Abstract
Purpose
To describe the motivations, barriers, and sociodemographic characteristics of healthy Chinese volunteers in phase I research and to demonstrate the factors influencing their willingness to participate in subsequent trials.
Methods
Healthy subjects who participated in seven phase I trials at two centres were invited to participate in the cross-sectional survey at discharge by anonymously and voluntarily completing the self-administered questionnaire.
Results
From 442 subjects asked to complete the questionnaire, a response rate of 94.8% (419) was obtained, and 72.8% of the respondents had participated in a mean of 2.0 ± 1.3 previous studies. Over 90% of the subjects indicated that the main motivations to participate trials were to help more people, to contribute to scientific research, and to obtain money. The top 5 barriers were time inconvenience, advertisement sources, potential risks associated with the drug, privacy, and the route of drug administration. Nearly half (49.6%) of the subjects were willing to participate in the next trial. The factors impacting the willingness of the subjects to participate in subsequent trials were gender, screening frequency, enrolment frequency, level of understanding of the research, two motivating factors (to make money and receive a free check-up), and ten barriers (e.g. risk, distance, living conditions, and trust).
Conclusions
The majority of healthy Chinese subjects were young, were less well educated, had low income levels, and had poor medical insurance coverage. Given the multiple sources of motivation and complex barriers to trial participation, investigators and recruitment staff should consider ethics aspects to guarantee volunteer safety and well-being.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A goal of human clinical trials is to develop new, safe, and effective therapies that have been rigorously tested [1]. This process also involves scientific, ethical, and commercial interests, which are interrelated. Clinical research subjects who suffer from diseases are often motivated to obtain possible therapeutic benefits and free medical treatment or to better understand the disease that afflicts them [2, 3]. However, unlike patients, healthy volunteers (please note that the terms ‘volunteers’, ‘participants’, and ‘subjects’ are used interchangeably throughout this article) are exposed to risk and discomfort without any expectation of health benefits from drug developmental trials and other experiments (the exception may be some novel vaccine trials in which healthy subjects may benefit medically from a publicly unavailable vaccine) [4]. Phase I trials typically rely on healthy volunteers to evaluate the safety and tolerability of investigational or preclinical medicinal products [5] and provide an opportunity for the general public to contribute to the advancement of medicine [5]. In addition to first-in-human trials, in practice, phase I studies also include healthy subjects in trials conducted later in the drug development process to evaluate food effects, drug-drug interactions, mass balance, bioequivalence between two formulations containing the same active ingredient, and so on. Under these conditions, healthy volunteers are essential to the development of new drugs and are invaluable for investigating drug safety, dosing, and pharmacokinetics in phase I clinical trials, especially first-in-human trials [5]. To ensure that the findings of clinical trials are meaningful and generalizable, they must enrol and retain a diverse and representative group of study participants [1].
Therefore, for recruiters and researchers, it is crucial to understand the subject-level factors that impact the participation of individuals in clinical studies [1]. Friesen [1] reported that participants’ research knowledge, motivations, awareness of risks and benefits of research, and previous trial involvement all provide information that can be used to improve subject recruitment. Subjects’ decisions to participate in some trials are often multifactorial and can be impacted by their age, cultural norms, motivations, and other internal and external factors.
In a literature review, Stuckel [6] showed that financial benefit was the primary motivation among many other reported motivating factors, including contributing to science or the health of others, free medical care, scientific interest, and curiosity. Volunteers also weigh a range of concerns when making a decision about participation, such as study goals, risks, time commitment, and inconvenience.
Over the past decade in Asian countries, the number of clinical trials has increased rapidly due to the importance of the drug market, the evidence that dosing is dependent on ethnicity, and the need to evaluate cost-effectiveness in clinical trials [7].
China, the largest developing country according to population, has a high demand for drugs for a diverse array of conditions and a large number of patients. Currently, the per capita income is lower in China than in developed countries, and the level of reimbursement for clinical trial subjects is relatively low. This has attracted an increasing number of international commercial companies, who are now conducting clinical trials in China, increasing the potential of the market. The number of clinical trials increased from 1685 in 2017 to 2756 in 2019 [8]. With the increasing number of new clinical research staff, the potential risk of inadequate regulatory oversight of research activities can reduce the power and quality of the trials, threaten the safety of the subjects, and reduce the external validity of the findings [9,10,11].
In addition, China is the largest Eastern country with a traditional culture that is thousands of years old; Chinese culture values diligence and hard work. The general public is sensitive regarding the concept of being a ‘guinea pig’ (which has a negative connotation) in an experiment, and the participants are often ignored or shamed. Therefore, it is challenging to recruit and retain sufficient participants, especially as the number of clinical trials increases. Nearly 8 years ago, Zhang stated that the occupational backgrounds of Chinese subjects were complex and that their education levels varied. College students, medical staff, and unemployed persons were the main groups of health volunteers in phase I clinical trials [12].
