Abstract
Purpose
To assess ceftriaxone population pharmacokinetics in a large pediatric population and describe the proper dose for establishing an optimized antibiotic regimen.
Methods
From pediatric patients using ceftriaxone, blood samples were obtained and the concentration was measured using high-performance liquid chromatography ultraviolet detection. The NONMEM software program was used for population pharmacokinetic analysis, for which data from 99 pediatric patients (2 to 12 years old) was collected and 175 blood concentrations were obtained.
Results
The best fit with the data was shown by the one-compartment model with first-order elimination. According to covariate analysis, weight had a significant impact on the clearance of ceftriaxone. Using Monte Carlo simulation, in a pediatric population with community-acquired pneumonia, a dose regimen of 100 mg/kg every 24 h produced satisfactory target attainment rates while remaining within the required minimum inhibitory concentration (2 mg/L).
Conclusion
Population pharmacokinetics of ceftriaxone was evaluated in children and an optimum dosing regimen was constructed on the basis of the pharmacokinetics-pharmacodynamics model-based approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In children, community-acquired pneumonia (CAP) is one reason for the immense rate of hospital admissions [1]. The occurrence of CAP infection is 5 to 11/1000 people/year in Europe and North America in adults [2]. In the USA, the average occurrence of CAP is about 2.5/1000 people/year in inpatient adults, in patients aged 65 to 79 years, the occurrence is 6.3/1000 people/year, and in patients 80 years old and older, the ratio of occurrence is as high as 16.4/1000 people/year [3]. According to research performed in 2013 in China, there are 16,585 CAP inpatient cases registered, among which high percentages are in patients ≤ 5 years (37.3%) and in individuals over 65 years old (28.7%). By comparison, only 9.2% of adults aged 26–45 years develop CAP. [4]. Pneumonia is a major cause of death in children less than 5 years old in China; among the 6.3 million pediatric patients who died, 14.8% are pneumonia cases in children under 5 years old [5].
Ceftriaxone belongs to 3rd-generation cephalosporins. In its chemical structure, as with other 3rd-generation members, there is substitution of the amino-thiazolyl group at the R1 position of the β-lactam ring. Ceftriaxone’s effectiveness against CAP has been confirmed by a wide range of clinical trials. It is effective against Haemophilus influenzae and Streptococcus pneumoniae at lower minimum inhibitory concentration (MIC) levels, and inhibits a wide range of pathogens in community-acquired pneumonia [6]. Ceftriaxone has a long half-life of about 6 to 8 h and a high ratio of protein binding (95%) [7]. The pharmacokinetic (PK) parameters of its unbound proportion (free drug) are linear and dose dependent [8, 9]. Its pharmacokinetics, based on the free concentration, is linear over the 150 to 1500 mg dose range [10]. The free drug plasma concentration increases in a disproportionate manner with increasing doses, so a once-a-day dosing regimen is appropriate [11]. The theoretical and physiological implications of these pharmacokinetic properties have been evaluated by McNamara et al. [12, 13].
Previously performed studies on ceftriaxone in this contest have a small number of patients, narrow age range, or less powerful PK analysis method, making it difficult to provide evidence-based dosing recommendation in children as summarized in Table 1 [14,15,16,17,18,19,20,21]. A recent systematic review also highlighted that there are insufficient evidences about the optimal dose of antimicrobials in children with CAP [22]. Ceftriaxone has high affinity for binding to plasma protein, and its pharmacokinetics varies among the children. This may lead to low microbiologic success [23]. Moreover, the recommended ceftriaxone dosage for children varies around the world as shown in Table 2 [23,24,25,26,27,28,29,30]. The current research work was performed to evaluate ceftriaxone population pharmacokinetics in Chinese children aged 2 to 12 years using modeling-simulation techniques to establish the optimum ceftriaxone dosage regimen for children with CAP.
Methodology
Design of the study
This was a prospective, open-label ceftriaxone pharmacokinetic study carried out at the Children’s Hospital of Hebei Province affiliated to Hebei Medical University, China. The inclusion criteria were as follows: pediatric patients (age 2 to 12 years) with confirmed or suspected community-acquired pneumonia, using ceftriaxone as part of their regular antibiotic regimen, and written parental consent for participation in the research work. The exclusion criteria were as follows: receiving other systemic trial drug therapy, using ceftriaxone not as a part of the regular antibiotic treatment, other factors that the principal investigator considered would cause patients to be unsuitable for inclusion. The ethics board of the Children’s Hospital of Hebei Province affiliated to Hebei Medical University approved this study.
