Abstract
Purpose
We aimed to summarize current evidence regarding the impact of a high-dose statin loading before percutaneous coronary intervention (PCI) on short-term outcomes in patients presenting with the acute coronary syndrome (ACS).
Methods
This meta-analysis was based on a search of the MEDLINE, Cochrane Central Register of Controlled Trials, Ovid Journals, and SCOPUS for randomized controlled trials that compared high-dose atorvastatin or rosuvastatin with no or low-dose statin administered before planned PCI in statin-naive patients with ACS. The primary endpoints were major adverse cardiovascular and cerebrovascular events (MACCE), myocardial infarction (MI), and all-cause mortality at 30 days. Prespecified subanalyses were performed with respect to statin and ACS type.
Results
A total of eleven trials enrolling 6291 patients were included, of which 75.4% received PCI. High-dose statin loading was associated with an overall 43% relative risk (RR) reduction in MACCE at 30 days (RR 0.57, 95% CI 0.41–0.77) in whole ACS population. This effect was primarily driven by the 39% reduction in the occurrence of MI (RR 0.61, 95% CI 0.46–0.80). No significant effect on all-cause mortality reduction was observed (RR 0.92, 95% CI 0.67–1.26). In the setting of ST-elevation myocardial infarction (STEMI), atorvastatin loading was associated with a 33% reduction in MACCE (RR 0.67, 95% CI 0.48–0.94), while in non-ST-elevation myocardial infarction ACS (NSTE-ACS), rosuvastatin loading was associated with 52% reduction in MACCE at 30 days (RR 0.48, 95% CI 0.34–0.66). The level of evidence as qualified with GRADE was low to high, depending on the outcome.
Conclusion
A high-dose loading of statins before PCI in patients with ACS reduces MACCE and reduces the risk of MI with no impact on mortality at 30 days. Atorvastatin reduces MACCE in STEMI while rosuvastatin reduces MACCE in NSTE-ACS at 30 days.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins are widely prescribed for lipid-lowering management in the setting of primary and secondary prevention of coronary artery disease (CAD). Among patients with the acute coronary syndrome (ACS) such as those with ST-elevation myocardial infarction (STEMI) and non-ST-elevation ACS (NSTE-ACS), statins hold IA class of recommendation (CoR) in major guidelines and are stipulated to be initiated as early as possible, in the absence of contraindications and regardless of baseline cholesterol levels at the time of the acute event [1–3]. In North-American guidelines for STEMI management from 2013, high-dose atorvastatin of 80 mg daily is recommended in all STEMI patients without contraindications (IB CoR) [4].
However, the exact timing of statin initiation and clinical benefit of high-dose statin loading (pretreatment) before primary or delayed percutaneous coronary intervention (PCI) among patients presenting with ACS is unclear. The biological rationale supporting the early or very early [5] use of statins during the initial (unstable) phase of the acute thrombotic event is based on pleiotropic effects beyond lipid lowering that might act early while awaiting PCI [6–10]. Data corroborates that, among patients with both stable angina and ACS, a high-dose statin loading before PCI was associated with improvement in clinical outcomes, mainly driven by the reduction in periprocedural MIs and major adverse cardiovascular and cerebrovascular events (MACCE) during the vulnerable short-term period [11–13]. The largest-to-date randomized controlled trial (RCT) evaluating the effect of high-dose atorvastatin loading before planned PCI in the population of ACS patients provided mixed results; however, significant MACCE reduction was observed among patients that received PCI and had STEMI [14, 15].
Due to the present evidence gap for this problem and the fact that none of the previous systematic reviews qualified evidence using Grading of Recommendations Assessment, Development, and Evaluation (GRADE), we performed a meta-analysis with GRADE evidence qualification. We included all relevant RCTs to date that assessed the efficacy of early high-dose statin loading vs. no statin/low-dose statin/placebo before PCI among statin-naive patients with ACS on endpoints of MACCE, recurrent MIs, and all-cause death during 30-day follow-up. Secondarily, we underwent to determine the effects of high-dose statin loading before PCI on 30-day MACCE concerning statin used (atorvastatin or rosuvastatin) and ACS subtype.
