Abstract
Introduction
Vancomycin is the usual antibiotic treatment in coagulase-negative staphylococcus sepsis in premature infants but causes renal toxicity. As linezolid is effective in Gram-positive cocci infection, and devoid of renal side-effects, it has been used in Nantes neonatal intensive care units and linezolid plasma concentrations were monitored.
Aim
The aims of this study are to report data on linezolid concentrations in premature infants, describe clinical and bacteriological evolution during treatment, and determine potential side effects.
Methods
A retrospective observational study of premature infants treated with linezolid in Nantes Hospital from January 2008 through November 2011 was conducted. Linezolid plasma concentrations, possible side effects due to linezolid, and clinical response to linezolid treatment were collected from folder review.
Results
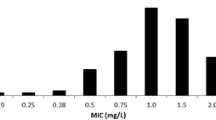
Twenty-four linezolid plasma concentrations were monitored in 16 premature patients, at steady state for continuous intravenous administration or 7 ± 1.5 h after last oral administration. Except for one case, linezolid plasma concentrations were ≥minimal inhibition concentration (MIC) for linezolid for both parenteral and oral administrations. We observed three cases of thrombocytopenia, two of leukopenia, three of neutropenia, and one of severe hyperlactacidemia, resolving after discontinuation of treatment. Clinical signs of infection resolved in 13/16 cases. Bacteria were coagulase-negative Staphylococci in 12/16 cases and were eradicated in 9/12 evaluable cases.
Conclusions
This study reports an adequate linezolid plasma concentration with regard to the linezolid MIC in extremely premature infants. However, considering adverse events reported, its use should be cautious and may concern only oral administration during the late phase of infection, to limit paradoxical catheter use to treat nosocomial infections. Moreover, safe and efficient anti-Staphylococcus therapies should be identified to treat this vulnerable population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Neonatal late-onset sepsis (LOS) is a low-incidence pathology, mostly caused by Gram-positive cocci [3, 19]. In premature infants, the chance of sepsis is increased when a central catheter is inserted [16]. The usual treatment for sepsis involves intravenous administration of vancomycin; however, this glycopeptide can cause renal toxicity [4, 13]. This is especially pertinent for premature infants because they present other risk factors for renal dysfunction (growth retardation, immature renal function, patent ductus arteriosus, hemodynamic failure, and other toxic drug treatments). When renal impairment occurs, vancomycin treatment is discontinued, and the next administration depends on vancomycin plasma concentration which is measured daily, after collection of blood from an invasive blood punction.
Linezolid treatment (an oxazolidinone-based drug) could provide an alternative to vancomycin. Linezolid exhibits good tissue penetration and 100 % bioavailability, which allows oral or parenteral administration and no need for dose adjustments or renal toxicity [5, 9, 10, 12, 18, 20]. One study reported no statistically significant difference in clinical efficacy between vancomycin and linezolid treatment in children aged from birth to 12 years [11]. However, side effects have been described, mostly from biological order (e.g., thrombocytopenia, myelosuppression [14], and lactic acidosis [17]), but also from clinical order, such as optic or peripheral neuropathy, headaches, and intestinal disorders.
In Nantes Medical School, increased linezolid plasma concentration and bactericidal activity from continuous administration of linezolid have been reported in a rabbit model of methicillin-resistant Staphylococcus aureus-induced endocarditis [8], compared to the same daily dose administered through intermittent dosing regimen. Therefore, linezolid was occasionally used in neonatal intensive care unit to treat known or suspected LOS induced by Gram-positive cocci through a continuous intravenous route (30 mg kg−1 day−1). Linezolid was used as a rescue treatment during the acute phase of infection, in cases of renal dysfunction, to prevent vancomycin toxicity and daily vancomycin plasma monitoring. Moreover, linezolid was also administered through an oral route (10 mg/kg every 8 h) when neonates were stabilized in the late phase of infection and intravenous access was no longer available for vancomycin treatment or needed to provide nutrition. This prevented insertion of a new catheter. During treatment, linezolid concentrations were monitored to compare the plasma concentrations with the minimum inhibitory concentration (MIC) when treatment was intravenously administered and to check for absorption when it was orally administered.
