Abstract
Purpose
Cytochrome P450 (CYP) is involved in the metabolism of valproic acid (VPA). Specifically, CYP2C9 and CYP2A6 are the main enzymes responsible for VPA metabolism. However, the correlation between plasma VPA concentrations and CYP2C9 and CYP2A6 gene variations is uncertain. This meta-analysis aimed to investigate the relationship between CYP2C9 and CYP2A6 variants and plasma concentrations of VPA.
Methods
The PubMed, Web of Science, and EMBASE databases were searched for qualifying studies published until July 2019. Cohort studies that included standardized plasma VPA concentrations and CYP2C9 and CYP2A6 genotypes were reviewed. The mean difference and 95% confidence intervals (CIs) were evaluated to assess the strength of the relationship. Data analysis was performed using Review Manager (version 5.3) and RStudio (version 3.6).
Results
In total, we analyzed data from six studies involving 807 patients. We found that CYP2C9*3 was associated with standardized plasma VPA concentration; *3 allele carriers had a 0.70-μg/mL higher concentration per mg/kg than non-carriers (95% CI 0.25, 1.15; P = 0.002). We also found a significant association between the CYP2A6*4 and standardized trough VPA concentration; patients with the *4 allele had a 0.48-μg/mL higher concentration per mg/kg than patients without the *4 allele (95% CI 0.10, 0.86; P = 0.01).
Conclusion
This meta-analysis demonstrated that CYP2C9*3 and CYP2A6*4 genetic variants affect plasma VPA concentrations. For epilepsy patients with these genotypes, dose adjustment may be necessary to ensure VPA’s therapeutic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Valproic acid (VPA) is a branched short-chain fatty acid widely given as a first-line antiepileptic drug. It is used in the treatment of many different types of epileptic seizures and psychiatric diseases, such as bipolar and schizoaffective disorders, social phobias, and neuropathic pains [1, 2]. Given VPA’s narrow therapeutic range (50–100 μg/mL) and severe hepatotoxicity, therapeutic drug monitoring is essential [3].
In humans, VPA metabolism is mainly comprised of three pathways, i.e., mitochondria β-oxidation, cytochrome P450 (CYP)–mediated oxidation, and glucuronidation by uridine 5′-diphospho-glucuronosyltransferases (UGTs) [4]. Among these, phase I (CYP oxidation) is an important metabolic pathway for VPA that is mainly mediated by CYP2C9 and CYP2A6 [5]. CYP2C9 and CYP2A6 are known to be genetically polymorphic, and the catalytic activities attributable to CYP2C9 and CYP2A6 can vary by 5-fold and 30-fold in human liver microsomes, respectively [6]. In addition, the two enzymes produce the unsaturated metabolite 4-ene-VPA, which causes hepatotoxicity along with VPA.
In clinical setting, VPA has shown high interindividual variability in steady-state serum concentration [7], resulting in various side effects, especially liver toxicity [8]. Therefore, dosage optimization would have a significant impact on clinical practice. Although oxidation by CYP is known to be an important pathway for VPA metabolism, the correlation between plasma VPA concentrations and CYP2C9 and CYP2A6 gene variants is uncertain [9]. Therefore, we conducted a meta-analysis that synthesizes results from all available cohort studies trying to provide necessary power for assessing the effects of CYP2C9 and CYP2A6 genetic variants on plasma VPA concentrations.
Methods and materials
Literature search strategy
Before this study was conducted, all processes for the meta-analysis were predetermined according to the checklist in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. An extensive search of electronic databases (PubMed, Web of Science, and EMBASE) was performed using the following search terms: (VPA OR valpro* OR divalproex OR Depakene OR Depakote OR dipropyl acetate) AND {(2C9 OR CYP2C9 OR cytochrome P450 2C9 OR cytochrome-p-4502C9 OR P4502C9 OR cytochrome p450 IIC9 OR CYP2C9*) OR (2A6 OR CYP2A6 OR cytochrome P450 2A6 OR cytochrome p-4502A6 OR CYP2A6*)}. Two reviewers independently conducted the data search, which included studies published until July 2019. There was no limitation on language or race.
Study inclusion and exclusion criteria
Studies were included if they had a cohort design, evaluated epilepsy patients who took VPA as monotherapy, included patients whose liver and kidney functions were normal, and assessed the relationship of CYP2C9 and CYP2A6 genotypes with standardized plasma trough concentrations of VPA (μg/mL per mg/kg). Standardized concentration was determined as the concentration divided by the dose of VPA given. Studies were excluded if they were reviews, comments, letters, news, or editorials; were conducted in vitro or in animals; or lacked results about the standardized trough concentrations of VPA. If data overlapped, only the most recent and comprehensive data were included in the meta-analysis.
