Abstract
Purpose
This study aimed to investigate the pharmacodynamic effects of indobufen and low-dose aspirin in patients with coronary atherosclerosis.
Methods
In the first phase, 218 patients with coronary atherosclerosis were randomly assigned to receive aspirin 100 mg once daily (standard dose); 100 mg once every 2 days; 100 mg once every 3 days; 50 mg twice daily; 75 mg once daily; 50 mg once daily; or indobufen 100 mg twice daily for 1 month. In the second phase, 20 healthy subjects were treated with indobufen 100 mg twice daily for 1 week followed after a 2-week washout by aspirin 100 mg once daily for 1 week. The primary outcome was arachidonic acid-induced platelet aggregation (PLAA), and the secondary outcomes included plasma thromboxane B2 (TXB2) and urinary 11-dehydro-TXB2 (11-dh-TXB2) levels at the end of each treatment.
Results
In the first phase, compared with aspirin 100 mg once daily: all aspirin groups had similar suppression of PLAA whereas indobufen group had significantly less suppressed PLAA. Aspirin given every second or third day, and indobufen produced less suppression of plasma TXB2. All treatment regimens produced similar inhibition of 11-dh-TXB2. In the second phase, compared with aspirin, indobufen produced less suppression of plasma TXB2 at 8 h and 12 h after the last dose.
Conclusions
Aspirin 50 mg twice daily, 75 mg once daily, and aspirin 50 mg once daily produce antiplatelet effects that are similar to aspirin 100 mg once daily. Aspirin given less often than once daily and indobufen 100 mg twice daily do not suppress platelets as effectively as aspirin 100 mg once daily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspirin [75 to 100 mg OD (once daily)] is widely used for secondary prevention of cardiovascular disease [1,2,3]. Aspirin inhibits platelet aggregation by irreversibly blocking platelet cyclooxygenase (COX-1), thereby preventing thromboxane formation [4, 5]. The most common side effects of aspirin are gastrointestinal intolerance, which occurs in more than one-half of the patients, and gastrointestinal bleeding which occurs in 1 to 2% of patients each year [6,7,8]. In patients who experience gastrointestinal side effects, a clinician may recommend lower doses or less frequent dosing of aspirin, or the use of an alternative antiplatelet drug such as indobufen.
Indobufen is an antiplatelet drug that inhibits thromboxane production by reversibly blocking platelet COX-1 [9, 10]. In randomized trials, indobufen has been reported to have fewer gastrointestinal side-effects than aspirin [11]. The improved safety of indobufen might be explained by reversible inhibition of COX-1, but the pharmacodynamics of indobufen compared with low-dose aspirin has not been thoroughly investigated, and no data yet has justified different low-dose aspirin regimens and use of indobufen versus standard dose aspirin among patients with coronary atherosclerosis.
In this study, we compared the pharmacodynamic effects of indobufen 100 mg twice daily and various aspirin dosing regimens with aspirin 100 mg once daily (standard dose) in patients with coronary atherosclerosis. We also compared the pharmacodynamic effects of indobufen 100 mg bid (twice daily) with aspirin 100 mg once daily in healthy volunteers. We measured the pharmacodynamic effects of the treatments using arachidonic acid (AA) induced platelet aggregation (PLAA), plasma thromboxane B2 (TXB2), and urinary 11-dehydro-TXB2 (11-dh-TXB2).
Methods
This study complies with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Approval Number2017-SR-226) and was registered at www.clinicaltrials.gov (Unique Identifier: NCT03230851). Written informed consent was obtained from each subject.
Subjects
We performed this study in 2 phases. In the first phase, we recruited patients with coronary atherosclerosis from the First Affiliated Hospital of Nanjing Medical University, and in the second phase, we recruited healthy volunteers.