Because there are few studies on linezolid plasma levels and linezolid use in the neonatal population, the main aim of this work was to present data on linezolid pharmacokinetics in premature infants, describe the clinical and bacteriological evolution during linezolid treatment, and determine the potential side effects.
Methods
Patients and study design
We conducted a retrospective observational study from the files of premature infants, born before 37 weeks of gestational age (GA) and treated with linezolid from January 2008 to November 2011, in the Nantes University Hospital. Within these files, the linezolid plasma concentrations, duration of administration, postnatal age, and MIC were described. MIC were determined using E-tests (BioMérieux France). Linezolid plasma concentrations were performed by a high-performance liquid-chromatography (HPLC) system (Alliance 2695 HPLC, Waters Corp., Milford, MA, USA) coupled with photo diode array (PDA) detector, at 258 nm (Waters 2996 PDA). Plasma samples (200 μL) were extracted by liquid-liquid extraction followed by evaporation to dryness and reconstitution in mobile phase solution. The chromatographic separation was carried out on reversed-phase column (Symetry C8, 4.6 × 150 mm, 5 μm (Waters Corp., Milford, MA, USA)) with an isocratic mobile phase consisting of dihydrogen phosphate buffer 50 mm (pH 3.6) and acetonitrile (75:25 v/v). Data were acquired using Empower 2 (Waters Corp., Milford, MA, USA). Under these conditions, a single chromatographic run could be completed within 10 min. Analytical methods was linear (r(2) > 0.99) over the calibration range of 0.5–20 mg/L. Limit of quantification was 0.5 mg/L. Intra- and inter-day precision (RSD %) and accuracy (%) were <15 %.
We first reported the potential biological side effects during linezolid treatment: considering the baseline hematologic values before treatment, abnormalities were defined as a decrease of >75 % compared to the baseline platelet counts and hemoglobin or a decrease >50 % compared to the baseline ranges for white blood cells and neutrophils, an increase in plasma creatinine levels of 10 mmol/L for renal failure, and a rise in lactate rate >3 mmol/L for hyperlactacidemia [15].
Clinicians in charge of children used the clinical definition to identify LOS: presence of clinical signs of infection (i.e., fever, hypothermia, tachycardia, apnea, heightened need for oxygen, increased ventilator settings, requirement for mechanical ventilation, and diminished oxygen saturation); modification of blood parameters (blood cell count and C-reactive protein increase); and pathogen identification from a sterile site (e.g., blood, trachea, or liquid from an articular puncture) (ANAES [1]). We described the evolution of the clinical signs of infection using the data reported by the clinicians. The bacteriological evolution was described when a pathogen had been identified and when a control culture was available.
Ethical considerations
Parents were informed by the clinicians at the onset of linezolid treatment to their children. We retrospectively conducted an anonymous observational cohort study on the medical file data. No parental written consent was required. As non-interventional retrospective study on folders, with complete anonymity of data was conducted, local ethics committee consultation was facultative.
Statistical analysis
Statistical analysis was performed using Stat Soft. Inc./USA Statistica 10.0 software. The demographic and clinical data were analyzed using the Mann–Whitney test for the continuous variables, and the chi-squared and Fisher’s exact tests were employed for the discontinuous variables. The data are reported as mean ± standard deviation, and a p value of <0.05 was considered significant.
Results
Sixteen children received linezolid treatment during the study period (Table 1). They were extremely premature; 12 were born before 28 weeks of GA. Mean postnatal age at the beginning of treatment was 3 weeks. Each child had previously received vancomycin treatment (15 mg/kg followed by 30 mg kg−1 day−1 through a continuous intravenous route). For children that experienced renal failure, 84.2 % received linezolid treatment. Oral treatments were given to 5/16 (31.3 %). Linezolid administered dose was 10 mg kg−1 every 8 h through oral route or 30 mg kg−1 day−1 through continuous intravenous route.