Data extraction and quality assessment
Two reviewers extracted data independently, and discrepancies were resolved by consensus. Extracted data included the following information: name of the first author, publication year, age and ethnicity of participants, genotyping methods, number of patients, and standardized trough concentrations of VPA.
Articles were assessed by two researchers based on the Newcastle-Ottawa Scale (NOS) for quality assessment. The NOS includes three categories, i.e., selection of the study sample, comparability between the case and control groups, and ascertainment of the outcome of interest [11]. Each study can earn a total score of 0–9.
Statistical analysis
The data review was conducted via Review Manager (version 5.3; The Cochrane Collaboration, Copenhagen, Denmark). For continuous variables (standardized trough concentrations of VPA), the mean difference (MD) and 95% confidence intervals (CIs) were used to identify the relationship between the existence of CYP2C9 and CYP2A6 variants and standardized trough concentrations of VPA. Only CYP2C9*3 and CYP2A6*4 were analyzed in the current meta-analysis because every study that met inclusion criteria examined these two only. CYP2C9 participants were divided into two groups, i.e., *3 allele carriers and non-carriers. CYP2A6 participants also were divided into two groups, i.e., *4 allele carriers and non-carriers.
The heterogeneity across studies was estimated by way of a chi-square test and an I2 statistic. I2 > 50% was considered to indicate significant heterogeneity. When there was no statistical evidence of heterogeneity, the fixed-effects model was used; otherwise, the random effects model was employed to calculate pooled estimates. Both Begg’s test and Egger’s regression test of the funnel plot were conducted using R Studio software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria) to identify publication bias [12]. A P value < 0.05 was considered statistically significant.
Results
Identification and characteristics of the included studies
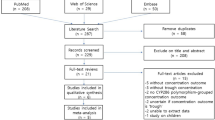
A detailed flow chart of the study selection process is presented in Fig. 1. A total of 684 studies were retrieved through the electronic databases. After the removal of duplicates, 566 records were initially identified, and the titles and abstracts were screened for inclusion in the study. From this initial review, the full texts of 26 studies were assessed for eligibility. Of these studies, 20 were excluded for the following reasons: the studies lacked relevant outcomes (n = 6), the researchers were unable to extract relevant data (n = 10), and the interventions were inappropriate (n = 4). Thus, six articles were identified for this meta-analysis. Two of the articles were written in Chinese, and the others were in English.
In total, this meta-analysis evaluated data from 807 patients. The characteristics of the included studies are presented in Table 1. The studies were published from 2006 to 2017, and all of them were conducted in China. Each patient received 10–30 mg/kg/day of VPA as monotherapy. The DNA source was blood, and the genetic variants were assayed by direct DNA sequencing. The NOS score for all included studies was 7 (Table 1).
Association between CYP genes and plasma concentration of VPA
Five studies with a total of 702 patients were evaluated for the association between CYP2C9 variants and standardized VPA concentration [3, 4, 12,13,14] (Fig. 2). The standardized trough concentration of VPA was 0.70 μg/mL per mg/kg higher in CYP2C9*3 carriers compared with non-carriers (3.95 vs 3.25 μg/mL per mg/kg, 95% CI 0.25, 1.15; P < 0.0001; Fig. 2). Moderate heterogeneity was found among studies (I2 53%; P = 0.07). The funnel plot was basically symmetrical and showed no publication bias (Fig. 4a). Begg’s test and Egger’s test also indicated that there was no evidence of publication bias (Begg’s test, P = 0.624; Egger’s test, P = 0.596).
Five studies evaluated the association between CYP2A6 variants and standardized trough concentration of VPA [3, 4, 12, 14, 15]. In these studies, standardized VPA concentration in CYP2A6*4 carriers was 0.48 μg/mL per mg/kg higher compared with the concentration in non-carriers (3.64 vs 3.16 μg/mL per mg/kg, 95% CI 0.10, 0.86; P = 0.01; Fig. 3). Heterogeneity was detected among studies (I2 = 91%; P < 0.0001). The funnel plot was basically symmetrical and indicated no publication bias (Fig. 4b). Neither Begg’s test nor Egger’s test showed significant publication bias (Begg’s test, P = 0.624; Egger’s test, P = 0.974).