Dosing regimen and pharmacokinetic sampling
Ceftriaxone (Rocephin, Roche Pharmaceuticals, Inc), as intravenous infusion, was administered either at a dose of 50–100 mg/kg once a day (QD) or two times a day (BID) over 30 min. A volume of 0.3 mL per blood sample was taken for PK analyses. For collecting pharmacokinetic samples, an opportunistic sampling design was selected [31]: samples were exclusively collected from blood remaining after routine biochemical and microbiological tests performed as part of patient clinical care. Infusion and sampling times were accurately noted. There was inclusion of samples with validated sampling information only. The samples of blood were obtained during routine clinical practice, then centrifuged at 2500×g at 4 °C for 10 min, and the samples of plasma were kept at − 70 °C.
Analytical method for ceftriaxone
For ceftriaxone, the analytical method was described previously [32]. Concisely, through high-performance liquid chromatography ultraviolet detection and as an internal standard using metronidazole, ceftriaxone concentration was measured. The calibration curve was 2.0–600.0 μg/mL. For controls, the intra-day and inter-day coefficients of variation (CV) were 4.5% and 2.4%, respectively. The lower limit of quantification was 2.0 μg/mL.
Ceftriaxone population pharmacokinetic modeling
For pharmacokinetic analysis, the nonlinear mixed-effects modeling program (NONMEM V 7.2, Icon Development Solutions, USA) was used. By using the first-order conditional estimation (FOCE) method with interaction, the pharmacokinetic parameters and their variability were assessed.
By using the exponential model, pharmacokinetic parameters’ inter-individual variability was assessed, shown as
Here, θi represents the ith subject parameter value, θ is the mean parameter typical value (within the population), while ηi is the variability between the subjects, supposed to allow normal distribution (with a mean of zero and variance). The forward and backward selection procedure was followed by covariate analysis. The likelihood ratio test was used for evaluation of the effects of variables on model parameters. Age, height, current weight, blood urea nitrogen, albumin, and concentration of creatinine (obtained in ≤ 48 h of PK samplings) were considered potential variables that could affect pharmacokinetic parameters, so their effects were evaluated. In the covariate model building initial stage, each covariate was considered if a significant (p < 0.05, X2 distribution with 1 degree of freedom) decreasing (decrease > 3.84) objective function value (OFV) from our basic model and a decrease in the PK parameter variability were achieved. Entire significant covariates were assembled instantly into the full model. Afterwards, the covariates were removed from the full model independently. If a rise greater than 6.635 (p < 0.01, X2 distribution) was observed in OFV, the covariates were considered significantly correlated with the PK parameters and were hence kept in the final model. Statistical and graphical criteria were used for validation of the model. Goodness-of-fit plots comprising conditional weighted residuals (CWRES) vs time, DV vs individual prediction (IPRED), observed DV vs population prediction (PRED), and PRED vs CWRES were primarily used for diagnostics [31]. Final model performance and stability were also investigated using a nonparametric bootstrap with replacement and resampling. Resampling was repeatedly performed five hundred times, then estimated parameter values achieved from the bootstrap process were compared to the original dataset values. The whole process was carried out in an automated fashion by using PsN (v2.30) [33]. Through normalized prediction distribution errors (NPDE), the final model was also evaluated statistically and graphically. Using parameters of the final population model, a simulation of 1000 datasets was performed. Results of the NPDE were graphically summarized by default according to the NPDE package (v1.2): (i) QQ plot of the NPDE and (ii) histogram of the NPDE. N (0, 1) distribution was expected to be followed for the NPDE [34].
