Abstract
Background
Tiotropium have been recommended as first-line maintenance therapy for chronic obstructive pulmonary disease (COPD) to reduce the frequency, duration, and severity of exacerbations and improve quality of life. Recently, it was reported that tiotropium use might link to cardiovascular risk in COPD patients. But it is controversial. We aimed to clarify the associations between tiotropium use and cardiovascular risk in patients with COPD.
Methods
We searched PubMed, EMBASE, Cochrane Library, and Clinical Trials.gov to identify potentially relevant articles. We included randomized controlled trials of any inhaled tiotropium versus non-anticholinergic treatment for COPD, with reporting of cardiovascular events as an adverse event. We conducted meta-analyses by the Peto and Mantel-Haenszel approaches with corresponding 95% CIs.
Results
Our work included 20 RCTs with more than 27,699 subjects. Pooled results indicated that tiotropium treatment did not increase the risk of cardiovascular events (Peto OR, 0.97, 95% CI, 0.84–1.12; I2 = 0%), overall mortality (RD, 0.00, 95% CI, − 0.00–0.01; I2 = 68%), and cardiovascular mortality (Peto OR, 1.58, 95% CI, 0.92–2.74; I2 = 0%) compared with controls. Then, subgroup analysis was performed according to the type of controls. The pooled results were consistent with the above (tiotropium vs LABA: Peto OR, 0.98, 95% CI, 0.81–1.19; I2 = 17%) (tiotropium vs placebo: Peto OR, 0.92, 95% CI, 0.75–1.44; I2 = 15%). In addition, there was also no association between cardiovascular risk and duration of tiotropium treatment.
Conclusions
Inhaled tiotropium does not increase the risk of cardiovascular events and cardiovascular mortality in patients with COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible airflow limitation and progressive incapacity, and usually accompanied by an increase in chronic inflammatory responses [1]. Frequent exacerbations of COPD contribute to impaired health status, increased hospitalization costs, and increased risk of death [2]. Long-acting bronchodilators, including long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), have been recommended as first-line maintenance therapy for COPD to reduce the frequency, duration, and severity of exacerbations and improve quality of life [3]. Tiotropium, a long-acting, anticholinergic, bronchodilator, has been widely used for treatment of COPD at all severity levels of the disease. It is usually recommended in combination with LABA and/or inhaled corticosteroid (ICS) to relieve symptoms and reduce exacerbations in COPD patients [3].
However, with the expected increasing use of tiotropium, the question of the potential cardiovascular risk arising from the tiotropium use becomes pertinent. Recent several studies have suggested that tiotropium use might link to cardiovascular risk. Gershon and colleagues reported that among older individuals with COPD, tiotropium use led to 1.24- to 1.80-fold increased risks of cardiovascular adverse events [4]. A nested case-control study also concluded that tiotropium added to LABA/ICS combination therapy was significantly associated with an increased cardiovascular risk in COPD patients and suggested that close monitoring of COPD patients requiring tiotropium is essential [5]. But a recent retrospective study reported that tiotropium did not increase risk of cardiac events, mortality, or serious cardiac adverse events [6]. A cohort study also revealed that adding long-acting muscarinic antagonists (LAMAs) in the real-world setting treatment of COPD does not increase the risk of most cardiovascular events [7]. Therefore, previous studies provided conflicting results. The cardiovascular risk of using tiotropium in COPD patients is still controversial.
Several systematic reviews and meta-analyses have been published, evaluating the association of tiotropium use and the risk of adverse cardiovascular events in patients with COPD [8,9,10,11,12]. Among these, two meta-analyses reported that inhaled anticholinergic drugs (tiotropium or ipratropium) significantly increased the risk of cardiovascular events and hinted that it would be essential to proactively identify participants at increased risk of cardiovascular events when treated with tiotropium or other anticholinergic drugs [8, 9]. But above results were limited owing to imprecision and small size. One meta-analysis reported that tiotropium use significantly reduces cardiovascular events and overall mortality. However, the data of this trial are not fully publicly available, and the study does not yet offer the number of events per intervention arm in each individual trial [11]. One meta-analysis concluded that tiotropium did not significantly increase the risk of adverse major cardiovascular events in patients with COPD [12]. This manuscript overcomes most of the limitations in the previous study. But this meta-analysis may be also outdated because it did not include the recently published large RCTs [15, 16, 21, 23, 27]. Therefore, there are doubts about the clinical significance of differences reported in the different meta-analyses. The relationship between tiotropium use and the risk of cardiovascular events in COPD patients is still uncertain. The aim of this meta-analysis was to objectively reappraise all available RCTs of cardiovascular risks with tiotropium use in COPD patients.