Results
Search results
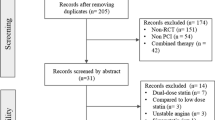
A total of 1520 records were identified from electronic databases. After duplicated were removed, 931 records were screened for eligibility, of which 893 were considered irrelevant. We obtained full texts for the 38 records and excluded 27 for reasons provided (Supplementary Table S1). This resulted in 11 randomized controlled trials being included in this review. The flowchart of study selection is shown in Fig. 1 according to updated PRISMA 2020 statement [17].
Included trials
We included 11 parallel design RCTs in this review, of which 5 were conducted in China [18–22], one in China and Korea [23], two in Italy [24, 25], one in the Netherlands [26], one in Korea [27], and one in Brazil [14]. Detailed information about the included studies is provided in Table 1. All included RCTs took place at the hospital setting and were reported between 2007 and 2018 while studies were conducted from 2005 to 2017. All studies were powered for at least 30-day follow-up.
Participants and clinical features
Overall, 11 RCTs enrolled a total of 6291 statin-naïve patients with ACS that were scheduled to undergo PCI, of which 3132 were randomized to high-dose statin loading before the procedure and 3159 comprised control group. Trials that examined rosuvastatin loading enrolled a total of 1300 patients of which 649 were randomized to high-dose rosuvastatin and 651 received no statin or placebo while trials involving atorvastatin loading enrolled a total of 4991 patients of which 2483 were randomized to high-dose atorvastatin while 2508 comprised control group. A total of 4743 (75.4%) patients received PCI while percutaneous coronary revascularization was planned in all enrolled patients. Among the remaining 1548 patients that did not receive PCI, the most common cause for not utilizing PCI was an indication for conservative medical treatment or referral to coronary artery bypass graft (CABG) surgery. In 9 out 11 studies, 100% of patients received PCI, while in one study, PCI receipt was 66% [25], and in another one, PCI receipt was 64.7% [14].
Across studies, mean age of enrolled patients ranged from 58 to 66.2 years, and a majority of those in the treatment group were men while similar distribution was present in the control group. Two trials enrolled only patients with STEMI, and eight trials enrolled only patients with NSTE-ACS while one trial included both patients with STEMI and NSTE-ACS (Table 2). Patients in both the treatment and control group were well-balanced in terms of distribution of comorbidities and global systolic function at baseline (Supplementary Table S2; Supplementary Figure S1). Likewise, the use of beta-blockers, ACE inhibitors and ARBs, and GPIIb/IIIa inhibitors during hospitalization was similar between two studied groups and across trials (Supplementary Table S3; Supplementary Figure S2).
Interventions
Patients were randomized to either receive high-dose atorvastatin or rosuvastatin. In all studies, statin was administered per os and before PCI. Atorvastatin was administered in at least 80-mg cumulative dose before PCI in all trials while rosuvastatin was administered in 40 mg cumulative dose before PCI in all trials except in a trial by Wang et al. [21] in which 20 mg rosuvastatin was administered at 2 to 4 h prior to PCI. Regarding the control group, patients in 6 trials [18, 20, 23–26] received no statin, and patients in 4 trials [14, 19, 21, 22] received placebo, while in one study [27], a low-dose statin (10 mg atorvastatin) was used as a control. Details of intervention vs. comparator across trials are shown in Table 1.
The timing of oral statin loading varied across trials—in most trials, loading dose was administered at 12 h before invasive management, whereas in some trials, this was commenced within few hours before the procedure. Furthermore, the concomitant standard of care antithrombotic treatment for ACS consisting of P2Y12 receptor antagonist (dominantly clopidogrel) and acetylsalicylic acid was administered to all patients before invasive management with the elective use of glycoprotein IIb/IIIa inhibitors (GPI) at the discretion of the operator. Maintenance antithrombotic and statin therapy were similar across trials and prescribed to all patients post-PCI (Supplementary Table S4).