Discussion
This study is the first meta-analysis to evaluate the influence of CYP2C9 and CYP2A6 variants on standardized trough VPA concentration. Patients carrying CYP2C9 or CYP2A6 variants showed higher standardized trough concentration of VPA than non-carriers.
In an in vitro study, the formation rates of 4-ene-VPA in human liver microsomes were reduced by 29% and 61% in samples with one and two variant CYP2C9 alleles, respectively [16]. CYP2C9 variations had a significant impact on 4-ene-VPA concentration; patients with the wild-type CYP2C9 (CYP2C9*1) had a greater capacity for VPA metabolism than those with the variant type CYP2C9*3 [4]. In terms of CYP2A6 and 4-ene-VPA, CYP2A6 is the principal human enzyme involved in the formation of the hepatotoxic metabolite [5]. Catalytic activities attributable to CYP2A6 have been reported to vary by 30-fold in human liver microsomes [6].
The present results are consistent with those reported by a previous meta-analysis that investigated the correlation between CYP2C9 variants and warfarin maintenance doses. According to that study, the presence of the *3 alleles was significantly associated with a lower warfarin maintenance dose [17]. Another study of the impact of CYP2C9 variants on phenytoin also supports our present result [18]. In that study, the existence of a loss-of-function (LOF) allele (*3) affected phenytoin metabolism and maintenance doses. As a result, it was recommended that phenytoin dose be reduced by 25–50% according to the phenotype.
A previous meta-analysis clarified that impaired nicotine metabolism was caused by genetic variants of the CYP2A6 gene, as the CYP2A6*4 allele completely lacks enzyme activity [19]. Another in vitro study showed that CYP2A6 genetic variation was significantly associated with the plasma concentration of letrozole [20]. These studies indicate that CYP2C9 and CYP2A6 variants affect the metabolism of various drugs.
Our meta-analysis showed that CYP2C9*3 or CYP2A6*4 allele carriers have 15–20% higher VPA concentrations than those in non-carriers, implying the need for dose adjustment. In a study with CYP2C9 status–guided VPA therapy in children, normal dose (30–40 mg/kg) was given to the *3 non-carriers, whereas reduced dose (10–20 mg/kg) was administered to the children with *3 carriers. This CYP2C9-guided treatment significantly reduced the incidence of serious side effects [21]. Further clinical studies using greater number of patients are necessary to make more detailed dose adjustment scheme.
It has been proposed that CYP enzymes are more important for VPA metabolism in children than in adults because children have higher CYP activity. Furthermore, it was found that CYP2C9 status–guided VPA therapy in children reduced adverse drug events from VPA [21, 22]. Therefore, further study in children is required to clarify the effects of CYP genetic variants on VPA therapy.
Although we performed considerable retrieval and analysis, several limitations should be considered. First, all studies included in this meta-analysis were conducted in Chinese people. As the allele frequencies of two variants (CYP2C9*3 and CYP2A6*4) in Caucasians (8.3% and 1.2%, respectively) and African-Americans (0.5% and 1.9%, respectively) are different from those reported in Asians (3.3% and 6.7%, respectively) [13, 23, 24], the interpretation of the results should be made with caution. Second, we were not able to conduct the analysis according to the CYP2C9*2 allele or of the combining effects of CYP2C9*3 and CYP2A6*4, due to a lack of available data. Third, despite the fact that drug trough levels can be influenced by drug release kinetics and prolonged VPA show varying release kinetics depending on the brand, we were unable to obtain brand names from the studies included in the current analysis. Additionally, the meta-analysis of CYP2A6 showed a substantial degree of heterogeneity, possibly due to the small number of studies included.