Methods
This is a meta-analysis of association between inhaled tiotropium and the risk of adverse cardiovascular events in COPD patients. This article was organized according to the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) statement [13].
Search strategy
Two reviewers (Mingjin Y. and Yuejun D.) independently and in duplicate searched PubMed, EMBASE, Cochrane Library, and Clinical Trials.gov (to September 1, 2019) to identify potentially relevant articles. The search terms were as follows: (“tiotropium” OR “bronchodilator” OR “anticholinergic drugs” OR “LAMA”) AND (“chronic obstructive pulmonary disease” OR“COPD” OR “chronic airflow obstruction” OR “AECOPD”) and randomized protocol design. There were no restrictions placed on geographic area or language.
Eligibility criteria
Our specific inclusion criteria for trials were as follows: (1) randomized controlled trials; (2) participants with COPD of any severity; (3) inhaled tiotropium as the intervention drug (e.g., tiotropium alone or in combination with LABA); (4) non-anticholinergic treatment (including LABA or placebo) as control; and (5) trials providing data on cardiovascular adverse events, including arrhythmia, coronary ischemia, myocardial infarction, or cardiovascular death (including 0 events). Exclusion criteria were as follows: (i) non-English manuscripts, (ii) a case-control or cohort design, (iii) abstracts or reviews, and (iv) participants with bronchiectasis or asthma.
Trial identification and data extraction
Two reviewers (Mingjin Y. and Yuejun D.) independently screened studies by title, abstract, and full text. Data were extracted from the included studies. Review Manager Software was used to analyze data and create tables. Disagreements were resolved by discussion by two reviewers until a consensus was reached.
Assessment of risk of bias
Risk of bias assessment was performed by two investigators (Mingjin Y. and Yuejun D.) independently using a Cochrane Toolkit [14]. Disagreements were resolved by discussion and consensus by two reviewers until a consensus was reached.
Statistical analysis
The Review Manager 5.3 software (v.5.3, Cochrane Collaboration, London, UK) was used to calculate pooled Peto odds ratio (OR) with 95% confidence intervals (CIs). We also calculated the pooled Mantel-Haenszel risk difference (RD) for included trials with 0 event in tiotropium treatment or control. The heterogeneity was calculated using the I2 test. If the findings indicate a substantial level of heterogeneity (I2 ≥ 50%), a random-effects model would be used; otherwise, a fixed-effects model would be used. We used the GRADEpro Guideline Development Tool to grade the quality of included evidence. The findings are shown in Table 2.
Results
Eligible trials
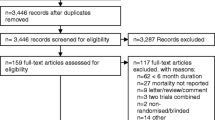
Our search identified 20 published studies that fulfilled the inclusion criteria after a detailed review [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The flow of the study is shown in e-Fig. 1 (Online Supplement). The 20 included trials enrolled 27,699 subjects, of whom 14,892 received inhaled tiotropium treatment and 12,807 received control therapy (including LABA or placebo).
Study characteristics
The eligible trials were published from 2000 to 2019. Population sizes ranged from 107 to 5992 participants. Thirteen RCTs assessed inhaled tiotropium vs placebo. Ten RCTs used active comparators (including inhaled indacaterol, salmeterol, or a combination inhaler containing salmeterol and fluticasone). There were 11 short-term RCTs with treatment duration between 1.5 and 6 months and 9 long-term RCTs with treatment duration over 6 months. Trial characteristics are shown in Table 1.
Risk of bias and quality of evidence
Details of the quality assessment of included RCTs are shown in e-Fig. 2a and b (Online Supplement). Eleven trials were evaluated as being at low risk of bias for all aspects. Allocation concealment was adequately described in 17 trials, and unclear in the remaining 3 trials. Four trials had an attrition bias. Three trials had unclear risk of bias for other bias. All trials were evaluated as being at low risk of bias for selective reporting and performance bias.
The GRADE analysis revealed that moderate quality of evidence (+++) was found for the effect of tiotropium therapy vs control (including LABA and placebo) with respect to the risk of cardiovascular events. Low quality of evidence (++) was found for the effect of tiotropium therapy vs LABA or placebo with respect to the risk of cardiovascular death. The findings of the GRADE assessment are reported in e-Table 1 (Online Supplement).