When conducting clinical trials, protecting the subjects and being culturally sensitive are the most important issues. Currently, many people from sponsor organizations or institutions lack an understanding of the local cultural background, behaviours, habits, and physiological characteristics of Chinese clinical trial volunteers. To some extent, this lack of understanding influences the subjects’ own understanding of the clinical trials, which can profoundly influence the success of trial recruitment and execution [13, 14].
In foreign countries, some studies [1, 4, 6, 9, 15,16,17,18,19,20,21] about the motivation or willingness of healthy volunteers to participate in phase I trials have been conducted, but only a few [22,23,24] have explored the barriers to participation, and a few other studies [22, 24, 25] have explored differences in motivation, barriers, and willingness among volunteers from different backgrounds. In China, there have been few studies about the motivations of and barriers experienced by healthy volunteers with regard to enrolling in phase I studies.
To successfully conduct phase I clinical trials in China, it is important to identify the factors that impact individuals’ willingness to participate in these studies and develop effective recruitment and retention strategies (for example, amending the design of the protocol, informed consent form, and advertisement) to target the healthy subjects needed for these phase I trials.
This study was performed to address these concerns. The primary aims were (1) to analyse subjects’ demographic information, their previous experience, and their willingness to participate in subsequent studies; (2) to measure volunteers’ knowledge of clinical trials; (3) to investigate volunteers’ motivations and the barriers they encounter; and (4) to compare the responses among ages, races, education levels, and so on.
Methods
Study design
This was a cross-sectional, descriptive survey using a self-administered questionnaire about the motivations and barriers of healthy volunteers participating in phase I clinical trials. To increase the generalizability of our results, we identified seven phase I trials of different types and with different characteristics by purposive sampling in two phase I clinical trial centres from March 2016 to Dec 2019, including pre-test trials. Each potential subject was informed of this investigation during the last discharge visit of the trial in which he/she was participating. The seven phase I trials were diverse and ranged across therapeutic areas, investigating drugs for cholesterol, diabetes, autoimmune diseases, tumours, and multiple myeloma. These trials also varied in purpose, design, and process and included pharmacokinetics trials, bioequivalence trials, mass balance trials, and first-in-human trials. The drugs were administered orally or intravenously with single or multiple doses. The drug types included chemical drugs and biosimilar drugs. All subjects were 18 years of age or older and had a body mass index (BMI) between 19.0 and 28.0 kg/m2; male and female subjects were included. All enrolled subjects were recruited by companies independent of the hospital institutions and sponsors, screened through a subject database to exclude subjects who had participated in another trial within the last 3 months, randomized into a trial group, administered drugs at least once, and asked to complete the questionnaire after providing informed consent. Any subjects who were participating in more than one of the seven trials were enrolled and completed the questionnaire only once.
Survey instrument
The survey instrument was developed by the authors through a stepwise process that included (a) a comprehensive literature review, (b) the generation of a draft survey instrument, (c) subject interviews, (d) revisions, (e) a pre-test with 48 healthy subjects participating in phase I clinical studies at one centre, and (f) development of the final version.
The questionnaire comprised a section with an informed consent form with the contact details of the research team on the front page, some items about general demographics and previous experience, and a section related to motivations and barriers with questions designed to assess subjects’ motivations and the barriers they experienced with regard to participating in clinical studies.
The survey questions covered five domains: (a) sociodemographic characteristics, including age, gender, race/ethnicity, education, employment, monthly income, location of residence, and medical insurance; (b) previous research experience; (c) knowledge pertaining to the clinical trials; (d) motivations; and (e) barriers influencing enrolment decisions. A dichotomous response with the options of yes or no was used in the knowledge, motivation, and barrier sections.
The aim of the research was to elucidate the motivations of and barriers experienced by healthy participants, enabling clinical trial agencies to better meet the needs of healthy volunteers. The questionnaires were administered and distributed without collecting identifying information and were returned to the investigator onsite in the last stage of the trial, and the participants were reassured of the confidentiality and anonymity of their participation.
Statistical analysis
Data were processed in an Excel database and examined for accuracy by two researchers. Data were analysed with SPSS (version 16.0). Internal consistency estimates were evaluated with Cronbach’s α to ensure reliability. The overall Cronbach’s α for the motivations and barriers from the pre-test (n = 48) was 0.944 (motivation 0.733 versus barrier 0.959) and that for all data was 0.920 (motivation 0.816 versus barrier 0.943). Frequency distributions and simple descriptive statistics (means and standard deviations) are used to describe the subjects’ general characteristics, previous experience, knowledge, motivations, and barriers. A chi-square test was conducted to determine the difference in the categorical variable (willingness to participate in future trials), and t tests and analysis of variance (ANOVA) were used to compare the mean values of variables (age, number of previous studies, and knowledge). Missing data were excluded from the analysis of some variables. Specifically, for each motivation and barrier variable, we looked for differences based on age, gender, ethnicity, education background, employment status, and number of clinical trials in which the participant had been enrolled. Given the statistical power and sample size, binary variables were created for some categorical and ordinal data. The results were considered statistically significant at a P value < 0.05.