Outcomes
The primary outcomes of this review were rates of MACCE, recurrent MI, and all-cause mortality at 30 days after ACS. MACCE at 30 days was explicitly stated or eligible for calculation in all included trials, as well as rates of recurrent MI and all-cause mortality, in a whole ACS population. In a secondary analysis stratified by ACS type, MACCE at 30 days in the setting of NSTE-ACS was available for analysis in all trials except in the Post et al. [26] and Kim et al. [27] that both investigated high-dose atorvastatin loading. On the other hand, in the setting of STEMI, 30-day MACCE could be analyzed only in 3 studies [14, 26, 27] that investigated high-dose atorvastatin loading while no studies examining the use of high-dose rosuvastatin in STEMI were available.
Funding
A majority of trials were free of industry-sponsored grants or funds. Six trials [14, 18, 21, 22, 25–27] reported receiving full funding, or at least partial grants from the government, universities, or private foundations. Two trials [19, 24] reported no external funding received while one trial [20] did not declare funding within the manuscript. Two trials [23, 26] declared receiving funds from the pharmaceutical industry (Pfizer Inc.).
RoB in included trials
Results of the RoB assessment in the included trials along with judgments and the supporting explanations are shown in Supplementary Table S5. Included trials generally had a low risk of bias with respect to performance, detection, attrition, reporting, and other potential biases while, on the other hand, most trials had unclear risk with respect to selection bias (random sequence generation and allocation concealment). Supplementary Figure S3 presents the summary of the RoB for each included trial. The percentage of high, low, or unclear risk of bias judgments across included trials is presented in Supplementary Figure S4.
Publication bias assessment and funnel plot asymmetry analysis were performed separately for the endpoints of MACCE, MI, and all-cause mortality at 30 days that included all 11 trials. This analysis revealed funnel plot asymmetry (Supplementary Figure S5) that was significant for the endpoint of MACCE at 30 days (Egger’s Z = -3.23, p = 0.001; Kendall’s τ = -0.24, p = 0.359) and MI at 30 days (Egger’s Z = -2.34, p = 0.020; Kendall’s τ = -0.31, p = 0.218) thus indicating a possibility of publication bias. Analysis of all-cause mortality at 30 days did not reveal significant funnel plot asymmetry (Egger’s Z = -0.64, p = 0.521; Kendall’s τ = -0.16, p = 0.54). Funnel plot asymmetry appeared to be largely driven by the absence of negative or neutral trials with respect to designated outcomes in trials that examined rosuvastatin loading (Supplementary Figure S6).
GRADE qualification of evidence
The quality of evidence for each primary and secondary outcome was qualified using GRADE. High, moderate, and low quality of evidence were determined for the primary outcomes of MI, MACCE, and all-cause death at 30 days, respectively. MACCE at 30 days endpoint was downgraded from high to moderate quality due to inconsistency—47% heterogeneity was detected and effects estimates from studies differed, especially concerning the study size. For the all-cause death endpoint, the level of certainty was downgraded to low quality due to imprecision—a very low number of events was observed; thus, the confidence intervals were large. Finally, findings that high-dose statin loading before PCI reduces the risk of MACCE at 30 days in NSTE-ACS and STEMI were based on the high and moderate quality of evidence, respectively. For the latter endpoint, the level of certainty was downgraded from high to moderate due to imprecision—only three studies were included in the analysis. The summary of findings based on GRADE qualification is provided in Table 3.
Effects of interventions
MACCE at 30 days
All studies contributed to effect estimates with an overall of 6291 patients. High-dose statin loading was associated with an overall 43% risk reduction of MACCE at 30 days (RR 0.57, 95% CI 0.44–0.71) in the whole ACS population (Fig. 2). This was predominantly driven by the effects of rosuvastatin (54% risk reduction) while atorvastatin reduced the risk of MACCE by 31%, although this was of borderline significance (p = 0.08). Low heterogeneity was detected while no differences between subgroups were observed (p = 0.15). Taken together, by combining the results of the meta-analysis and considering the level of certainty of the evidence, we found that high-dose statin loading before PCI reduces MACCE at 30 days.