In the first phase, we included patients who met the following eligibility criteria: (i) age at least 18 years; (ii) evidence of coronary atherosclerosis, as demonstrated by coronary angiography (CAG), and with a guideline indication for aspirin treatment [1, 12]; and (iii) taking aspirin 100 mg OD (once daily) for more than 5 days. We excluded the following patients: (i) “resistance” to aspirin 100 mg once daily (see below); (ii) uncontrolled hypertension (> 160/100 mmHg); (iii) anemic patients with hemoglobin < 100 g/L; (iv) known bleeding diathesis or high risk of bleeding (e.g., platelet count < 100 × 109/L); (v) taking non-steroidal anti-inflammatory drugs; (vi) active cancer; (vii) active peptic ulcer disease; (viii) history of percutaneous coronary intervention or coronary artery bypass grafting; (ix) any medication taken within the preceding week that could potentially interfere with the anti-platelet effects of the drugs under study; and (x) other reasons that researchers considered inappropriate to participate in this study. Aspirin resistance was defined as an arachidonic acid-induced platelet aggregation > 20% in patients taking aspirin 100 mg once daily for more than 5 days [13].
In the second phase, we included healthy individuals over the age of 18 years. We excluded the following: (i) known allergy or intolerance to aspirin or indobufen; (ii) high risk of bleeding (e.g., thrombocytopenia), known bleeding diathesis, active peptic ulcer disease; (iii) current smoker; (iv) diabetes; (v) pregnancy; and (vi) consumption of any drug within the preceding week that could potentially interfere with the anti-platelet effects of the study drugs.
Study interventions
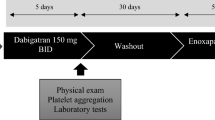
In the first phase, patients were treated with aspirin 100 mg once a day for at least 5 days, after which they were randomly assigned to any of the following 7 treatment groups: (i) aspirin 100 mg once daily; (ii) aspirin 100 mg once every 2 days; (iii) aspirin 100 mg once every 3 days; (iv) aspirin 50 mg twice daily; (v) aspirin 75 mg once daily; (vi) aspirin 50 mg once daily; or (vii) indobufen 100 mg twice daily (Fig. 1A). The pharmacodynamic effects of each treatment regimens were compared with the regular dose of aspirin 100 mg once daily for 1 month.
Study flow chart. A and B demonstrate the study flow charts of the first and second phase studies, respectively. CAG = coronary angiography; PLAA = arachidonic acid-induced platelet aggregation; TXB2 = thromboxane B2; 11-dh-TXB2 = 11-dehydro- thromboxane B2; ASA = acetylsalicylic acid; INDO = indobufen; qd = once daily; qod = once every 2 days; q72h = once every 3 days; bid = twice daily
In the second phase, healthy volunteers received indobufen 100 mg twice daily for 7 days, followed by a 14-day washout period, after which they were given aspirin 100 mg once daily for another 7 days (Fig. 1B). The pharmacodynamic effects of indobufen and aspirin were compared at the end of each 7-day treatment period.
In both phases, the pharmacodynamic outcome measures were PLAA, TXB2, and urinary 11-dh-TXB2.
Aspirin was purchased from Baijingyu Nanjing Pharmaceutical Co., Ltd., Nanjing, China (25 mg/tablet for 50 mg or 75 mg aspirin regimens), or Bayer Schering Pharmaceutical AG. Leverkusen, Germany (100 mg/tablet for 100 mg aspirin regimens). Indobufen was donated by Huadong Medicine Co., Ltd. Hangzhou, China (200 mg/tablet).
Blood and urine collection
Venous blood was collected into two 2.7-mL draw vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ USA) containing 0.105 M buffered sodium citrate (3.2%). Simultaneously, urine was collected into a 10-mL sterile tube, and an aliquot was transferred into a 1.5-mL tube and frozen at −80 °C until further analysis.
In the first phase, samples were collected after the initial 5-day treatment with aspirin and again at 1 month after the completion of randomized study treatments. All samples were collected before the next dose. In the second phase, samples were collected before initiating study drug and repeated at 2 h, 8 h, and 12 h after the last dose of study drug.
Laboratory procedures
We measured platelet aggregation in response to AA and ADP (Chrono-Log Corporation, Havertown, PA, USA) using light transmittance aggregation (Model 700, Chrono-Log Corporation, Havertown, PA, USA). The calibration of optical circuits was performed according to the instruction of the machine, which was released on the website (http://chronolog.com/IFU.html).
Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared shortly after blood collection by spinning the sample at 200 g for 5 min in the centrifuge machine. Then, the PRP was carefully removed, and the remaining blood was centrifuged at 2465 g for 10 min to obtain PPP. The centrifuge temperature was maintained at 22 °C. Platelet counts were adjusted by addition of PPP to the PRP to achieve a count of 250 × 109 /L. Then, 500 μL adjusted PRP was transferred into a test tube, with 500 μL PPP set as a control. After warming for 2 min, PRP and PPP were put into the testing places and were warmed for a further 2 min, then 10 μL AA (with a final concentration of 1 mmol/L) were added to the adjusted PRP, after which the maximum platelet aggregation rates in 8 min were recorded, which represented the PLAA [14]. Platelet aggregation was completed within 3 h of preparation of PRP. Finally, AA-induced PRP was centrifuged at 1000 rpm for 10 min to obtain plasma TXB2 samples, which were then stored at −80 °C for further analysis.
Plasma TXB2 was measured using the Thromboxane B2 Express ELISA Kit-Monoclonal (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Urinary 11-dh-TXB2 was analyzed using a commercially available 11-dehydro-thromboxane B2 ELISA Kit-Monoclonal (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Standards were adopted to make standard curves (R2 > 99%) to achieve values of plasma TXB2 and urinary 11-dh-TXB2. Besides, all samples were set in triplicate, and blank controls were added to ensure the accuracy of the experiment referring to the manufacturer’s instructions.
Outcomes
In the first phase, the primary outcome was PLAA; the secondary outcomes were plasma TXB2 and urinary 11-dh-TXB2 levels, as well as bleeding events and medically intervened gastrointestinal intolerance at 1 month. Bleeding events were defined according to the Bleeding Academic Research Consortium criteria [15]. In the second phase, the primary outcome was PLAA; the secondary outcomes were plasma TXB2 and urinary 11-dh-TXB2 levels at 2 h, 8 h, and 12 h after the last dose. Clinical events were adjudicated by Professor Chunjian Li who was blinded to the grouping of the patients.
Statistical analysis
SPSS statistical software version 22.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. Normally distributed data are presented as mean ± standard deviation (SD) and analyzed by Student’s t-test or one-way analysis of variance (ANOVA) as appropriate. Non-normally distributed data were presented as median (25th, 75th percentile). In the first phase of the study, the Kruskal–Wallis test was used to compare the levels of PLAA, plasma TXB2, and urinary 11-dh-TXB2 at baseline (prior to the randomization) and 1 month among the 7 groups, and the Bonferroni-corrected Wilcoxon rank-sum test was used to compare the levels of PLAA, plasma TXB2, and urinary 11-dh-TXB2 at 1 month between the low-dose/frequency aspirin groups or indobufen group and the aspirin 100 mg daily group. In the second phase of the study, the Wilcoxon matched-pairs signed-rank test was used to compare the corresponding levels of PLAA, plasma TXB2, and urinary 11-dh-TXB2 at 2 h, 8 h, and 12 h after taking the last dose of indobufen and aspirin. Categorical variables were presented as numbers (percentage) and analyzed by Chi-square test. The two-sided p-value of < 0.05 was considered statistically significant.
Results
In the first phase, 219 patients underwent baseline platelet function tests, and 1 patient was excluded from the study due to aspirin resistance. As a result, 218 patients were randomized, and all randomized patients completed 1-month clinical follow-up.
Patients’ demographic parameters and clinical characteristics that may impact on their clinical outcomes are listed in Table 1. By statistical analysis, patients’ gender, age, body mass index (BMI), history of hypertension, diabetes, smoking, concomitant medications [proton pump inhibitor (PPI), nitrates, calcium-channel blocker (CCB), beta-blockers, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACEI/ARB), statins], platelet count (PLT), left ventricular end-diastolic diameter (LVDd), and left ventricular ejection fraction (LVEF) were well balanced among the 7 randomized treatment groups (Table 1). Likewise, baseline pharmacodynamic measures, including PLAA, plasma TXB2, and urinary 11-dh-TXB2, were comparable among the 7 randomized treatment groups (Supplemental Table 1).
In the first phase, 167 patients underwent blood and urine tests for pharmacodynamic evaluation at 1 month. We compared the characteristics of the patients who completed the tests and those who dropped out in the first phase study; no statistical difference was found (Supplemental Table 2). Besides, we compared the characteristics of the 167 subjects who completed the tests among the 7 groups, neither statistical difference was found (Supplemental Table 3).