Results
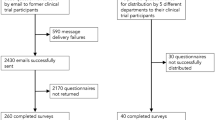
Among the 452 subjects of seven trial projects, 442 were prospective participants, and 10 participated in two of the seven projects. A total of 419 subjects agreed to complete the questionnaires, yielding a response rate of 94.8%, while 13 declined, with time inconvenience given as the most common reason. Some participants did not answer all of the questions, but the questionnaires were still considered valid as they contained enough information for some variables.
The general characteristics of the subjects are presented in Table 1, and their research experience is presented in Table 2. Their knowledge of clinical trials is presented in Table 3, and their motivations and barriers are presented in Tables 4 and 5. The relations between willingness to participate in subsequent trials and the motivation and barriers are shown in Tables 6 and 7.
Sociodemographic/general information
The general sociodemographic characteristics of the participants are listed in Table 1. The mean age of the participants was 28.5 ± 5.6 years. Participants were predominantly male (64%). The participants were predominantly of Han ethnicity (93.9%), and the proportion of participants who were of Han ethnicity was higher than that in the 2010 National Census (91.51%) [26]. The majority of subjects were single (63.2%) and did not have children (65.8%), and approximately two-thirds had less than a high school education. Only 10% of the participants were students. Almost two-thirds reported a monthly income equivalent to or less than RMB ¥5000, and 93.9% reported an annual income less than or equivalent to RMB ¥8000. Most respondents (97.2%) reported having an excellent or good health status.
Research experience
Regarding the participants’ research experience (Table 2), six subjects reported being screened for and enrolled in N trials, which indicated that the number was too numerous to recall. This finding was similar to that in the USA (80 enrolment events) [27]. These individuals are usually called professional subjects, as they make a living by participating in trials. Except for these six participants (N times), the number of enrolments ranged from 0 to 12. Approximately 72.8% of the participants reported previous clinical research enrolment experience, with a mean of 2.0 ± 1.3 previous studies. Only 21.5% of the participants had had only one experience with clinical trial screening and enrolment. Nearly half (43.4%) of the participants had been administered drug after enrolment more than twice. However, when the subjects were divided into two groups according to the frequency of screening and enrolment experiences (> two and ≤ two), there were significant differences between the two groups with regard to age, race, job, and monthly income. Repeat participation was less likely in the subjects who were younger, minority, students, or had the lowest income.
With regard to the participants’ sources of information about the clinical trials, over half of the respondents reported responding to posters. If the volunteers were interested, 45.4% chose friends as the people with whom they would discuss the decision with, and 31.8% consulted relatives before participating in one trial. After trial project participation, 49.6% of the subjects reported they would like to take part in the study again, while 45.4% said ‘maybe’ and 4.9% said ‘no’. Nearly all subjects (98.5%) expressed trust in the doctors during their trials.
Knowledge of clinical trials
Seven survey items examined the participants’ overall knowledge of clinical trials, with all participants obtaining high percentages (over 90%). Nearly all respondents correctly understood ‘I can participate in the trial voluntarily’ (99.5% (412/414)) and ‘Screening occurs after the informed consent form is signed’ (98.5% (407/413)). The majority of the subjects (over 385) were also aware that the objective of the clinical trials was to provide better medical service for patients (96.5%) and that they could withdraw from a trial without cause at any time (93.9%); most participants also correctly answered ‘what is clinical trial?’ (93.0%). Of all the participants, 373 understood they were supposed to participate in trials that had been approved by the Institutional Review Board with regard to aspects of scientific and ethical conditions to ensure their safety (91.2%) and that each new drug should be investigated in the human body before it enters the market (90.5%).
When the overall score for knowledge of clinical trials was seven in total, the mean score was 6.5 ± 1.0 among the participants, and subjects who were younger, of Han ethnicity, and non-labourers were more likely to have a higher score (Table 3).
Study participant motivations
Eight items were answered as ‘yes’ or ‘no’ by volunteers in this section. The top three most common motivations (over 90%) were to help more people, contribute to scientific research, and obtain money (Table 4). Other motivations included curiosity (86.7%), receiving a free check-up (84.4%), and free living quarters and food (68.7%). Two motivations with response rates lower than 50% were being financially desperate for money (44.3%) and needing a job to make money (34.3%).