Myocardial infarction at 30 days
All studies contributed to effect estimates with an overall of 6291 patients. High-dose statin loading before PCI was associated with an overall 39% risk reduction of MI at 30 days (RR 0.61, 95% CI 0.46–0.80) in the whole ACS population compared to the control group (Fig. 3). No significant heterogeneity across studies and no subgroup differences were detected (p = 0.33). Results of meta-analysis along with the certainty of evidence qualification together show that high-dose statin loading before PCI reduces the risk of MACCE at 30 days.
All-cause death at 30 days
All studies contributed to effect estimates with an overall of 6291 patients. Overall results obtained in the analysis suggested an 8% reduction of death at 30 days; however, due to imprecise results, no significant difference between high-dose statin loading and control group was found (RR 0.92, 95% CI 0.67–1.26; Fig. 4). No significant differences between subgroups were found (p = 0.34), and no heterogeneity was detected across included studies. This result of meta-analysis along with qualification of certainty of evidence shows that high-dose statin loading before PCI does not reduce the risk of all-cause death at 30 days, compared to no statin loading.
MACCE at 30 days in NSTE-ACS
Nine studies contributed to the effect estimates with an overall of 4955 patients. High-dose statin loading before PCI was associated with a cumulative 45% risk reduction of MACCE at 30 days in the NSTE-ACS (RR 0.55, 95% CI 0.38–0.80; Fig. 5A). This effect was significantly driven by rosuvastatin (52% risk reduction) while atorvastatin was associated with 35% risk reduction, although of borderline significance. No significant difference between subgroups treated with two different statins was detected in this population (p = 0.38, I2 = 0%). There was a moderate heterogeneity (60%) present among studies in this setting, largely due to the study of Yu et al.; however, we excluded this study in the sensitivity analysis, and similar effect estimates were obtained. Such results combined with a high level of certainty of evidence show that high-dose statin loading, particularly that with rosuvastatin, reduces the risk of MACCE at 30 days in the NSTE-ACS population.
MACCE at 30 days in STEMI
Three studies of which all studied atorvastatin as intervention contributed to the effect estimates with an overall of 1255 patients. No studies for rosuvastatin were eligible for the analysis. No significant heterogeneity across studies was detected. Taken together with qualification of certainty of the evidence, a result of this meta-analysis shows that high-dose atorvastatin loading before PCI reduces MACCE at 30 days in the STEMI population, and this was attributed to 33% relative risk reduction (RR 0.67, 95% CI 0.48–0.94; Fig. 5B).
Exploratory PCI analysis
The analysis of PCI-only cohorts included a total of 4306 patients of which 2144 received statin loading while 2162 received no statin loading before PCI. We observed that, among patients with ACS, of which all received PCI, statin loading was associated with a 44% relative risk reduction of MACCE at 30 days (RR 0.56, 95% CI 0.43–0.74) (Fig. 6). This effect was consistent and significant for both rosuvastatin (RR 0.46, 95% CI 0.33–0.65) and atorvastatin (RR 0.65, 95% CI 0.44–0.97). Finally, a low degree of overall heterogeneity (I2 = 33%) across studies was detected, and there was no significant difference observed between trials concerning statin type used (p = 0.20).
Discussion
Data obtained from 6291 patients from 11 RCTs that were included in this meta-analysis showed that high-dose statin loading before PCI reduces the risk of MACCE and reduces the risk of MI at 30 days in the whole ACS population. High-dose loading of either statin does not reduce the risk of all-cause death at 30 days. These findings are based on evidence of moderate certainty concerning MACCE, high certainty with respect to MI, and low certainty concerning all-cause death. In the context of STEMI, high-dose atorvastatin loading before PCI reduces the risk of MACCE at 30 days, and this is backed by evidence of moderate certainty while no studies with rosuvastatin were available in this setting. In patients with NSTE-ACS, evidence of high certainty shows that high-dose rosuvastatin loading before PCI reduced the risk of MACCE at 30 days while atorvastatin had a borderline impact on this endpoint. These findings suggest that high-dose atorvastatin loading before PCI likely confers benefits in STEMI while high-dose rosuvastatin loading reduces adverse events in NSTE-ACS patients. These effects seem to be primarily driven by the significant reduction of recurrent MIs during the vulnerable 30-day period which translates to an overall reduction of MACCE, however, without impact on mortality. Of note, trials studying atorvastatin loading had larger patient enrollment compared to those studying rosuvastatin loading thus inferring its use in ACS potentially more valid. Finally, our exploratory analysis focused on patients with ACS, of whom all received PCI, confirmed our main findings since both atorvastatin and rosuvastatin loading consistently reduced MACCE at 30 days in this cohort of patients.