Optimization and evaluation of the dosing regimen
The effect of ceftriaxone on bacteria is time dependent, so the pharmacokinetic and pharmacodynamic relationship is the duration that the concentration of the free fraction of the drug remains above the MIC (fT > MIC). For cephalosporins, to achieve the maximum antibacterial activity, a probability of target attainment of about 70% was needed for a 60–70% dosing interval [35, 36]. European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines reported that pathogens which may cause community-acquired pneumonia, along with the relevant range of MIC values, were also included in dosing optimization [37]. The ceftriaxone MIC range for most of the common causative pathogens was between 0.06 and 2.0 mg/L, so a MIC value of 2 mg/L was chosen as the pharmacokinetic-pharmacodynamic breakpoint. Ceftriaxone pediatric dose simulations were performed on the basis of milligrams per kilogram. Monte Carlo simulations were carried out using parameter estimates achieved from the final model. Simulation of the original dataset was performed one hundred times, and for each original pediatric patient, the time period above the MIC was obtained. To ensure that population distribution characteristics were the same, therefore the original pediatric patients were considered for the simulation. Given the unique pharmacokinetic properties, a simulation was performed for each once-daily and twice-daily dosing regimen (50, 75, and 100 mg/kg). Calculation of the probability of target attainment was performed to optimize the antibiotic therapy for each dosing regimen. From total concentrations (Ctot), the free concentrations (Cfree) were estimated using in vivo binding parameters of ceftriaxone [38] and the following given equation [39]:
where nP 517 μmol/L is the total concentration of protein binding sites and the binding affinity constant (Kaff) is 0.0367 L/μmol. [P] is the individual albumin concentration.
Results
Study population
In our study, 99 pediatric patients with hospital admission between 2017 and 2018 were recruited. Parental informed consent was provided, and the criteria for inclusion were met by all patients selected for the study. The total dose administered was 240–1500 mg/24 h. The mean (standard deviation [SD]) values of age and weight were 5.14 (2.59) (range 2.00–11.7) years and 19.3 (6.89) (range 10.5–45.0) kg at the time of study, respectively. Table 3 summarizes the patient characteristics.
Model building
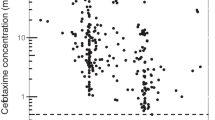
For PK modeling, 175 ceftriaxone concentrations obtained from 99 pediatric patients (range 1–2 PK samples per patient) were available. The mean sampling time since last dose was 7.66 h (range 0.50–28.08 h). Ceftriaxone sample concentrations were in range from below the value limit of quantification (< LOQ) to 284.39. One concentration was lower than the LOQ, and this value was replaced by a constant value of 1 μg/mL, which in statistical analyses is equal to half of the LOQ (2 μg/mL). Figure 1 shows ceftriaxone concentration vs time profile.
The one-compartment model with first-order elimination was the best fit for the obtained data. Compared to the one-compartment model (OFV = 1346.868), the two-compartment model has a similar OFV (OFV = 1343.485), but results were not stable. In addition, the two-compartment model has high residual variability (37.0%, one compartment 33.7%). In terms of ceftriaxone clearance (CL) and volume of distribution (V), this model was parameterized. Using the exponential model, the inter-individual variability was best described and then determined for CL and V. For describing residual variability, the proportional model was the best.
Covariate analysis
Current weight was a priori incorporated into the basic model using the allometric size approach (where allometric coefficients were 0.75 for CL and 1 for V), which significantly dropped the OFV value by 57.64 points (1289.23–1346.87), subtracting the OFV value of the current body weight from the OFV value of the structural model. Height, blood urea nitrogen, albumin, and creatinine were also evaluated in this modeling, but they had no effect on the OFV. The density plots of the main covariates tested in the model are shown in Fig. 2.
Final PK model parameter estimates are summarized and given in Table 4. At a steady state, the median (range) of the estimated weight normalized clearance and the volume of distribution was 0.03 (0.01–0.05) L/h/kg and 0.16 (0.12–0.18) L/kg respectively. The AUC0–24, for the assessed dosage regimen, was in range from 460.4 to 2291.3 mg*h/L at steady state.
Evaluation of the model
Model diagnostics for the final model of ceftriaxone displayed the acceptable goodness of fit. Figure 3a, b shows that the predictions are unbiased. Diagnostic plots of CWRES vs time and PRED demonstrated no trends as shown in Fig. 3c, d. Additionally, estimates of the median parameter achieved from the bootstrap procedure agreed quite closely with the respective values of the final population model, which indicates that our final model is sufficiently stable and can redetermine the population pharmacokinetic parameter estimates, as shown in Table 4. Figure 3e, f shows the NPDEs. NPDE distribution and histogram agreed with the theoretical N (0, 1) distribution and density, which shows a good fit of the model to the individual data. The NPDE mean was 0.0546 [Wilcoxon’s signed rank test (p = 0.604)] while the variance was 0.921 [Fisher’s variance test (0.469)].