Cardiovascular safety of tiotropium use versus control (including LABA and placebo)
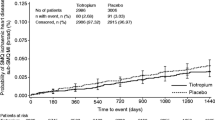
Data from 20 studies that reported cardiovascular events during treatment were pooled. Meta results revealed that tiotropium treatment did not increase the risk of cardiovascular events vs control in a meta-analysis of 20 RCTs enrolled 27,699 subjects (Peto OR, 0.97, 95% CI, 0.84–1.12; I2 = 0%) (Fig. 1). Further subgroup analysis was performed next according to duration of administration. Of the eligible trials, nine RCTs assessed long-term use of tiotropium (> 6 months). The pooled results revealed that long-term use of tiotropium was not associated with an increased risk of cardiovascular events when compared with control (Peto OR, 0.99, 95% CI, 0.85–1.15; I2 = 10%) (Fig. 2). Eleven RCTs assessed short-term use of tiotropium (≤ 6 months). The pooled results were also consistent with the above (Peto OR, 0.81, 95% CI, 0.53–1.25; I2 = 0%) (Fig. 3).
Cardiovascular safety of tiotropium use versus LABA or placebo
Data from 13 placebo-controlled studies were pooled. Compared with placebo, inhaled tiotropium did not increase the risk of cardiovascular events (Peto OR, 0.92, 95% CI, 0.75–1.44; I2 = 15%) (Fig. 4). Further subgroup analysis was also performed according to duration of administration. Meta results revealed that long-term use of tiotropium did not increase risk of cardiovascular events when compared with placebo (Peto OR, 0.91, 95% CI, 0.74–1.13; I2 = 30%) (Fig. 5); this was so with short-term use of tiotropium (Peto OR, 1.75, 95% CI, 0.34–8.86; I2 = 0%) (Fig. 6). Ten RCTs assessed inhaled tiotropium vs LABA. The pooled results were also consistent with the above (Peto OR, 0.98, 95% CI, 0.81–1.19; I2 = 17%) (Fig. 7).
Use of tiotropium and the risk of overall mortality
There were 16 trials providing information on overall mortality during treatment (Table 2). The pooled results exhibited that inhaled tiotropium did not significantly increase the risk of overall mortality vs control (including LABA and placebo) (RD, 0.00, 95% CI, − 0.00–0.01; I2 = 68%) (Fig. 8). Considering that statistical heterogeneity among the included studies might reduce the reliability of above results, subgroup analyses were performed next according to the type of controls. Data from ten placebo-controlled studies were pooled. Meta results revealed that tiotropium use did not increase the risk of overall mortality vs placebo (Peto OR, 0.91, 95% CI, 0.79–1.03; I2 = 46%) (Fig. 9). Eight RCTs assessed tiotropium vs LABA. The results were also consistent with the above (Peto OR, 1.16, 95% CI, 0.82–1.63; I2 = 39%) (Fig. 10).
Use of tiotropium and the risk of cardiovascular mortality
Of the eligible trials, eight trials provided information on cardiovascular mortality during treatment (Table 2). The pooled results exhibited that tiotropium treatment did not significantly increase the risk of cardiovascular mortality vs control (including LABA and placebo) (Peto OR, 1.58, 95% CI, 0.92–2.74; I2 = 0%) (Fig. 11). Then, subgroup analysis was performed according to the type of controls. Compared with placebo, tiotropium treatment did not increase the risk of cardiovascular mortality (RD, − 0.00, 95% CI, − 0.00–0.00; I2 = 0%) (Fig. 12); this was so with tiotropium vs LABA (RD, 0.00, 95% CI, − 0.00–0.01; I2 = 81%) (Fig. 13).
Discussion
This is a meta-analysis designed to assess the safety of regular use of tiotropium in COPD patients. Our work included 20 RCTs with more than 27,699 subjects, of whom 14,892 received inhaled tiotropium and 12,807 received control therapy (including LABA or placebo). The results here indicated that tiotropium did not increase the risks of cardiovascular events, overall mortality, and cardiovascular mortality compared with controls. Then, subgroup analysis was performed according to the type of controls. There were 13 RCTs comparing tiotropium with placebo; the pooled results were consistent with the above. When we incorporated data from ten RCTs in the meta-analysis that compared tiotropium with LABA, the summary effect estimate did not change. In addition, there was also no association between duration of tiotropium treatment and cardiovascular risk.
Recent studies have reported that inflammatory cytokines may potentially play a role in mediating the systemic cardiovascular effects [35]. IL-8, as one of the most common inflammatory cytokines, has been found to participate in inducing the occurrence of cardiovascular events by destabilizing existing atherosclerotic plaque [36]. Tiotropium treatment can significantly increase the levels of sputum IL-8 [37]. Therefore, it is seemingly plausible that tiotropium use increases the risk of cardiovascular events in COPD patients. Several studies also suggested that tiotropium use might link to cardiovascular risk [8, 9]. However, the results of our review are the opposite of the above. The pooled results revealed that tiotropium did not significantly increase the risk of cardiovascular events in patients with COPD. In addition, tiotropium treatment did not also increase the risk of cardiovascular mortality. Given the individual differences and diversity of conditions, we also believe that there may be a different cardiovascular response to tiotropium in individual patients. Although the issue is not novel, it is important for developing COPD management protocols. Our findings could be used for reference in the management of COPD.