For those who completed the 1-month follow-up in the first phase study, blood and urine sampling were performed at a mean of 23.21 ± 2.86 h (aspirin 100 mg once daily); 47.04 ± 1.92 h (100 mg once every 2 days); 71.11 ± 2.30 h (100 mg once every 3 days); 11.98 ± 0.75 h (50 mg twice daily); 23.05 ± 2.32 h (75 mg once daily); 23.46 ± 2.55 h (50 mg once daily); and 12.27 ± 1.24 h (indobufen 100 mg twice daily) after the last dose of the study drug at 1 month.
In the second phase, 20 healthy volunteers, 9 (45%) male, with a mean age of 25.7 ± 2.2 years and a mean BMI of 21.0 ± 1.9 kg/m2, were recruited. One volunteer dropped out due to intolerance to aspirin and the remaining volunteers completed the study (Table 1).
Platelet aggregation
In the first phase, patients randomized to receive indobufen had significantly less suppression of PLAA than those receiving aspirin 100 mg once daily (Fig. 2A). There were no significant differences in PLAA levels among the aspirin groups (Fig. 2A).
Baseline and 1-month PLAA, plasma TXB2, and 11-dh-TXB2 levels in subjects in the first phase study. Data are presented as median (25th, 75th percentile). A–C demonstrate alterations of PLAA, plasma TXB2, and 11-dh-TXB2 at 1 month after receiving the 7 different treatment regimens, respectively. PLAA = arachidonic acid-induced platelet aggregation; TXB2 = thromboxane B2; 11-dh-TXB2 = 11-dehydro-thromboxane B2; ASA = acetylsalicylic acid; INDO = indobufen; qd = once daily; qod = once every 2 days; q72h = once every 3 days; bid = twice daily. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the corresponding level of standard dose aspirin group
In the second phase, 19 volunteers underwent pharmacodynamic evaluation. At 2 h, 8 h, and 12 h after the last dose of indobufen and aspirin, PLAA levels were comparable (Fig. 3A).
Baseline and post-treatment PLAA, plasma TXB2, and 11-dh-TXB2 levels in subjects in the second phase study. Data are presented as median (25th, 75th percentile). A–C demonstrate alterations of PLAA, plasma TXB2, and 11-dh-TXB2 in healthy volunteers at 2 h, 8 h, and 12 h after receiving indobufen or aspirin respectively. PLAA = arachidonic acid-induced platelet aggregation; TXB2 = thromboxane B2; 11-dh-TXB2 = 11-dehydro- thromboxane B2; *P < 0.05, **P < 0.01, ***P < 0.001 compared with the corresponding level of indobufen. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding baseline level
Plasma TXB2
In the first phase, plasma TXB2 was less inhibited at 1 month in patients randomized to indobufen, aspirin 100 mg every 2 days, and 100 mg every 3 days compared with aspirin 100 mg once daily (Fig. 2B). There were no significant differences in plasma TXB2 levels in patients randomized to receive aspirin 50 mg twice daily, aspirin 75 mg once daily, and aspirin 50 mg once daily compared with aspirin 100 mg once daily (Fig. 2B).
In the second phase, 19 volunteers underwent plasma TXB2 measurement. The plasma TXB2 levels were comparable at 2 h after taking the last dose of indobufen and aspirin (Fig. 3B). However, the plasma TXB2 levels at 8 h and 12 h after the last dose of indobufen were significantly higher than the corresponding results for aspirin (Fig. 3B).
Urinary 11-dehydro TXB2
In the first phase, 167 patients underwent the 1-month urinary 11-dh-TXB2 test. There were no significant differences in 1-month urinary 11-dh-TXB2 levels among the 7 groups (Fig. 2C).
In the second phase, 19 volunteers underwent the urinary 11-dh-TXB2 test. There were no significant differences comparing urinary 11-dh-TXB2 levels at 2 h, 8 h, and 12 h after taking the last dose of indobufen and aspirin (Fig. 3C).
Clinical outcomes
In the first phase, there was 1 major bleeding incidence in the indobufen group while no major bleeding in any of the aspirin groups. There were 6 minor bleeding incidence and 7 adverse gastrointestinal events without any significant differences between the groups (Table 2).