Optimization of dosing regimen
Target attainment rates of the free ceftriaxone concentration above the MIC target of 2 mg/L at 60–70% of the dosage interval increased with simulated dosing. For cephalosporins, maximum effects have been observed consistently at a dosing interval of 60–70% [36]. Using 60% fT > MIC as the PD target, the target attainment rates of dosing regimens of 50 mg/kg BID, 50 mg/kg QD, 75 mg/kg BID, 75 mg/kg QD, 100 mg/kg BID, and 100 mg/kg QD were 99.4%, 51.2%, 100%, 68.9%, 100%, and 81.8%, respectively. Considering the target attainment rate, safety, and applicability of different dose regimens, 100 mg/kg QD was enough to produce a satisfactory target attainment rate for CAP therapy.
Discussion
In this study, the population pharmacokinetics of ceftriaxone was assessed in a large representative cohort of children with CAP using the strategy of opportunistic sampling [40]. To optimize the dosage regimen of antimicrobial therapy, the developmental pharmacokinetics and pharmacodynamics, safety, and microbiology are important considerations [41]. The pharmacokinetic and pharmacodynamic parameters, which correlate with beta-lactam bacteriological and clinical efficacy, are the percentages of time that the concentration of the serum-unbound drug exceeds the MIC value for the concerned pathogens (TMIC) [42]. Generally in adults, a TMIC of at least 40–50% of the dosing interval is acceptable [43]. However, children need a higher TMIC target to prevent the occurrence of antibiotic resistance and achieve the required efficacy [44]. In cephalosporins, maximum effects have been observed consistently at a dosing interval of 60–70% [36]. In the present research, as a more conservative endpoint, a target T > MIC of 60% of the dosing interval was chosen. Ceftriaxone is a typical agent of the cephalosporin group, having stronger activity against Streptococcus pneumoniae and Haemophilus influenzae, the most common pathogens responsible for causing CAP [45]. MIC breakpoints of Streptococcus pneumoniae and Haemophilus influenzae were 0.5 mg/L and 0.125 mg/L, and for most of the relevant pathogens, the susceptibility breakpoints were less than 2 g/mL, according to the EUCAST guidelines [37]. We thus selected a high MIC of 2 mg/L as a breakpoint to determine the TMIC for ceftriaxone use in CAP.
Most of the adverse dug events (ADEs) caused by cephalosporins were because of the inappropriate drug use especially in the pediatric population, among which the highest number of ADEs has been reported for ceftriaxone among the group members [46]. Moreover, the ceftriaxone dosage regimen established by various referenced sources and guidelines varies considerably, as shown in Table 2. These variations in the observations of routine health care practices demonstrate the urgent need for authentic pharmacokinetic data for pediatric patients. Pharmacometrics approach relevance and importance for quantitative evaluation of factors that may explain the inter-individual variability of drug disposition, and thus, doses have been well established currently, particularly in the pediatric population [47]. The European Medicines Agency (EMA) in recent times reported the need for population pharmacokinetic approaches because of their ability to determine covariates related to individualizing doses for specific patients and maturating individuals [48]. Monte Carlo simulations were used to calculate percent fT > MIC to obtain effective concentrations. The results demonstrated that the optimum concentration was achieved with doses of 100 mg/kg QD. Ceftriaxone has a special pharmacokinetics profile with a long half-life because its pharmacokinetic parameters of the free drug proportion are linear and dose dependent [6, 9]. Based on long half-life and sustained levels in plasma, a once-a-day dosing regimen of ceftriaxone in children with pneumonia was more effective against the causative pathogens [12]. Studies performed in adults also highlighted that once-daily administration of ceftriaxone can be effective [7, 8]. Its once-daily dosing regimen provides a distinctive benefit over the other agents of the cephalosporin group, which require administration several times a day. This advantage can contribute to the acceptance of ceftriaxone in out-patient departments and cost-effectiveness as well [6].
However, our study had some limitations; the optimum dosing regimen established on the basis of modeling and simulation approaches needs to be evaluated more in clinical practices for confirmation of its clinical benefits. Controlled trials will be necessary to establish the clinical efficacy of such therapeutic regimens.
Conclusion
A ceftriaxone population pharmacokinetic model was assessed while using a large sample size covering the entire age range of children with CAP. An optimal dosing regimen of 100 mg/kg QD was established using developmental pharmacokinetic-pharmacodynamic modeling and simulation.