The analysis of differences in general characteristics showed that labourers, compared with non-labourers, were more motivated by being desperate for money and were less motivated by contributing to scientific research. Minority groups were more likely to be motivated by being desperate for money than non-minority groups, and subjects without children were more likely to be motivated by curiosity than subjects with children.
In this study, younger volunteers and individuals with low income levels were more likely to be motivated by curiosity than their counterparts. The reports of other motivations, including monetary incentives, were not significantly different among healthy volunteers in different groups. Regarding the frequency of participation, motivation was not significantly different between volunteers who had participated in more than two trials and those who had participated in two or fewer trials. Some reports [16, 21] listed financial reward as a primary motivation for repeat volunteers.
Barriers influencing enrolment decisions
Twenty barriers were selected by subjects, and the top choice was time inconvenience (50%). The next four choices (over 45%) were having a lack of recruitment information, perceiving potential risks of the drug, fearing their participation would be known by relatives and friends, and administrating drugs by the intravenous route. The factor of poor living conditions is only selected by 21.7%.
After analysing the influence of different characteristics, 12 barriers were found to be significantly different among various groups of participants (Table 5). The subjects of Han ethnicity and those who had no children were less likely to participate due to time inconvenience. The potential risks associated with the drug were more likely to impede the participation of subjects who were single or divorced, had children, and had local medical insurance. Only labourers were more likely to worry about their participation being known by relatives and friends, to feel that the money was dishonourable, and to consider inconvenient transportation and expensive fares in the context of trial participation. The subjects with children were less likely to participate because of the intravenous route of drug administration. Male subjects were less likely to choose to participate in trial projects with long trial periods, long stays, many visits, and conflicts with work. If multiple blood draws were required and the participants were located a long distance from the institution, students were less likely to be involved in the trial project. Lack of trust in the source of information was more likely to be the primary barrier for minorities and individuals with local medical insurance. Regarding trust in the medical institution, individuals with a younger age, lower income, and local medical insurance were likely to have a negative attitude towards participation. Ethnic minority individuals were more likely to doubt the authority of the recruitment staff. The living conditions were more likely to be the barrier for subjects who were male, married, or had children.
Willingness to participate in subsequent trials
After participation in the current trial, only 49.6% of the participants were willing to participate in the next trial. Regarding the factors affecting their willingness, subjects who were male, had undergone screening and enrolment more than twice, had a high score on trial knowledge, viewed trial participation as a job, and wanted a free check-up were more likely to participate in a subsequent trial (Table 6). Moreover, factors such as potential risks associated with the drug, the intravenous route of drug administration, living a long distance from the institution, poor living conditions, lack of trust in the medical institution, doubt regarding the authority of the recruitment staff, mistrust regarding the information sources, perception of the money obtained by participation as dishonourable, unsatisfactory responses to questions, and innate uncertainty were considered barriers (Table 7).
Discussion
With 419 volunteers from seven different trials, this is currently the largest retrospective study in China to investigate the sociodemographic characteristics, willingness to participate in subsequent trials, experience, knowledge, motivations, and barriers among healthy adult volunteers participating in phase I studies. Understanding these factors is vital to protect human subjects and facilitate better recruitment and retention in clinical trials. To date, there has been only one study that was conducted with 100 healthy volunteers involved in phase I clinical studies to assess clinical research and related factors in China [28]. The results of the two studies showed a positive attitude towards medical research in the context of the scientific contributions [28]. The current study provides insight into the general characteristics, willingness, experience, knowledge, motivations, barriers, and factors related to participation in phase I clinical research; the results of this study differed from those reported in studies performed in other counties, perhaps due to cultural differences. Some studies [1, 4, 6, 9, 15,16,17,18,19,20,21] conducted in other countries have evaluated the motivations, willingness, and views of healthy volunteers in phase I studies with regard to their participation in research.
In our study, the male to female ratio was approximately 2:1, which may be a result of the protocol requirements. Most protocols require a higher percentage of healthy male volunteers in phase I clinical studies. The composition of our sample is similar to that in a study in South Korea, with the majority of participants being men (64% and 70.2%, respectively), and the mean age was also similar (28.5 ± 5.6 years and 26.6 ± 6.8 years, respectively) [7]; however, a study conducted in the USA enrolled a greater proportion of men (87.6%), and the participants were older (35.7 ± 9.8 years) than those in our study [17]. This finding indicates that these healthy subjects are of reproductive age, but all these trials require volunteers to use contraception for at least 1 month and up to 1 year. Therefore, in the recruitment and informed consent phase, sufficient explanation, frequent reminders from the hospital, and the timely management of pregnancies are essential. China is a multi-ethnic country with 56 ethnicities, and this study showed that participants were predominantly of Han ethnicity (93.9%), accounting for a higher proportion than in the 2010 National Census (91.51%) [26]. In our study, we found that 58.6% of our participants had received less than a senior high school education. This was a higher proportion than that in the report by Grady from Belgium, Singapore, and the USA (42.0%, 30.7%, and 32.9%, respectively) [17].
