Abstract
Antipredator behavior of the paralarval stages of merobenthic octopuses is largely unknown. Reported here is an investigation of the behavioral response of Octopus sinensis paralarvae to the sympatric predator fish Girella punctata. Fishes of this species exhibited predatory behavior by engulfing individual paralarvae, but most fishes almost immediately rejected the paralarva from the mouth. No paralarvae died after being engulfed and ejected. The number of such temporary engulfings of a paralarva by a fish during the test period (10 min) decreased with the increase in the number of times a paralarva was engulfed. The frequency of engulfing attacks by the fish also declined. The paralarvae did not eject ink before being engulfed, but 43% of the paralarvae exhibited inking immediately after ejection from the fish’s mouth. The fishes ingested the ink masses released by the paralarvae, and the proportion of fish ingesting the ink masses increased with increasing experience of encountering the ink. When the paralarvae ejected ink, the time interval between first engulfing and subsequent engulfing increased, apparently in relation to distraction of the fish by the ink. These results suggest that paralarvae have an antipredatory mechanism which causes predatory fish to eject them after they have been engulfed. In addition, the paralarvae use the stimulus of being ejected from the mouth of a fish as a cue for ink ejection. The ink mass may be attractive to the fish as a food item because of its potential action as a phagomimetic distraction, enabling the paralarvae to escape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benthic octopuses possess various antipredator responses including withdrawing into crevices, camouflage (changing their skin color and texture to match the background), threatening (by using deimatic skin patterning), mimicking other animals, and ejection of ink, which may induce chemical and visual disruption of predators (Hanlon and Messenger 2018). These responses rely upon the morphological traits characteristic of octopuses, such as a soft body, arms with many suckers, an ink sac, and versatile skin with numerous specialized elements (chromatophores, iridophores, and leucophores; Messenger 2001; Hanlon and Messenger 2018). Many octopus species have a merobenthic life history, in which the offspring spend a period of time as part of the plankton before taking up a benthic life. Although the planktonic form shares the same basic body plan as the adult (and thus is termed a “paralarva”; Young & Harman 1988), they require antipredator strategies during their planktonic life which differ from those during benthic life. However, very few studies have reported on the antipredator behavior of octopus paralarvae (Villanueva and Norman 2008).
Several morphological traits of the paralarvae have been considered relevant to antipredator strategy. The transparent musculature and silvery membranous layer of opaque viscera reduce contrast against the background and thereby reduce visual detection of paralarvae by predators in the water column. In addition, chromatophores and ink ejection are known to be functional even in newly hatched paralarvae (Villanueva and Norman 2008). Using ejection of an ink decoy known as a “pseudomorph” in combination with camouflage enabled by the chromatophores, along with the transparent body and escape by jetting, is effective to confuse predators and enable successful escape. A similar sequence has been reported in squid and cuttlefish species, both in the natural habitat and in the laboratory, which is known as the “blanch-ink-jet manoeuvre” (Hanlon and Messenger 1988, 2018; Crook et al. 2014; York and Bartol 2016). In this sequence, the potential prey animal detects the approach of a predator and shifts from a passive mode of preventing visual detection by the predator (such as camouflage; i.e., primary defense) to an active mode of avoiding attack by the predator, such as the blanch-ink-jet maneuver (i.e., secondary defense). There is an apparent dilemma in that the transparent paralarva becomes more detectable after switching to the blanch-ink-jet maneuver sequence: if its timing is earlier than (or close to) the timing of detection by the predator, this maneuver may not work. Indeed, it has been reported that the squid paralarvae did not exhibit inking behavior when they encountered with predatory fish (York and Bartol 2016). It is largely unknown if or how the octopus paralarva can detect the decision of the predator to attack, and (upon detection of an imminent attack by the predator) how they switch from primary defense to secondary defense.
This study aimed to investigate the behavioral response of octopus paralarvae to predatory fish. The East Asian common octopus Octopus sinensis d’Orbigny, 1841, was selected as the model species. Because this octopus is an important fishery resource and has an important role in the marine benthic ecosystem as a predator (Boyle and Rodhouse 2005; Sauer et al. 2021), it is important to understand their antipredator strategy during their early life history, especially since it may drive the population dynamics of the species.