References
Clark JE, Hammal D, Hampton F, Spencer D, Parker L (2007) Epidemiology of community-acquired pneumonia in children seen in hospital. Epidemiol Infect 135(2):262–269
Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Jeune IL et al (2009) Pneumonia guidelines committee of the BTS standards of care committee. Thorax 64, Suppl 3: iii1-iii55
Jain S , Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM et al (V) Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427
Hui L, Xincai X, Lu J, Zongxi C, Luo L, Yang Z (2013) Epidemiological characteristics and etiology of community-acquired pneumonia in Guangzhou from 2009 to 2012. Chinese J Prevent Med 47(12):1089–1094
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE (2016) Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet 388(10063):3027–3035
Lamb HM, Ormrod D, Scott LJ, Figgitt DP (2002) Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs 62(7):1041–1089
Cabellos C, Viladrich PF, Verdaguer R, Pallares R, Liñares J, Gudiol F (1995) A single daily dose of ceftriaxone for bacterial meningitis in adults: experience with 84 patients and review of the literature. Clin Infect Dis 20(5):1164–1168
Brogden RN, Ward A (1988) Ceftriaxone. A reappraisal of its antibacterial activity and pharmacokinetic properties, and an update on its therapeutic use with particular reference to once-daily administration. Drugs 35(6):604–645
Yuk JH, Nightingale CH, Quintiliani R (1989) Clinical pharmacokinetics of ceftriaxone. Clin Pharmacokinet 17(4):223–235
Stoeckel K, McNamara PJ, Brandt R, Plozza Nottebrock H, Ziegler WH (1981) Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther 29:650–657
Stoeckel K, Koup JR (1984) Pharmacokinetics of ceftriaxone in patients with renal and liver insufficiency and correlations with a physiologic nonlinear protein binding model. Am J Med 77(4C):26–32
McNamara PJ, Gibaldi M, Stoeckel K (1983a) Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding. I Theoretical considerations. Eur J Clin Pharmacol 25:399–405
McNamara PJ, Gibaldi M, Stoeckel K (1983b) Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding. II Physiological significance. Eur J Clin Pharmacol 25:407–412
Grubbauer HM, Dornbusch HJ, Dittrich P, Weippl G, Mutz I, Zobel G, Georgopoulos A, Fotter R (1990) Ceftriaxone monotherapy for bacterial meningitis in children. Chemotherapy 36:441–447
Steele RW, Eyre LB, Bradsher RW, Weinfeld RE, Patel IH, Spicehandler J (1983) Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob Agents Chemother 23:191–194
Fukumoto K, Aida S, Oishi T, Ueno K (2009) Pharmacokinetics of ceftriaxione, a third-generation cephalosporin, in pediatric patients. Biol Pharm Bull 32:1139–1141
Schaad UB, Hayton WL, Stoeckel K (1985) Single-dose ceftriaxone kinetics in the newborns. Clin Pharmacol Ther 37:522–528
Chadwick E, Yogev R, Shulman S, Weinfeld R, Patel I (1983) Single dose ceftriaxone pharmacokinetics in pediatric patients with central nervous system infections. J Pediatr 102:134–137
Del Rio M, MacCracken G, Nelson J, Chrane D, Shelton S (1982) Pharmacokinetics and cerebrospinal fluid bactericidal activity of ceftriaxone in the treatment of pediatric patients with bacterial meningitis. Antimicrob Agents Chemother 22:622–627
Standing JF, Anderson BJ, Holford NH, Lutsar I, Metsvaht T (2015) Comment on pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 54:1287–1288
Isla A, Trocóniz IF, Canut A, Abora A, Martín-Herrero JE, Pedraz J, et al (2011) Evaluación farmacocinética/far-macodinámica de agentes antimicrobianos para el tratamiento de la otitis media aguda en España. Enferm Infecc Microbiol Clin 29:167–173
Mullholland S, Gavranich J, Chang AB (2010) Antibiotics for community-acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database Syst Rev 7(7):CD004875
Fukumoto K, Aida S, Oishi T, Ueno K (2009) Pharmacokinetics of ceftriaxione, a third-generation cephalosporin, in pediatric patients. Biol Pharm Bull 32(7):1139–1141
Sharland M (2016). Manual of childhood infection, the blue book. 912 Pages ISBN: 9780198729228. Oxford Specialist Handbooks in Pediatrics
Gary P. Wormser, Roger G. Faix. 2007. Red book: 2006 report of the Committee on Infectious Diseases, 27th Edition Edited by Larry K. Pickering, Carol F. Baker, Sarah S. Long, and Julia A. McMillan Elk Grove Village, IL: American Academy of Pediatrics, 2006. 992 pp. $124.95 (cloth), Clinical infectious diseases. 44. 892–892
AHFS drug information essentials. Bethesda, MD (2004) American Society of Health-System Pharmacists
Helen K (2015). The Harriet Lane handbook: a manual for pediatric house officers, 17th edition, Philadelphia, PA 19103-2899, ISBN: 978-0-323-39955-5
BNF for children 2016-2017. (2016). 1st ed. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Ltd.