Twenty-four linezolid plasma concentrations were monitored in 16 patients, representing 17 episodes of infection, as patient 15 was treated for two different infections, one documented with Staphylococcus epidermidis, and the second one not documented (Table 2). Blood sampling to quantify linezolid plasma concentrations occurred at 89.5 ± 63.6 h after start of treatment, which is considered to be at steady state for continuous intravenous administration in most cases. Concentration after oral administration was monitored at 7 ± 1.5 h after the last dose. Except for one case, linezolid plasma concentrations were ≥MIC for linezolid for both parenteral and oral administrations. No association was observed between linezolid plasma concentration and side effects.
We observed three cases of thrombocytopenia, two of leukopenia, and three of neutropenia. There was one case of severe hyperlactacidemia (maximal lactate rate of 10.4 mmol/L) after 4 days of intravenous treatment in a premature infant born at 24.2 weeks of GA. This child had received linezolid because he suffered from acute renal failure and oliguria. All of these side effects were resolved after discontinuation of treatment.
The clinical evolution was assessed in all 16 cases. The clinical symptoms of infection were resolved in 13 cases; two patients died (extremely premature, presenting bronchopulmonary dysplasia, patent ductus arteriosus, and sepsis state), and one had no resolution of symptoms during linezolid treatment (this child presented a posteriori a Pseudomonas aeruginosa infection and was given ceftazidime 24 h later).
Bacteria were identified in 14/16 cases and were coagulase-negative staphylococci in 75 % of the cases (6/16 S. epidermidis and 6/16 Staphylococcus haemolyticus) and S. aureus in 1/16 cases. MIC values were known for every case except two. We described the bacterial eradication in 9/12 of the evaluable cases.
Discussion
To the best of our knowledge, this is the first study to report on concentration data of linezolid use in a premature population. Considering elimination half-life of 5 h in premature population, we observed an adequate linezolid plasma concentration with regard to the linezolid MIC, at steady state when continuous infusion was used, or at residual point, before following dose, when it was intermittent oral administration. These results in premature population are of interest, as linezolid is a time-dependent antimicrobial and duration concentration is superior to MIC is a good predictor of treatment efficacy.
We described the biological side effects with treatment; however, the imputability of linezolid in this small cohort of ill newborns was difficult to assess. Nevertheless, the occurrence of hyperlactacidemia after only a short treatment is of concern. This side effect could be a result of the immaturity of mitochondrial protein synthesis in premature children [7]. The clinical and bacteriological evolution was adequate, but the relationship between efficacy and linezolid treatment cannot be established because of the limited number of patients in the study. A significant proportion of patients died during treatment (12.5 %), but previously published data indicated that extremely premature infants (born before 28 weeks of GA) present a high mortality rate, from 30 to 62 % depending on birth weight [6].
One of the main interests in linezolid use for LOS in premature infants is the possibility of oral administration with good bioavailability, which would avoid the catheter-associated risk of sepsis. Indeed, oral administration could be a solution to the paradoxical issue of further infection induced by insertion of catheters to treat nosocomial infections.
Nevertheless, it is important to identify other drug options to treat Gram-positive cocci-induced infections in the premature population because of the potential side effects associated with linezolid treatment. Daptomycin, a lipopeptide antibiotic, could be a candidate to treat Staphylococcus, as well as Enterococcus-resistant infections [2]. Ceftaroline, a new broad-spectrum cephalosporin, might be another alternative, exhibiting interesting bactericidal activity against Gram-positive organisms; however, there are no data in the premature population available yet.