Our findings were derived from RCTs that included patients with ACS that we typically see in real-world clinical practice. Of note, we only included trials that enrolled patients naive to statin treatment and with the unstable disease. Equally important, three-quarters of all included patients underwent PCI while those that did not were in most instances referred to CABG surgery or conservative treatment. However, our exploratory analysis of PCI-only patients was in line with the main findings and showed that both statins reduced MACCE at 30 days.
We also focused our analyses on trials using the two most commonly used high-potency statins in clinical practice, atorvastatin, and rosuvastatin. Previous meta-analyses [28–30] were heterogeneous concerning such clinical parameters—some studies mixed stable and unstable CAD patients or included both statin-naive and chronic statin users. Furthermore, actual receipt of PCI widely differed in a substantial number of included trials or analyses were focused on one particular or multiple statin types. Thus, the quality of evidence in this setting was never critically evaluated before. Therefore, findings obtained from previous systematic reviews might not entirely apply to real-world ACS patients.
Cardioprotective effects of early high-dose statin loading in ACS patients with planned percutaneous revascularization are not fully elucidated, but it is suggested that statins exert pleiotropic effects that are beneficial to the cardiovascular system and are beyond the dominant lipid-lowering mechanism [31]. The seminal CANTOS trial validated the inflammatory hypothesis of atherothrombosis since monoclonal antibody blocking inflammatory interleukin-1β pathway managed to reduce rates of recurrent cardiovascular events, compared to placebo, among patients that had a history of MI and increased systemic inflammation as evidenced by high-sensitivity C-reactive protein (CRP) levels ≥ 2 mg/L [32]. Drugs interfering with inflammatory and immune pathways such as colchicine, methotrexate as well as drugs antagonizing IL-6 receptors, were tested for the prevention of adverse cardiovascular events with mixed success in clinical trials [33]. Since statins act anti-inflammatory and reduce CRP levels independent of low-density lipoprotein cholesterol (LDL-C) reduction [34], it is plausible that synergistic anti-inflammatory and lipid-lowering mechanisms of statins administered in high dose during the early phase of ACS may yield beneficial cardiovascular effects, although such mechanisms are yet to be fully explained.
Earlier clinical studies showed that intensive statin use can reduce MACCE in patients with ACS undergoing PCI [35] and prevent procedural myocardial injury in elective PCI among patients with stable CAD [36]. However, questions such as how early to initiate these agents and in which dose during the acute presentation of the coronary event have been less clear [37]. The signal of benefit was more clearly revealed when Patti and colleagues published a collaborative patient-level meta-analysis showing that preprocedural use of statins led to a significant reduction of periprocedural MIs and adverse events at 30 days in a heterogeneous patient cohort receiving PCI [11]. More recent meta-analyses suggested the benefits of atorvastatin [28] and rosuvastatin [29] loading on periprocedural myocardial injury and MACCE among ACS patients undergoing PCI.