Drugs.com [Internet]. Ceftriaxone information from Drugs.com; Dec 12, 2017 https://www.drugs.com/dosage/ceftriaxone.html
Athenahealth, Inc. [US] https://online.epocrates.com/drugs/162302/ceftriaxone/Peds-Dosing
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24:2187–2197
Verdier M-C, Tribut O, Tattevin P, Le Tulzo Y, Michelet C, Bentué-Ferrer D (2011) Simultaneous determination of 12 beta-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: application to therapeutic drug monitoring. Antimicrob Agents Chemother 55:4873–4879
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN): a Perl module for NONMEM related programming. Comput Methods Prog Biomed 75:85–94
Comets E, Brendel K, Mentré F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Prog Biomed 90:154–166
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10
Craig WA (1995) Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22:89–96
EUCAST (2019) Antimicrobial susceptibility testing. Clinical breakpoints— bacteria (v 9.0)
McNamara PJ, Trueb V, Stoeckel K (1990) Ceftriaxone binding to human serum albumin. Indirect displacement by probenecid and diazepam. Biochem Pharmacol 15:1247–1253
Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, Perrotin D, Dequin P-F, Le Guellec C (2011) Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol 72:758–767
Leroux S, Turner MA, Barin-Le Guellec C, Hill H, van den Anker JN, Kearns GL, Jacqz-Aigrain E, Zhao W (2015) Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 54:1273–1285
Barker CIS, Germovsek E, Hoare RL, Lestner JM, Lewis J, Standing JF (2014) Pharmacokinetic/pharmacodynamic modelling approaches in paediatric infectious diseases and immunology. Adv Drug Deliv Rev 73:127–139
Jacobs MR (2001) Optimization of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin Microbiol Infect 7:589–596
Craig WA (2001) Does the dose matter? Clin Infect Dis 33(Suppl 3):S233–S237
MacGowan A (2004) Elements of design: the knowledge on which we build. Clin Microbiol Infect 0:6–11, 10
Ohno A, Ishii Y, Kobayashi I, Yamaguchi K (2007) Antibacterial activity and PK/PD of ceftriaxone against penicillin-resistant Streptococcus pneumoniae and beta-lactamase-negative ampicillin-resistant Haemophilus influenzae isolates from patients with community-acquired pneumonia. J Infect Chemother 13(5):296–301
Ren X, Liu D, Ding N, Huang K, Xiong Y, Du G, Zeng F (2012) Safety evaluation of cephalosporins based on utilization and adverse drug events: analysis of two databases in China. Expert Opin Drug Saf 11:689–697
Tod M, Jullien V, Pons G (2008) Facilitation of drug evaluation in children by population methods and modelling. Clin Pharmacokinet 47:231–243
European Medicines Agency (2009) Guideline on the investigation of medicinal products in the term and preterm neonate. European Medicines Agency, London, United Kingdom http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003750.pdf
Funding
This work was supported by the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2017ZX09304029-002, 2017ZX09304029-005), Scientific Research Foundation for the High-Level Returned Overseas Chinese Scholars (Ministry of Human Resources and Social Security, CG2016030001) and Hundred-Talent Program (The People’s Government of Hebei Province, E2015100010), Young Taishan Scholars Program of Shandong Province, Qilu Young Scholar Program of Shandong University, and Science and Technology Planning Project of Hebei Province (15277705D), We declare that we have no conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Contributions
LYT, BPX, ADS, EJA, and WZ designed the study; YKW, YEW, and LYT organized the trial and recruited patients; MK, YZ, and HYS set up the analytical method; MWK, BHT, and WZ performed PK analysis; MWK, YKW, LYT, and WZ drafted the first version of the manuscript and it was critically reviewed by all the other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was conducted at the Children’s Hospital of Hebei Province and was approved by the ethics board of the Children’s Hospital of Hebei Province affiliated to Hebei Medical University. This study was also registered at ClinicalTrials.gov (NCT03113344).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.W., Wang, YK., Wu, YE. et al. Population pharmacokinetics and dose optimization of ceftriaxone for children with community-acquired pneumonia. Eur J Clin Pharmacol 76, 1547–1556 (2020). https://doi.org/10.1007/s00228-020-02939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02939-4