Discussion
Our study indicates that compared with aspirin 100 mg once daily, aspirin doses of 50 mg or 75 mg once daily and 50 mg twice daily produced similar platelet inhibition, whereas aspirin given less often than once daily and indobufen were less effective. Although our study was not powered for clinical outcomes, this pharmacodynamic data suggests that aspirin given every second or third day, or indobufen given 100 mg twice a day cannot be the alternate therapy for standard dose aspirin in cardiovascular disease prevention.
Aspirin and indobufen produce their antiplatelet effect by acetylating platelet COX-1, thereby inhibiting thromboxane A2 (TXA2) synthesis [16]. Thus, their antiplatelet efficacy can be preferentially assessed via the indirect effect of TXA2-induced platelet aggregation by adding arachidonic acid to blood samples [17]. TXB2 is the hydration product of biologically active TXA2 [18]. Plasma TXB2 reflects the plasma TXA2 level produced by the platelets after being induced by AA during the PLAA testing. In vivo, TXA2 is rapidly converted into the more stable and inert metabolite TXB2, which is further metabolized to 11-dh-TXB2, the major product found in urine. Measurement of urinary 11-dh-TXB2 can facilitate an indirect assessment of the capacity of platelets to form TXA2 [19].
Fan et al. reported that aspirin resistance, evaluated by PLAA, was associated with an increased risk of adverse clinical events [20, 21]. Eikelboom et al. reported that urinary thromboxane concentration had predictive value for major adverse cardiovascular events in patients with cardiovascular diseases [16]. However, while using PLAA and urinary thromboxane measurements to evaluate the platelet-inhibitory effects of aspirin and indobufen, we found fewer differences than with plasma TXB2. This is not surprising because plasma thromboxane is an extremely sensitive measure of the effects of aspirin and indobufen that directly reflects the effects of these agents on their platelet target [14]. Although both PLAA and urinary thromboxane are inhibited by aspirin and indobufen, neither are specific measures of their effects on platelet COX-1, and urinary thromboxane has previously been shown to correlate poorly with other measures of the anti-platelet effects of aspirin [22]. We have previously reported that PLAA remains fully inhibited on days 1 and 2 after stopping aspirin, whereas plasma TXB2 recovers in a linear fashion immediately after stopping aspirin [14]. Consistent with these findings, we show here that PLAA and urinary thromboxane remain fully suppressed for at least 2 days in patients receiving reduced aspirin every second or third day, whereas there is partial recovery of plasma TXB2 levels between doses.
The gastrointestinal side effects of aspirin are dose-dependent [8], and indobufen’s reversible inhibition of COX-1 could potentially explain why it is better tolerated than aspirin. Because the lower doses of aspirin tested in our study were as effective as standard dose in inhibiting platelet function, lowering the dose might be a suitable option in patients with gastrointestinal intolerance. However, the use of indobufen or aspirin once in 2 or 3 days in these patients could compromise efficacy even though it reduces gastrointestinal side effects.
In the first phase study, blood and urine samplings were performed at an average of 12.27 ± 1.24 h after taking indobufen, and it is possible that delayed blood sampling might explain the attenuated antiplatelet effect of twice daily indobufen. Accordingly, we further explored the timing of offset of indobufen’s platelet-inhibitory effect and showed in the second phase of our study that recovery of plasma thromboxane had already begun at 8 h post-dose, which is also consistent with what has been previously reported [23].
Our study has potential limitations. First, we only tested a single indobufen dosing regimen in patients with coronary atherosclerosis, and we are uncertain whether a higher dose or more frequent dosing would maintain platelet inhibition while potentially reducing gastrointestinal side effects. Previous pharmacodynamic studies with high dose indobufen contradicted with each other. Cipollone et al. reported that, in patients with unstable angina, indobufen 200 mg twice daily provided all-day superior platelet inhibition compared with aspirin 320 mg daily by urinary 11-dh-TXB2 test [24]. However, Lee et al. found that indobufen 200 mg twice daily was inferior to aspirin 200 mg daily 12 h after withdrawal of the study drug by PLAA detection in healthy volunteers [23]. Further study is warranted to investigate the clinical efficacy or pharmacodynamic effect of high dose indobufen by multiple platelet function tests in patients with coronary atherosclerosis. Second, our study was not powered for clinical outcomes, and we cannot draw conclusions regarding the efficacy and safety of the treatment regimens under evaluation. However, in post hoc sample size calculations (based on means and standard deviations observed in our study), we have confirmed that our sample size provides at least 90% power to demonstrate a significant difference between standard dose aspirin and the other six groups (as measured by platelet aggregation testing) at a given time point. Third, there were numerical differences in the prevalence of age, diabetes, and the administering of proton pump inhibitor (PPI) across the indobufen and all aspirin groups during follow-up in the first phase study, which might impact on our results. However, none of the above difference reached statistically significant, and to the best of our knowledge, we have not found any adverse effects of PPI on the efficacy of aspirin or indobufen. Fourth, we tested aspirin from two different companies and cannot exclude differences in potencies of the two preparations. Fifth, we only included Chinese patients and volunteers in this study, and the results may not be generalizable to other populations. Finally, our study results may not be applicable to patients with a reduced response to aspirin, who were excluded from our study.