Current European and American guidelines for the management and diagnosis of STEMI [2, 4] and NSTE-ACS [1, 3] recommend the initiation of statins as early as possible; however, no specific remarks to high-dose loading before PCI and exact timing of statin initiation are made. To answer these questions, the largest-to-date, double-blind, placebo-controlled, multicenter RCT (SECURE-PCI) that evaluated the use of 80 mg of atorvastatin before and 24 h after coronary angiography among patients with ACS was neutral with respect to the primary outcome of MACCE at 30 days. However, when the data were analyzed concerning those patients that in the end received PCI, it was shown that high-dose atorvastatin loading was associated with a significant 34% reduction in 30-day MACCE (HR 0.66, 95% CI 0.48–0.98), and this effect was sustained in STEMI, but not NSTE-ACS patients [15] which is in line with our findings. Similarly, the observed greater impact of rosuvastatin on MACCE reduction in NSTE-ACS compared to atorvastatin could be explained by the more robust reduction in systemic and microvascular inflammation by rosuvastatin compared to atorvastatin among patients with ACS [38], and this might translate to improved clinical outcomes. Of note, elevated hs-CRP levels were shown to be a prognostic indicator of new MACE and cardiovascular and all-cause mortality in patients with ACS [39]. However, the mechanistic pathways explaining the potential advantage of rosuvastatin in NSTE-ACS have not been fully elucidated yet.
This meta-analysis has some limitations. No protocol was published before this analysis and we only included RCTs in the English language without searching grey literature. While trials were overall at low risk of bias, the majority of studies were of unclear risk concerning selection bias. Similarly, due to the subjective nature of the risk of bias assessment and lack of some information provided by the authors of the studies, uncertainty regarding some evaluations may exist. Moreover, most of the studies had discrepancies concerning a number of analyzed cases vs. those that were randomized to treatment; however, this is in line with the clinical practice since it is expected that not all of the patients randomized to PCI will receive PCI since they might have other indications. Also, it should be acknowledged that most of the evidence of the benefit of statin loading comes from smaller trials whereas the largest trial included (SECURE-PCI) was neutral concerning the benefit of statin loading in ACS. Due to the predominant weight of this trial and the great number of events compared to other trials, it is likely that it significantly impacted on overall results, although our sensitivity analyses in which this trial was removed confirmed our initial findings. Similarly, the body of evidence in terms of patient size was more robust for NSTE-ACS than STEMI population, and this might have an effect on obtained results. For some endpoints, we encountered moderate heterogeneity and discrepancies between effects of larger vs. small studies; however, to account for this, we used a random-effects statistical model that provides more conservative and generalizable results as opposed to a fixed model. Publication bias might also be present due to the detected funnel plot asymmetry, and this observation was mostly driven by trials examining rosuvastatin loading since there appears to be a lack of studies reporting neutral or negative effects with respect to rosuvastatin loading on outcomes of interest. Finally, we should account for a potential geographical bias since most of the included studies were conducted in East Asia.
Conclusions
The results of this meta-analysis, based on moderate and high certainty of the evidence, suggest that early high-dose loading of atorvastatin and rosuvastatin before planned PCI was in association with a significant reduction of MACCE and recurrent MI, in particular, during the short-term period, among statin-naive patients with ACS. This effect seemed to be pronounced among STEMI patients loaded with high-dose atorvastatin and NSTE-ACS patients loaded with high-dose rosuvastatin before planned PCI. However, the results from this meta-analysis should be interpreted with caution due to the potential presence of publication bias and limitations such as small studies contributing to most of the observed effects and the largest trial demonstrating an overall neutral effect of statin loading on clinical outcomes, although more granular analyses of this trial suggested a benefit of early atorvastatin loading in patients receiving PCI and particularly if they had STEMI.
Taken together, these findings suggest a personalized, rather than “one size fits all” approach regarding the early high-potency statin loading in ACS. Therefore, to identify which specific ACS populations would benefit the most from early statin loading strategy, future RCTs should enroll a large number of patients receiving high-dose rosuvastatin and atorvastatin loading in NSTE-ACS, as well as high-dose rosuvastatin loading in the context of STEMI for which the largest evidence gap currently exists.
Availability of data and material
The data that supports the findings of this study are available in the article and the supplementary material of this article.
Code availability
Not applicable.