Many healthy volunteers in our cohort had a low income level and were unemployed. Approximately one-third of the participants reported individual incomes of more than RMB ¥60,000/year, which was much lower than the income level of healthy volunteers in developed countries, nearly half of whom had a household income over USD$25,000/year (the exchange rate between US dollars and RMB is 1 to 7) [17], but higher than the national average in 2019 (approximately RMB ¥30,733 annually) [29]. This may indicate that the subjects were mostly working in large cities where most phase I trial centres are located.
A total of 42.8% of the healthy volunteers had no permanent job in our study. This finding is relatively similar to the 44% in Singapore, higher than the 34.8% in Belgium, and lower than the 72.5% in the USA [17]. The proportion of volunteers who were students was 10.8%, which is similar to the proportions in Belgium, Singapore, and the USA in 2017 (12.0%, 17.0%, and 12.4%, respectively) but much lower than that in Portugal (over 50% in 2007 and 2008) [30,31,32,33]. In the case report form (CRF) for these trials, job characteristics, which may affect drug metabolism, are usually asked about and recorded by the research staff. This study showed that 82.8% of subjects were not manual labourers. Perhaps due to the limitations imposed by income and job, 31.3% of our subjects lacked medical insurance, which is similar to the percentages in Belgium, Singapore, and the USA (29.7%, 38.1%, and 33.0%, respectively) [17]. Our study showed that 72.8% of the participants with previous clinical research experience had participated in a mean of 2.0 previous studies, which was less than the number (4.6) reported by Grady [17]. A total of 21.5% of the subjects had no experience with screening or drug administration before this trial. Over half of the subjects had been involved in one or two screenings (56.1%) and drug administrations (55.1%), which is less than proportions reported in Korea (87.6%) [7]. This indicates that the internal environment of the majority of Chinese subjects has not been exposed to many experimental drugs.
Before making a decision about participating in a clinical trial, volunteers often ask for someone else’s opinion. Friends were consulted by 45.4% of the subjects, and relatives were consulted by 31.8%. This finding is different from the proportions in a study in Portugal [30,31,32,33], in which more participants consulted with family members/partners (53%) and fewer consulted with friends (40%). These findings show that relatives and friends are influential with regard to the participation of healthy volunteers in clinical trials [7].
A previous study showed that money was the primary motivation for most phase I healthy volunteers [6]. In our study, the subjects reported that their primary motivation for participation was altruistic (to contribute to scientific research and help others), followed by the desire to receive free medical care and financial incentives, which is similar to the findings of a previous study [6]. We did not find any impacts of age, gender, income, education, or medical insurance on the motivations (including money) to participate in phase I research. We further explored motivation in regard to financial incentives. Subjects who were desperate for money (44.3%) and who participated in research as a job to make money (34.3%) were concerned with ethics protections and informed consent. Some studies also reported that providing monetary compensation to subjects could result in coercion of those in need [19, 34, 35]. However, it seems only fair to compensate healthy research subjects because they gain no other benefit from their participation [34, 36]. From a research perspective, it appears that without monetary compensation, it would not be possible to recruit a sufficient number of healthy volunteers to fill the growing demand [10, 16, 21, 33, 37, 38]. This practice can raise ethics concerns because the vulnerable characteristics of economically disadvantaged volunteers may be exploited [9, 39]. It has become more common for healthy volunteers participating in phase I clinical trials to earn at least part of their living that way [40,41,42,43].
Some individuals pursue research participation as a full-time job and travel across the country to enrol in studies [27]. These subjects, called professional research participants, have developed their own social networks [27]. In our study, the 34.3% of the subjects whose participation was motivated by the need for a job to make money are likely to become professional research participants in the future. They should receive sufficient attention during recruitment and retention [7]. Researchers must use a clear and honest approach to explain the risks associated with trials to volunteers when obtaining informed consent and during the subsequent processes [7]. In different drug clinical trials, due to the differences among individuals, some individuals may metabolize or eliminate drugs more slowly than others. If the next trial in which they are participating overlaps with the current trial, adverse drug reactions are possible [27]. There is an ethical responsibility to protect these participants from the risks of overlapping trial enrolment, even if they make false statements about their prior study participation [27]. As in other countries [27], the clinical research subject database system in China can identify whether subjects have participated in another trial during a certain time period that is set by investigators to avoid frequent participation in trials.