As a predator, the nibbler fish Girella punctata Gray, 1835 was selected because it is sympatric with O. sinensis and recognized as an omnivorous fish fed on various organisms including zooplankton (Yasuda 1960; Yagishita and Nakabo 2003; Fishelson et al. 2014). Newly hatched paralarvae of O. sinensis were offered to the nibbler fish, and their behavior was video recorded. The goals were to clarify: (1) the behavioral response of the paralarvae when they are detected by the predator; and (2) how the blanch-ink-jet maneuver works by changing the behavior of predatory fish.
Materials and methods
Animals
Two wild female O. sinensis were caught by octopus trap and pot in the Seto Inland Sea, off Oita (33° 46 N, 131°22 E) and Okayama (34° 25 N, 133°47 E), Japan, respectively. They were reared individually in a rectangular fiberglass tank or a cylindrical polyethylene tank with flow-through water systems until spawning in the Fisheries Research Division, Oita Prefectural Agriculture, Forestry and Fisheries Research Center or the Research Institute for Fisheries Science, Okayama Prefectural Technology Center for Agriculture, Forestry, and Fisheries. After they started spawning, ten egg strings were sampled from each female and transported to the laboratory at Tokyo University of Marine Science and Technology (TUMSAT) on 28 September 2022 from Oita and 4 October 2022 from Okayama. The egg strings were maintained until hatching in aquaria according to the method of Dan et al. (2023). Paralarvae (aged 0 d) hatched from 12 to 30 October 2022 were used for the experiments on the day of hatching.
Thirty G. punctata were collected from the natural habitat at the boulder shore of Izu City, Shizuoka, Japan (34° 55 N, 138°47 E) on 27 September 2022. They were transported to the laboratory at TUMSAT and reared until use in four glass aquaria (450 × 300 × 350 mm, water volume 40-L) filled with artificial seawater (33 g L−1 salinity; Sealife, Marinetech Co. Ltd., Tokyo, Japan). They were supplied with the frozen shrimp Acetes japonicus as feed. Total length (mean ± SD) of the fishes was 67.8 ± 1.8 mm.
Dormant dried cysts of Artemia franciscana (Utah strain; Ocean Star International Inc., Burlingame, USA) were hatched and cultured for more than a month according to the method of Dan et al. (2018). The total length of the Artemia used for the experiments was 6.39 ± 0.73 mm.
Experiment 1: antipredator behavior of O. sinensis paralarvae in response to G. punctata
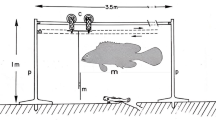
Before conducting this experiment, various fish imitations were offered to the paralarvae to test behavioral response, but no behavioral traits were observed. Therefore, to investigate the nature of antipredator response of O. sinensis paralarvae, the experiment offering the paralarvae to the sympatric potential predator fish G. punctata was carried out, while using the fewest number of individuals possible to ensure results. Individual G. punctata were placed in an acrylic rectangular aquarium (150 × 150 × 150 mm, water depth 130 mm) filled with artificial seawater. Prior to the experiment, the fish was starved for 12 h. The test aquarium was enclosed by black PVC boards or screens. Stable lighting was supplied from an LED light source in a white PVC tank lid. A white PVC board was also placed along one side of the aquarium as a background for video recording (Fig. S1). The fish was acclimated for 1 h in the test aquarium, and then a paralarva was introduced using a large-bore glass pipette. The behavior of the paralarva and fish was recorded using a video camera (HC-VX992M; Panasonic Corporation). The test period was terminated at 10 min from the introduction of the paralarva, and then the paralarva was removed from the test aquarium. After a wait of 5 min, another paralarva was offered to the same test fish, and this procedure was repeated 5 times (Trials 1 − 5) for 30 fishes (total 150 paralarvae). The seawater was changed when the test fish was changed.
