written informed consent.
Electronic supplementary material
The online version of this article contains supplementary material, which is available to authorized users.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, XH., Shao, K., An, HM. et al. The pharmacokinetics of tacrolimus in peripheral blood mononuclear cells and limited sampling strategy for estimation of exposure in renal transplant recipients. Eur J Clin Pharmacol 78, 1261–1272 (2022). https://doi.org/10.1007/s00228-021-03215-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03215-9