Despite these motivations, the majority of participants faced several barriers that influenced their enrolment decisions. Compared to the motivations, the overall percentages of participants who reported barriers were much lower. The most common barrier was time inconvenience, which was reported by 50%. Time, as the most common barrier, in addition to risk and the complexity of trials, is an important consideration for healthy volunteers when they are making their decisions about enrolment [44]. Many volunteers reported risk as an important factor, and some said there was an ‘absolute limit’ that was the ultimate deciding factor regarding the risk they were willing to accept [16].
Currently, in most phase I centres (such as our two centres) in China, healthy volunteers are recruited by another commercial company that is independent of the institution, and volunteers learn about the opportunity from posters, chat tools, and word-by-mouth. There is little information available from the government or the institution nor is there any specific website. Few specific staff are authorized to recruit participants by the principal investigators in phase I centres. This may result in a lack of trust in the source of the information and institution, especially among subjects with a limited understanding of hospitals. In our study, one-third of the subjects encountered this barrier, which may further influence their recruitment and subsequent involvement. In the future, advertisements that include the contact information of the investigators who are working through official channels would improve the confidence of the subjects and increase participation.
Confidentiality and privacy are important for the subjects who wish to avoid their relatives and friends learning that they are participating. In China, healthy volunteers involved in trials are called guinea pigs (which has a negative connotation) in public and private discussions and even in the news on the internet. Therefore, in the informed consent phase, emphasis should be placed on the ways in which the privacy of the subjects is safeguarded to increase their participation and retention.
Regarding barriers to participation in future research, with the exception of the potential risk associated with the drugs, the route of administration and the distance from the centre, other barriers could be modified by the institution and staff. In particular, the barrier of unsatisfactory responses could be addressed by ensuring appropriate and friendly interactions with volunteers on the part of the research staff. In this study, only 21.8% of the volunteers considered living conditions to be a participation barrier, but this issue had a significant impact on participation in subsequent studies (P = 0.001). In China, deficient medical resources result in overcrowding of hospitals and institutions, which makes the living conditions less comfortable. Therefore, from the perspective of recruitment, institution staff could try to design and decorate living conditions to resemble a home to attract and retain prospective subjects.
Conclusion
In this study, the majority of healthy subjects were relatively young, were less well educated, had low levels of income, and had poor medical insurance coverage. To guarantee the safety and well-being of this population, the design of the informed consent form, the information provided on advertisements, and the availability of insurance to cover trial-related medical expenses are vital ethical concerns for investigators. The main motivation is not solely monetary reward but also to contribute to science. However, several barriers, especially time and risk, may influence the ultimate decision regarding participation.
Limitations
Our study subjects were recruited from participants enrolled in trial and therefore may differ from the population of potential volunteers because the reasons for declining to participate after giving informed consent are unknown. Each motivation may be weighted differently for each participant. Perhaps asking subject to rank their motivation could provide more information about the most important motivation. The risk associated by the drug was discussed in further detail or ranked by the subjects. In future studies, participants could be asked to select and weigh the importance of motivations and barriers using a scoring system.
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
References
Friesen LR, Williams KB (2016) Attitudes and motivations regarding willingness to participate in dental clinical trials. Contemp Clin Trials Commun 2:85–90. https://doi.org/10.1016/j.conctc.2015.12.011
Agrawal M, Grady C, Fairclough DL, Meropol NJ, Maynard K, Emanuel EJ (2006) Patients’ decision-making process regarding participation in phase I oncology research. J Clin Oncol 24:4479–4484. https://doi.org/10.1200/JCO.2006.06.0269
Madsen SM, Mirza MR, Holm S, Hilsted KL, Kampmann K, Riis P (2002) Attitudes towards clinical research amongst participants and nonparticipants. J Intern Med 251:156–168. https://doi.org/10.1046/j.1365-2796.2002.00949.x