Conclusion
This study reports an adequate linezolid plasma concentration with regard to the linezolid MIC and could be an interesting therapeutic alternative in extremely premature newborns because of its oral delivery. However, the reported adverse events associated with this treatment, especially hyperlactacidemia, must be taken into account before prescribing. Use of this treatment in premature infants should be cautious and may concern only oral administration during the late phase of infection, allowing clinicians to limit paradoxical catheter use to treat nosocomial infections. Because of the burden of coagulase-negative Staphylococcus infections in neonates, it is urgently necessary to conduct studies that compare different anti-Staphylococcus therapies and identify the best compromise between efficacy and toxicity in this vulnerable population.
References
ANAES (2002) Recommandation pour la pratique clinique: Diagnostic et traitement curatif de l’infection bactérienne précoce du nouveau-né
Beiras-Fernandez A, Vogt F, Sodian R et al (2010) Daptomycin: a novel lipopeptide antibiotic against Gram-positive pathogens. Infect Drug Resist 3:95–101
Cohen-Wolkowiez M, Moran C, Benjamin DK et al (2009) Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J 28:1052–1056
Fratarelli DAC, Ergun H, Lulic-Botica M et al (2005) Vancomycin elimination in human infants with intra uterine growth retardation. Pediatr Infect Dis J 24:979–983
French G (2003) Safety and tolerability of linezolid. J Antimicrob Chemother 51(S2):ii45–ii53
Guellec I, Lapillonne A, Renolleau S et al (2011) Neurological outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics 127:e883–e891
Honzik T, Wenchich L, Böhm M et al (2006) Activities of respiratory chain complexes and pyruvate dehydrogenase in isolated muscle mitochondria in premature neonates. Early Hum Dev 84:269–276
Jacqueline C, Batard E, Perez L et al (2002) In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob Agents Chemother 46:3706–3711
Jones RN, Stilwell MG, Hogan PA et al (2007) LEADER surveillance program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers). Diag Microbiol Infect Dis 309–317
Jungbluth GL, Welshman IR, Hopkins NK (2003) Linezolid pharmacokinetics in pediatric patients: an overview. Pediatr Infect Dis J 22:S153–S157
Kaplan SL, Deville JG, Yogev R et al (2003) Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr Infect Dis J 22:677–685
Kearns GL, Jungbluth GL, Abdel-Rahman SM et al (2003) Impact of ontogeny on linezolid disposition in neonates and infants. Clin Pharmacol Ther 74:413–422
McKamy S, Hernandez E, Jahng M et al (2011) Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr 158:422–426
Meissner HC, Towsend T, Wenman W et al (2003) Hematologic effects of linezolid in young children. Pediatr Infect Dis J 22:S186–S192
Saiman L, Goldfarb J, Kaplan SA et al (2003) Safety and tolerability of linezolid in children. Pediatr Infect Dis J 22:S193–S200
Sohn AH, Garrett DO, Sinkowitz-Cochran RL et al (2001) Prevalence of nosocomial infections in neonatal intensive care unit patients: results from the First National Point-Prevalence Survey. J Pediatr 139:821–827
Su E, Crowley K, Carcillo JA, Michaels MG (2011) Linezolid and lactic acidosis: a role for lactate monitoring with long-term linezolid use in children. Pediatr Infect Dis J 30:804–806
Swaney SM, Aoki H, Ganoza MC et al (1998) The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother 42(12):3251–3255
Van den Hoogen A, Gerards LJ, Verboon-Maciolek MA et al (2010) Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology 97:22–28
Vardakas KZ, Ntziora F, Falagas ME (2007) Linezolid: effectiveness and safety for approved and off-label indications. Expert Opin Pharmacother 8:2381–2400
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sicard, M., Launay, E., Caillon, J. et al. Pharmacokinetics of linezolid treatment using intravenous and oral administrations in extremely premature infants. Eur J Clin Pharmacol 71, 611–615 (2015). https://doi.org/10.1007/s00228-015-1813-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1813-3