In conclusion, our results suggest that, in patients with coronary atherosclerosis, aspirin 50 mg twice daily, 75 mg once daily, and aspirin 50 mg once daily produce antiplatelet effects that are similar to aspirin 100 mg once daily. Aspirin given less often than once daily and indobufen 100 mg twice daily do not suppress platelets as effectively as aspirin 100 mg once daily, which should not be regularly administered for patients with coronary atherosclerosis.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B (2019) 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 140(11):e596–e646. https://doi.org/10.1161/CIR.0000000000000678
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Group ESCSD (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40(2):87–165. https://doi.org/10.1093/eurheartj/ehy394
Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ (2018) ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC Guideline Comparison. J Am Coll Cardiol 72 (23 Pt A): 2915–2931. https://doi.org/10.1016/j.jacc.2018.09.057
Binderup HG, Houlind K, Brasen CL, Madsen JS (2019) Identification of aspirin resistance using a PDW-miR92a-score: Validation in an intermittent claudication cohort. Clin Biochem 64:30–36. https://doi.org/10.1016/j.clinbiochem.2018.12.009
Schrör K (1997) Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost 23(4):349–356. https://doi.org/10.1055/s-2007-996108
Group ASC, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J (2018) Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 379(16):1529–1539. https://doi.org/10.1056/NEJMoa1804988
Laheij RJ, Jansen JB, Verbeek AL, Verheugt FW (2001) Helicobacter pylori infection as a risk factor for gastrointestinal symptoms in patients using aspirin to prevent ischaemic heart disease. Aliment Pharmacol Ther 15(7):1055–1059. https://doi.org/10.1046/j.1365-2036.2001.01016.x
Devillier P (2001) [Pharmacology of non-steroidal anti-inflammatory drugs and ENT pathology]. Presse medicale (Paris, France : 1983) 30(39-40 Pt 2):70–79
Liu J, Xu D, Xia N, Hou K, Chen S, Wang Y, Li Y (2018) Anticoagulant activities of indobufen, an antiplatelet drug. Molecules (Basel, Switzerland) 23(6):1452. https://doi.org/10.3390/molecules23061452
Barillà F, Pulcinelli FM, Mangieri E, Torromeo C, Tanzilli G, Dominici T, Pellicano M, Paravati V, Acconcia MC, Gaudio C (2013) Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets 24(3):183–188. https://doi.org/10.3109/09537104.2012.686072
Marzo A, Crestani S, Fumagalli I, Giusti A, Lowenthal DT (2004) Endoscopic evaluation of the effects of indobufen and aspirin in healthy volunteers. Am J Ther 11(2):98–102. https://doi.org/10.1097/00045391-200403000-00004
Task Force on Chinese Guidelines for the Prevention of Cardiovascular D, Editorial Board of Chinese Journal of C, (2018) [Chinese guidelines for the prevention of cardiovascular diseases(2017)]. Zhonghua Xin Xue Guan Bing Za Zhi 46(1):10–25. https://doi.org/10.3760/cma.j.issn.0253-3758.2018.01.004
Saw J, Madsen EH, Chan S, Maurer-Spurej E (2008) The ELAPSE (evaluation of long-term clopidogrel antiplatelet and systemic anti-inflammatory effects) study. J Am Coll Cardiol 52(23):1826–1833. https://doi.org/10.1016/j.jacc.2008.08.047
Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW (2012) Reversal of the anti-platelet effects of aspirin and clopidogrel. J Thromb Haemost 10(4):521–528. https://doi.org/10.1111/j.1538-7836.2012.04641.x
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123(23):2736–2747. https://doi.org/10.1161/circulationaha.110.009449
Eikelboom J, Hirsh J, Weitz J, Johnston M, Yi Q, Yusuf S (2002) Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105(14):1650–1655. https://doi.org/10.1161/01.cir.0000013777.21160.07
Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, Cataldo G, Milanesi G, Lavezzari M, Pamparana F, Coccheri S (1997) Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (Studio Italiano Fibrillazione Atriale) Investigators. Stroke 28(5):1015-1021. https://doi.org/10.1161/01.str.28.5.1015
Catella F, Healy D, Lawson J, FitzGerald G (1986) 11-Dehydrothromboxane B2: a quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci USA 83(16):5861–5865. https://doi.org/10.1073/pnas.83.16.5861
Harrison P, Frelinger A, Furman M, Michelson A (2007) Measuring antiplatelet drug effects in the laboratory. Thromb Res 120(3):323–336. https://doi.org/10.1016/j.thromres.2006.11.012
Liu T, Zhang J, Chen Y, Feng X, Wang L, Liu M (2015) ASSA14-03-38 Comparison of urinary11-dehydrothromboxaneB2 detection and plasma LTA assay for evaluating aspirin response in the Aged Patients with Coronary Artery Disease. Heart 101(Suppl 1):A22–A22. https://doi.org/10.1136/heartjnl-2014-307109.56
Fan L, Cao J, Liu L, Li X, Hu G, Hu Y, Zhu B (2013) Frequency, risk factors, prognosis, and genetic polymorphism of the cyclooxygenase-1 gene for aspirin resistance in elderly Chinese patients with cardiovascular disease. Gerontology 59(2):122–131. https://doi.org/10.1159/000342489
Pastori D, Nocella C, Farcomeni A, Bartimoccia S, Santulli M, Vasaturo F, Carnevale R, Menichelli D, Violi F, Pignatelli P (2017) Relationship of PCSK9 and urinary thromboxane excretion to cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol 70(12):1455–1462. https://doi.org/10.1016/j.jacc.2017.07.743
Lee JY, Sung KC, Choi HI (2016) Comparison of aspirin and indobufen in healthy volunteers. Platelets 27(2):105–109. https://doi.org/10.3109/09537104.2015.1042853
Cipollone F, Patrignani P, Greco A, Panara M, Padovano R, Cuccurullo F, Patrono C, Rebuzzi A, Liuzzo G, Quaranta G, Maseri A (1997) Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation 96(4):1109–1116. https://doi.org/10.1161/01.cir.96.4.1109
Funding
This work was supported by grants from the Jiangsu Province’s Key Provincial Talents Program (ZDRCA2016013), the Second Level of 333 High Level Talent Training Project in Jiangsu Province (BRA2019099), the Nanjing Science and Technology Development Plan 2016 (201605071), the Special Fund for Key R & D Plans (Social Development) of Jiangsu Province (BE2019754), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD).
Author information
Authors and Affiliations
Contributions
Mingwen Yang, Lianlian Mei, and Chunjian Li designed the study. Mingwen Yang, Lianlian Mei, Zekang Ye, and Inam Ullah performed experiments. Chuchu Tan, Guoyu Wang, Qian Gu, Yi Lu, and Jianling Bai analyzed the data. Samee Abdus, Lu Shi, and Xiaoxuan Gong discussed the results. Mingwen Yang wrote the original manuscript. John W Eikelboom and Chunjian Li reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Approval Number2017-SR-226).
Consent to participate
All enrolled subjects signed the informed consent form (ICF) before the study was conducted.
Consent for publication
All co-authors have reviewed and approved this manuscript for publication.
Conflict of interest
Dr. Chunjian Li received the donation of indobufen from Huadong Medicine Co., Ltd., China.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, M., Ye, Z., Mei, L. et al. Pharmacodynamic effects of indobufen compared with aspirin in patients with coronary atherosclerosis. Eur J Clin Pharmacol 77, 1815–1823 (2021). https://doi.org/10.1007/s00228-021-03177-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-021-03177-y