References
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL et al (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 42(14):1289–1367
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr et al (2014) 2014 AHA/ACC Guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 64(24):e139–e228
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61(4):485–510
Angeli F, Reboldi G, Mazzotta G, Garofoli M, Cerasa MF, Verdecchia P (2012) Statins in acute coronary syndrome: very early initiation and benefits. Ther Adv Cardiovasc Dis 6(4):163–174
Hwang DS, Shin ES, Kim SJ, Lee JH, Kim JM, Lee SG (2013) Early differential changes in coronary plaque composition according to plaque stability following statin initiation in acute coronary syndrome: classification and analysis by intravascular ultrasound-virtual histology. Yonsei Med J 54(2):336–344
Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK et al (2015) Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med 13(1):229
Taguchi I, Oda K, Yoneda S, Kageyama M, Kanaya T, Toyoda S et al (2013) Evaluation of serial changes in tissue characteristics during statin-induced plaque regression using virtual histology-intravascular ultrasound studies. Am J Cardiol 111(9):1246–1252
Moscardó A, Vallés J, Latorre A, Madrid I, Santos MT (2013) Reduction of platelet cytosolic phospholipase A2 activity by atorvastatin and simvastatin: biochemical regulatory mechanisms. Thromb Res 131(4):e154–e159
Takata K, Imaizumi S, Zhang B, Miura S, Saku K (2016) Stabilization of high-risk plaques. Cardiovasc Diagn Ther 6(4):304–321
Patti G, Cannon CP, Murphy SA, Mega S, Pasceri V, Briguori C et al (2011) Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: a collaborative patient-level meta-analysis of 13 randomized studies. Circulation 123(15):1622–1632
Zhai C, Cong H, Liu Y, Zhang Y, Liu X, Zhang H et al (2015) Effect of high-dose statin pretreatment on the incidence of periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention: grading the evidence through a cumulative meta-analysis. Clin Cardiol 38(11):668–678
Navarese EP, Kowalewski M, Andreotti F, van Wely M, Camaro C, Kolodziejczak M et al (2014) Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol 113(10):1753–1764
Berwanger O, Santucci EV, de Barros ESPGM, Jesuíno IA, Damiani LP, Barbosa LM et al (2018) Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: the SECURE-PCI randomized clinical trial. JAMA 319(13):1331–1340
Lopes RD, de Barros ESPGM, de Andrade JI, Santucci EV, Barbosa LM, Damiani LP et al (2018) Timing of loading dose of atorvastatin in patients undergoing percutaneous coronary intervention for acute coronary syndromes: insights from the SECURE-PCI randomized clinical trial. JAMA Cardiol 3(11):1113–1118
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Yun KH, Jeong MH, Oh SK, Rhee SJ, Park EM, Lee EM et al (2009) The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 137(3):246–251
Yu XL, Zhang HJ, Ren SD, Geng J, Wu TT, Chen WQ et al (2011) Effects of loading dose of atorvastatin before percutaneous coronary intervention on periprocedural myocardial injury. Coron Artery Dis 22(1):87–91
Luo J, Li J, Shen X, Hu X, Fang Z, Lv X et al (2013) The effects and mechanisms of high loading dose rosuvastatin therapy before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 167(5):2350–2353
Wang Z, Dai H, Xing M, Yu Z, Lin X, Wang S et al (2013) Effect of a single high loading dose of rosuvastatin on percutaneous coronary intervention for acute coronary syndromes. J Cardiovasc Pharmacol Ther 18(4):327–333
Xie W, Li P, Wang Z, Chen J, Lin Z, Liang X et al (2014) Rosuvastatin may reduce the incidence of cardiovascular events in patients with acute coronary syndromes receiving percutaneous coronary intervention by suppressing miR-155/SHIP-1 signaling pathway. Cardiovasc Ther 32(6):276–282
Jang Y, Zhu J, Ge J, Kim YJ, Ji C, Lam W (2014) Preloading with atorvastatin before percutaneous coronary intervention in statin-naïve Asian patients with non-ST elevation acute coronary syndromes: a randomized study. J Cardiol 63(5):335–343
Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G et al (2007) Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 49(12):1272–1278
Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F (2014) Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the PRATO-ACS Study (protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome). J Am Coll Cardiol 63(1):71–79
Post S, Post MC, van den Branden BJ, Eefting FD, Goumans MJ, Stella PR et al (2012) Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo-controlled pilot trial. Catheter Cardiovasc Interv 80(5):756–765
Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG et al (2010) Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv 3(3):332–339
Ma M, Bu L, Shi L, Guo R, Yang B, Cao H et al (2019) Effect of loading dose of atorvastatin therapy prior to percutaneous coronary intervention in patients with acute coronary syndrome: a meta-analysis of six randomized controlled trials. Drug Des Devel Ther 13:1233–1240
Pan Y, Tan Y, Li B (2015) Li X (2015) Efficacy of high-dose rosuvastatin preloading in patients undergoing percutaneous coronary intervention: a meta-analysis of fourteen randomized controlled trials. Lipids Health Dis 14:97
Benjo AM, El-Hayek GE, Messerli F, DiNicolantonio JJ, Hong MK, Aziz EF et al (2015) High dose statin loading prior to percutaneous coronary intervention decreases cardiovascular events: a meta-analysis of randomized controlled trials. Catheter Cardiovasc Interv 85(1):53–60
Oesterle A, Laufs U, Liao JK (2017) Pleiotropic effects of statins on the cardiovascular system. Circ Res 120(1):229–243
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131
Ma J, Chen X (2021) Anti-inflammatory therapy for coronary atherosclerotic heart disease: unanswered questions behind existing successes. Front Cardiovasc Med 7:631398
Albert MA, Danielson E, Rifai N, Ridker PM (2001) Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 286(1):64–70
Gibson CM, Pride YB, Hochberg CP, Sloan S, Sabatine MS, Cannon CP (2009) Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome. PCI-PROVE IT: A PROVE IT-TIMI 22 (pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22) substudy. J Am Coll Cardiol 54(24):2290–5
Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G (2004) Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (atorvastatin for reduction of myocardial damage during angioplasty) study. Circulation 110(6):674–678
Mega S, Patti G, Cannon CP, Di Sciascio G (2010) Preprocedural statin therapy to prevent myocardial damage in percutaneous coronary intervention: a review of randomized trials. Crit Pathw Cardiol 9(1):19–22
Kumar B, Shah MAA, Kumar R, Kumar J, Memon A (2019) Comparison of atorvastatin and rosuvastatin in reduction of inflammatory biomarkers in patients with acute coronary syndrome. Cureus 11(6):e4898
Mani P, Puri R, Schwartz GG, Nissen SE, Shao M, Kastelein JJP et al (2019) Association of initial and serial C-reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA-16 Trial. JAMA Cardiol 4(4):314–320
Acknowledgements
The authors would wish to express gratitude to an independent methodology consultant Tina Poklepovic Pericic (T.P.P.), DMD, PhD, from Cochrane Croatia, for participating in risk of bias assessment performed for this study and help with manuscript preparation.
Author information
Authors and Affiliations
Contributions
J.A.B. concieved the study and wrote the first original draft of the manuscript in its entirety. J.A.B., M.L.O., and M.K. performed research, data collection, and manuscript preparation. All authors (J.A.B., M.L.O., M.K., D.D., K.S., D.G., and J.B.) commented on the original draft and contributed to the writing of manuscript. All authors participated in the manuscript revision process. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with humans or animal subjects performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Early loading of high-dose statins in patients with the acute coronary syndrome (ACS) naive to statin treatment and before percutaneous coronary intervention was associated with a reduction in major adverse cardiovascular and cerebrovascular events during the 30 days.
• This effect was primarily driven by the reduction in recurrent myocardial infarctions with no impact on all cause mortality. These effects were observed for the rosuvastatin use in the setting of non-ST-elevation ACS and atorvastatin in ST-elevation ACS.
• Caution in interpreting these findings should be exercised due to the possibility of publication and geographical bias as well as the small sample size of the most of included trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borovac, J.A., Leth-Olsen, M., Kumric, M. et al. Efficacy of high-dose atorvastatin or rosuvastatin loading in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a meta-analysis of randomized controlled trials with GRADE qualification of available evidence. Eur J Clin Pharmacol 78, 111–126 (2022). https://doi.org/10.1007/s00228-021-03196-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03196-9