Compared with other studies
The risk of cardiovascular events and/or death associated with tiotropium treatment has been assessed in several meta-analyses of RCTs. Singh and colleagues performed a meta-analysis on the basis of 17 RCTs and concluded that inhaled tiotropium was associated with a significantly increased risk of myocardial infarction, stroke, or cardiovascular death [9]. However, there are some significant differences between this analysis and our current manuscript. First, Singh and colleagues performed a pooled analysis of a mix of RCTs that compared tiotropium vs. controls (including LABA, albuterol, and placebo). The active controlled trials were pooled together with placebo-controlled trials. Subgroup analysis was not further performed according to the type of controls. But this problem was solved in our analysis. Second, the data of including trials are not fully publicly available, and this analysis does not yet offer the number of events per intervention arm in each individual trial. Third, their results were limited owing to imprecision, significant heterogeneity, and small size. In 2009, Rodrigo and colleagues published a systematic review with meta-analysis including a total of 19 RCTs enrolling 18,111 subjects [12]. Findings in their study reported that tiotropium treatment did not significantly increase the risk of cardiovascular events in patients with COPD. But this meta-analysis may be outdated because it did not include the recently published large RCTs [15, 16, 21, 23, 27, 29,30,31,32,33]. The included RCTs had also some bias in reporting bias or incomplete data. Furthermore, their analysis also did not perform further subgroup analysis based on duration of administration. In 2019, a new meta-analysis was published to investigate the relationship between triple therapy and the risk of cardiovascular events [10]. Findings in their study reported that adding tiotropium to ICS/LABA therapy did not increase the risk of cardiovascular events in patients with COPD compared with ICS/LABA therapy. But the main aim of this analysis was to explore the role of adding LAMA to ICS/LABA combination in COPD. Studies designed to compare tiotropium versus placebo were not included in their analysis.
Strengths and limitations
This is the largest meta-analysis designed to assess the cardiovascular risk of regular use of tiotropium in COPD patients. A comprehensive search process and clear inclusion criteria were developed and applied to this meta-analysis. Furthermore, it included 20 RCTs with more than 27,699 patients, which meet the basic requirements for further sequential and subgroup analysis. All RCTs were also graded strictly on the basis of the quality of evidence by the GRADE approach.
This paper also has some limitations. First, the inescapable clinical heterogeneity weakened the conclusions of this study. This paper included studies from 2000 to 2019. The diagnosis and therapeutic techniques of COPD, including inhaler device, type of drug, course of treatment, and management plan, have evolved. Second, baseline characteristics, including age, body mass index, underlying disease, severity of COPD, and smoking history, are also important factors affecting the occurrence and progression of cardiovascular events. Most of the included studies did not provide detailed data on age, body mass index, underlying disease, severity of COPD, and smoking history. Further subgroup analysis cannot be performed based on the above information. Third, due to the exclusion of the relevant studies with insufficient information, selection bias could not be avoided.
Conclusions
Our works indicate that inhaled tiotropium does not increase the risks of cardiovascular events, all-cause mortality, and cardiovascular mortality in patients with COPD. In addition, there is also no association between cardiovascular risk and duration of tiotropium treatment.