In conclusion, standardized concentrations of VPA were found to be significantly higher in CYP2C9 or CYP2A6 LOF allele carriers than in non-carriers. These findings provide further evidence for genetic effects of the CYP2C9 and CYP2A6 genes on the pharmacokinetics of VPA, which will help improve individualized therapy in clinics.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Perucca E (2002) Pharmacological and therapeutic properties of valproate. CNS Drugs 16:695–714
Jawien W, Wilimowska J, Klys M, Piekoszewski W (2017) Population pharmacokinetic modelling of valproic acid and its selected metabolites in acute VPA poisoning. Pharmacol Rep 69:340–349
Zhao M, Zhang T, Li G, Qiu F, Sun Y, Zhao L (2017) Associations of CYP2C9 and CYP2A6 polymorphisms with the concentrations of valproate and its hepatotoxin metabolites and valproate-induced hepatotoxicity. Basic Clin Pharmacol Toxicol 121:138–143
Wang C, Wang P, Yang LP, Pan J, Yang X, Ma HY (2017) Association of CYP2C9, CYP2A6, ACSM2A, and CPT1A gene polymorphisms with adverse effects of valproic acid in Chinese patients with epilepsy. Epilepsy Res 132:64–69
Sadeque AJ, Fisher MB, Korzekwa KR, Gonzalez FJ, Rettie AE (1997) Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J Pharmacol Exp Ther 283:698–703
Wrighton SA, Vandenbranden M, Stevens JC, Shipley LA, Ring BJ, Cashman JR, Rettie AE (1993) In vitro methods for assessing human hepatic drug metabolism: their use in drug development. Drug Metab 25:453–484
Baillie TA, Sheffels PR (1995) Valproic acid chemistry and biotransformation. In: Levy RH, Mattson RH, Meldrum BS (ed) Antiepileptic drugs, 4th edn. New York: Raven Press, pp 589–604
Nanau RM, Neuman MG (2013) Adverse drug reactions induced by valproic acid. Clin Biochem 46:1323–1338
Amini-shirazi N, Ghagremani MH, Ahmadkhaniha R, Mandegary A, Dadgar A, Abdollahi M, Shadnia S, Pakdaman H, Kebriaeezadeh A (2010) Influence of CYP2C9 polymorphism on metabolism of valproate and its hepatotoxin metabolite in Iranian patients. Toxicol Mech Methods 20:452–457
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 6:264–269
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Tan L, Yu JT, Sun YP, Ou JR, Song JH, Yu Y (2010) The influence of cytochrome oxidase CYP2A6, CYP2B6, and CYP2C9 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Clin Neurol Neurosurg 112:320–323
Guo Y, Hu C, He X, Qiu F, Zhao L (2012) Effests of UGT2B7, and CYP2C9 genotypes on plasma concentration of valproic acid in Chinese children with epilepsy. Drug Metab Pharmacokinet 27:536–542
Liao Q, Shi J, Zhang Y, Xiaolei LI, Liu S, Qiu J (2013) Effects of cytochrome P450 isozymes 2A6, 2B6, 2C9 and 2C19 genetic polymorphisms on plasma concentration of sodium valproate. Chin J Neurol 46:82–86
Sun Y, Tan L, Song J (2005) Effect of CYP2A6 genetic polymorphisms on serum concentration of sodium valproate. Chin J Neurol 11:745–747
Ho PC, Abbott FS, Zanger UM, Chang TK (2003) Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenomics J 3:335–342
Zhang J, Tian L, Huang J, Huang S, Chai T, Shen J (2017) Cytochrome P450 2C9 gene polymorphism and warfarin maintenance dosage in pediatric patients: a systematic review and meta-analysis. Cardiovasc Ther 35:26–32
Silvado CE, Terra VC, Twardowschy CA (2018) CYP2C9 polymorphisms in epilepsy: influence on phenytoin treatment. Pharmgenomics Pers Med 11:51–58
Yoshida R, Nakajima M, Watanabe Y, Kwon JT, Yokoi T (2002) Genetic polymorphisms in human CYP2A6 gene causing impaired nicotine metabolism. Br J Clin Pharmacol 54:511–517
Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V, Hoffmann E, Tyndale RF, Flockhart DA (2011) Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther 90:693–700
Budi T, Toth K, Nagy A, Szever Z, Kiss A, Temesvari M, Hafra E, Garami M, Tapodi A, Monostory K (2015) Clinical significance of CYP2C9-status guided valproic acid therapy in children. Epilepsia 56:849–855
Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, Smolenski S, Goble R (2002) Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 66:185–200
Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, Miners JO, Birkett DJ, Goldstein JA (1996) The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6:341–349
Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF (2004) Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics 14:615–626
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the work and have read and approved the manuscript for publication. Ha Young Yoon, Min Hyoung Ahn, and Hye Sun Gwak were responsible for the study concept and design. Ha Young Yoon and Min Hyoung Ahn participated in data extraction. Ha Young Yoon, Min Hyoung Ahn, Jeong Yee, Nari Lee, and Ji Min Han analyzed the data. Ha Young Yoon and Min Hyoung Ahn contributed to the manuscript writing, and Hye Sun Gwak finalized it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PNG 801 kb)
Rights and permissions
About this article
Cite this article
Yoon, H.Y., Ahn, M.H., Yee, J. et al. Influence of CYP2C9 and CYP2A6 on plasma concentrations of valproic acid: a meta-analysis. Eur J Clin Pharmacol 76, 1053–1058 (2020). https://doi.org/10.1007/s00228-020-02872-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02872-6