Manton KJ, Gauld CS, White KM, Griffin PM, Elliott SL (2019) Qualitative study investigating the underlying motivations of healthy participants in phase I clinical trials. BMJ Open 9:e24224. https://doi.org/10.1136/bmjopen-2018-024224
Fisher JA, McManus L, Wood MM, Cottingham MD, Kalbaugh JM, Monahan T, Walker RL (2018) Healthy volunteers’ perceptions of the benefits of their participation in phase I clinical trials. J Empir Res Hum Res 13:494–510. https://doi.org/10.1177/1556264618804962
Stunkel L, Grady C (2011) More than the money: a review of the literature examining healthy volunteer motivations. Contemp Clin Trials 32:342–352. https://doi.org/10.1016/j.cct.2010.12.003
Chu SH, Jeong SH, Kim EJ, Park MS, Park K, Nam M, Shim JY, Yoon Y (2012) The views of patients and healthy volunteers on participation in clinical trials: an exploratory survey study. Contemp Clin Trials 33:611–619. https://doi.org/10.1016/j.cct.2012.02.018
Center for drug evaluation,NMPA (2020) The total number registered yearly (CTR available). http://www.chinadrugtrials.org.cn/eap/clinicaltrials.informationstatistics. Accessed 6 Apr 2020
Tishler CL, Bartholomae S (2002) The recruitment of normal healthy volunteers: a review of the literature on the use of financial incentives. J Clin Pharmacol 42:365–375
Kass NE, Myers R, Fuchs EJ, Carson KA, Flexner C (2007) Balancing justice and autonomy in clinical research with healthy volunteers. Clin Pharmacol Ther 82:219–227. https://doi.org/10.1038/sj.clpt.6100192
Grady C (2005) Payment of clinical research subjects. J Clin Invest 115:1681–1687. https://doi.org/10.1172/JCI25694
Zhang ZF, Shen YH, Li ZQ (2012) The issue in recruitment and management of subjects in phase I clinical trials. Chin J Clin Pharmacol Ther 17:481–484
Ulrich CM, James JL, Walker EM, Stine SH, Gore E, Prestidge B, Michalski J, Gwede CK, Chamberlain R, Bruner DW (2010) RTOG physician and research associate attitudes, beliefs and practices regarding clinical trials: implications for improving patient recruitment. Contemp Clin Trials 31:221–228. https://doi.org/10.1016/j.cct.2010.03.002
Cao YM, Cao Y, Xu Y, Dong J, Li LL, Gong T, Zhang YZ (2017) Prevalence survey of cognition of potential participants for clinical trials. Herald Med 36:226–230
Townsend A, Cox SM (2013) Accessing health services through the back door: a qualitative interview study investigating reasons why people participate in health research in Canada. Bmc Med Ethics 14:40. https://doi.org/10.1186/1472-6939-14-40
Hassar M, Pocelinko R, Weintraub M, Nelson D, Thomas G, Lasagna L (1977) Free-living volunteer’s motivations and attitudes toward pharmacologic studies in man. Clin Pharmacol Ther 21:515–519
Grady C, Bedarida G, Sinaii N, Gregorio MA, Emanuel EJ (2017) Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research. Clin Trials 14:526–536. https://doi.org/10.1177/1740774517722130
Nappo SA, Iafrate GB, Sanchez ZM (2013) Motives for participating in a clinical research trial: a pilot study in Brazil. BMC Public Health 13:19. https://doi.org/10.1186/1471-2458-13-19
Tishler CL, Bartholomae S (2003) Repeat participation among normal healthy research volunteers: professional guinea pigs in clinical trials? Perspect Biol Med 46:508–520. https://doi.org/10.1353/pbm.2003.0094
Luzurier Q, Damm C, Lion F, Daniel C, Pellerin L, Tavolacci M (2015) Strategy for recruitment and factors associated with motivation and satisfaction in a randomized trial with 210 healthy volunteers without financial compensation. BMC Med Res Methodol 15:2. https://doi.org/10.1186/1471-2288-15-2
Bigorra J, Baños JE (1990) Weight of financial reward in the decision by medical students and experienced healthy volunteers to participate in clinical trials. Eur J Clin Pharmacol 38:443–446. https://doi.org/10.1007/BF02336681
Mbunda T, Bakari M, Tarimo EAM, Sandstrom E, Kulane A (2014) Factors that influence the willingness of young adults in Dar es Salaam, Tanzania, to participate in phase I/II HIV vaccine trials. Glob Health Action 7:22853. https://doi.org/10.3402/gha.v7.22853
Fisher JA, McManus L, Cottingham MD, Kalbaugh JM, Wood MM, Monahan T, Walker RL (2018) Healthy volunteers’ perceptions of risk in US phase I clinical trials: a mixed-methods study. PLoS Med 15:e1002698. https://doi.org/10.1371/journal.pmed.1002698
Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E (2000) Public attitudes regarding willingness to participate in medical research studies. J Health Soc Policy 12:23–43. https://doi.org/10.1300/J045v12n02_02
Chen SC, Sinaii N, Bedarida G, Gregorio MA, Emanuel E, Grady C (2017) Phase 1 healthy volunteer willingness to participate and enrollment preferences. Clin Trials 14:537–546. https://doi.org/10.1177/1740774517722131
National Bureau Statistics of China (2011) Bulletin of main data from sixth national population survey in 2010,(No.1)http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm .Accessed 28 Apr 2011