References
Adeloye D, Chua S, Lee C, Global Health Epidemiology Reference Group (GHERG) (2015) Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 5(2):020415
Raherison C, Girodet PO (2009) Epidemiology of COPD. Eur Respir Rev 18(114):213–221
Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2019) Global strategy for the diagnosis, management and prevention of COPD, 2019. Available from: http://www.goldcopd.org. Accessed May 2019
Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, Stukel TA (2013) Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 173(13):1175–1185
Liou J-T, Lin CW, Tsai C-L, Wang Y-H (2018) Risk of severe cardiovascular events from add-on tiotropium in chronic obstructive pulmonary disease. Mayo Clin Proc 93(10):1462–1473
Tashkin DP, Leimer I, Metzdorf N, Decramer M (2015) Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trial. Respir Res 16:65
Suissa S, Dell'Aniello S, Ernst P (2017) Concurrent use of long-acting bronchodilators in COPD and the risk of adverse cardiovascular events. Eur Respir J 23:49(5)
Cazzola M, Calzetta L, Rogliani P, Matera MG (2017) Tiotropium formulations and safety: a network meta-analysis. Ther Adv Drug Saf 8(1):17–30
Singh S, Loke YK, Furberg CD (2008) Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 300:1439–1450
Calzetta L, Cazzola M, Matera MG, Rogliani P (2019) Adding a LAMA to ICS/LABA therapy: a meta-analysis of triple combination therapy in COPD. Chest 155(4):758–770
Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP (2009) Mortality in the 4-year trial of tiotropium (uplift) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180:948–955
Rodrigo GJ, Castro-Rodriguez JA, Nannini LJ, Plaza Moral V, Schiavi EA (2009) Tiotropium and risk for fatal and nonfatal cardiovascular events in patients with chronic obstructive pulmonary disease: systematic review with meta-analysis. Respir Med 103(10):1421–1429
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) Cochrane bias methods group; Cochrane statistical methods group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Bateman ED, Ferguson GT, Barnes N (2013) Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 42(6):1484–1494
Decramer ML, Chapman KR, Dahl R (2013) Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 1(7):524–533
Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359:1543–1554
Wedzicha JA, Calverley PMA, Seemungal TA (2008) The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 177(1):19–26
Brusasco V (2005) Health outcomes following treatment for 6 months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 61(1):91–91
Casburi R (2002) A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 19(2):217–224
Chan CK, Maltais F, Sigouin C (2016) A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J 14(8):465
Voshaar T, Lapidus R, Maleki-Yazdi R (2008) A randomized study of tiotropium Respimats soft mist inhaler vs. ipratropium pMDI in COPD. Respir Med 102(1):0–41
Sethi S, Kerwin E, Watz H, Ferguson GT, Mroz RM, Segarra R, Molins E, Jarreta D, Gil EG (2019) AMPLIFY: a randomized, phase III study evaluating the efficacy and safety of aclidinium/formoterol vs monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int J COPD 14:667–682
Niewoehner DE, Rice K, Cote C (2005) Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 144(2):317–326
Moita J, Barbara C, Cardoso J (2008) Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulm Pharmacol Ther 21(1):0–151
Casaburi R, Briggs DD, Donohue JF (2000) The spirometric efficacy of once-daily dosing with tiotropium in stable COPD. Chest 118(5):1294–1302
Covelli H, Bhattacharya S, Cassino C (2012) Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacother J Hum Pharmacol Drug Ther 25(12):1708–1718
Bateman ED, Dyk MV, Sagriotis A (2008) Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: a pilot study. Pulm Pharmacol Ther 21(1):0–25
Maltais F, Hamilton A, Voß F (2019) Dose determination for a fixed-dose drug combination: a phase II randomized controlled trial for tiotropium/olodaterol versus tiotropium in patients with COPD. Adv Ther 36(4):962–968
Chunxue B, Masakazu I, Haak LS (2017) Lung function and long-term safety of tiotropium/olodaterol in East Asian patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 3329-3339
Zhou Y, Zhong N-s, Li X, Chen S, Zheng J, Zhao D, Yao W, Zhi R, Wei L, He B, Zhang X, Yang C (2017) Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med 377(10):923–935
Buhl R, Magder S, Bothner U (2017) Long-term general and cardiovascular safety of tiotropium/olodaterol in patients with moderate to very severe chronic obstructive pulmonary disease. Respir Med 122:58–66
Covelli H, Pek B, Schenkenberger I (2016) Efficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular risk. Int J Chron Obstruct Pulmon Dis 11:1–12
Donohue JF, Van Noord JA, Bateman ED (2002) A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 122(1):47–55
Sin DD, Man SF (2003) Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 107(11):1514–1519
Boekholdt SM, Peters RJ, Hack CE (2004) IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 24(8):1503–1508
Powrie DJ, Wilkinson TMA, Donaldson GC (2007) Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J 30(3):472–478
Acknowledgments
The authors are indebted to all members of the Respiratory Diseases Laboratory of Chengdu Second People’s Hospital and Department of Pulmonary Diseases of West China Hospital.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Mingjin Yang, Jianqing He. Performed the experiments: Mingjin Yang. Analyzed the data: Mingjin Yang, Yan Zhang. Contributed reagents/materials/analysis tools: Hong Chen, Jianqing He. Wrote the first draft of the manuscript: Mingjin Yang, Zhibo Xu. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
As this is a meta-analysis, ethics committee approval is not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingjin Yang is the first author
Rights and permissions
About this article
Cite this article
Yang, M., Zhang, Y., Cheng, H. et al. Association of tiotropium use and the risk of adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 76, 795–805 (2020). https://doi.org/10.1007/s00228-020-02853-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02853-9