Resnik DB, Koski G (2011) A national registry for healthy volunteers in phase 1 clinical trials. JAMA 305:1236–1237. https://doi.org/10.1001/jama.2011.354
Wei YD, Yang L, Zhang S, Guo JC, Li HY, Sun YM (2014) Healthy volunteers’ knowledge and perception of clinical research. Chin J Clin Pharmacol 30:828–829
National Bureau Statistics of China(2020) Statistical Communique of the 2019 National Economic and Social Development of the People’s Republic of China. http://www.stats.gov.cn/tjsj/zxfb/202002/t20200228_1728913.html. Accessed 28 Feb 2020
Almeida L, Coelho R, Albino-Teixeira A, Soares-da-Silva P (2008) Adverse non-drug-related complaints by healthy volunteers in phase I studies compared to the healthy general population. Int J Clin Pharmacol Ther 46:574–583. https://doi.org/10.5414/CPP46574
Almeida L, Kashdan TB, Coelho R, Albino-Teixeira A, Soares-da-Silva P (2008) Healthy subjects volunteering for phase I studies: influence of curiosity, exploratory tendencies and perceived self-efficacy. Int J Clin Pharmacol Ther 46:109–118. https://doi.org/10.5414/CPP46109
Almeida L, Kashdan TB, Nunes T, Coelho R, Albino-Teixeira A, Soares-da-Silva P (2008) Who volunteers for phase I clinical trials? Influences of anxiety, social anxiety and depressive symptoms on self-selection and the reporting of adverse events. Eur J Clin Pharmacol 64:575–582. https://doi.org/10.1007/s00228-008-0468-8
Almeida L, Azevedo B, Nunes T, Vaz-da-Silva M, Soares-da-Silva P (2007) Why healthy subjects volunteer for phase I studies and how they perceive their participation? Eur J Clin Pharmacol 63:1085–1094. https://doi.org/10.1007/s00228-007-0368-3
Lemmens T, Elliott C (1999) Guinea pigs on the payroll: the ethics of paying research subjects. Account Res 7:3–20. https://doi.org/10.1080/08989629908573939
Vere DW (1991) Payments to healthy volunteers--ethical problems. Br J Clin Pharmacol 32:141–142. https://doi.org/10.1111/j.1365-2125.1991.tb03872.x
Lemmens T, Elliott C (2001) Justice for the professional guinea pig. Am J Bioeth 1:51–53. https://doi.org/10.1162/152651601300169095
Czarny MJ, Kass NE, Flexner C, Carson KA, Myers RK, Fuchs EJ (2010) Payment to healthy volunteers in clinical research: the research subject’s perspective. Clin Pharmacol Ther 87:286–293. https://doi.org/10.1038/clpt.2009.222
Hermann R, Heger-Mahn D, Mahler M, Seibert-Grafe M, Klipping C, Breithaupt-Grogler K, de Mey C (1997) Adverse events and discomfort in studies on healthy subjects: the volunteer’s perspective. A survey conducted by the German Association for Applied Human Pharmacology. Eur J Clin Pharmacol 53:207–214. https://doi.org/10.1007/s002280050364
Viens AM (2001) Socio-economic status and inducement to participate. Am J Bioeth 1:1f–2f. https://doi.org/10.1162/152651601300169202
Resnik DB (2012) Limits on risks for healthy volunteers in biomedical research. Theor Med Bioeth 33:137-149. https://doi.org/10.1007/s11017-011-9201-1
Johnson RA, Rid A, Emanuel E, Wendler D (2015) Risks of phase I research with healthy participants: A systematic review. Clin Trials 13:149–160. https://doi.org/10.1177/1740774515602868
Gupta V, Kerry VB, Goosby E, Yates R (2015) Politics and universal health coverage — the post-2015 global health agenda. NEJM 373(13):1189–1192
van Gelderen CEM, Savelkoul TJF, van Dokkum W, Meulenbelt J (1993) Motives and perception of healthy volunteers who participate in experiments. Eur J Clin Pharmacol 45(1):15–19
Agoritsas T, Deom M, Perneger TV (2011) Study design attributes influenced patients’ willingness to participate in clinical research: a randomized vignette-based study. J Clin Epidemiol 64:107–115. https://doi.org/10.1016/j.jclinepi.2010.02.007
Acknowledgements
The authors thank the participants for their responses to the survey, the research staff at the Beijing Shijitan Hospital and the Aerospace Center Hospital for their invaluable support with regard to collecting and entering the data. The opinions expressed are those of the authors.
Funding
This research was funded by the Beijing Shijitan Hospital Funds (2014-C20), which helped pay for materials and training.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, especially Jin Wang and Gang Chen. Material preparation and data collection were performed by Xiaona Liu and Chen Liu, and the analysis was performed by Gang Chen and Qingkun Song. The first draft of the manuscript was written by Zejuan Wang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethics approval
This study was reviewed by the Ethics Committee of the Beijing Shijitan Hospital (Beijing, China) and approved as a non-interventional study (2014-C20).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Chen, G., Liu, X. et al. The motivations, barriers, and sociodemographic characteristics of healthy Chinese volunteers in phase I research. Eur J Clin Pharmacol 77, 557–568 (2021). https://doi.org/10.1007/s00228-020-03040-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-03040-6