After the experiment, the test animals were returned to the rearing tank and was not used for any additional experiments. The mantle length of 30 randomly sampled paralarvae was measured under a microscope: the value was 1.92 ± 0.23 mm. Before taking measurements, the paralarvae were anesthetized in ethanol at a concentration of 10 mL L−1, following the guidelines established by Directive 2010/63/EU (Andrews et al. 2013; Gleadall 2013; Fiorito et al. 2015; Escánez et al. 2018). Water temperature, pH, dissolved oxygen concentration, salinity, and photon flux density were measured at the end of the test sequence for each fish, producing the following respective mean values: 20.6 ± 0.7 °C, pH 8.32 ± 0.20, 8.37 ± 0.52 mg L−1, 32.0 ± 0.8 g L−1, and 25.1 ± 4.7 µmol m−2 s−1.
Video-recorded behavior of the paralarvae and predatory fish was observed using a VLC media player (version 3.0.18), and the time (s) at which the fish engulfed the paralarva (by its suction feeding mechanism: see, for example, Alexander 1967; Lauder 1982) was recorded, along with the number of engulfments. Many fish engulfed the paralarva but rejected it (Fig. 1, see Results section). Some of the paralarvae ejected an ink mass and the fish ate it. Therefore, the following events were recorded: whether or not the paralarva was rejected after being engulfed by the fish; whether or not the paralarva ejected an ink mass; and whether or not the fish ingested the ink mass.
Experiment 2: difference in predatory behavior of G. punctata on O. sinensis paralarvae and Artemia
To investigate the characteristic predatory behavior of the predatory fish on O. sinensis paralarvae, the feeding behavior of G. punctata was compared between paralarvae and Artemia. Each G. punctata individual was placed in a test aquarium (as in Experiment 1) and acclimated for 1 h. Then a paralarva and five Artemia were introduced into the aquarium simultaneously using a large-bore pipette, and their behavior was video-recorded for 10 min. Video recording was conducted once for 15 fishes, which were randomly selected from the 30 fishes used in Experiment 1. The number of Artemia preyed on by the fish, and whether or not the paralarvae were preyed on by the fish, was observed from the video recordings.
Experiment 3: predatory behavior of G. punctata on immobilized O. sinensis paralarvae
To investigate the effect of behavior (movement) of O. sinensis paralarvae on the predatory behavior of G. punctata, immobilized paralarvae were offered to the fish and the behavior was video recorded. Individual fish was placed individually into a test aquarium (as for Experiment 1). After acclimation for 1 h, an immobilized paralarva was introduced using a large-bore pipette and the engulfing behavior of the fish was observed, as well as whether or not the paralarva was eaten by the fish. Paralarvae were immobilized by decreasing water temperature gradually from 21 °C to 4 °C in a 100 mL glass beaker placed in an incubator (hypothermia: cf. Agnisola et al. 1996; Andrews and Tansey 1981; Gleadall 2013) to avoid the introduction of anesthetic agents that may affect the predatory fish. The test was conducted once for each of 9 fishes which were randomly sampled from the 30 fishes used in Experiment 1.
Data analysis
Statistical analyses were performed using R statistical software (R4. 2. 2; R Core Team 2022) using a significance level of 5%.
To evaluate whether the incidences of the behavior of the predatory fish and paralarvae occurred randomly or intentionally, a binomial test (binom.test function) was applied to test the null hypothesis for the ratios of the paralarvae eaten, the paralarvae engulfed (captured), the paralarvae subsequently rejected, and the paralarvae releasing an ink mass (H0; ratio = 0.5).
To assess the factors affecting the predatory behavior of the fish G. punctata on O. sinensis paralarvae, a generalized linear mixed-effect model (GLMM) was applied using the glmer function in the lme4 package (Bates et al. 2015; Zuur et al. 2009). In Experiment 1, the fish captured the paralarva repeatedly during the 10 min test period. Therefore, to test the effect of capture experience on capture frequency of the fish, the number of captures over a 10 min period was used as a response variable with a Poisson distribution (log link), while the trial number (Trials 1 − 5, in chronological order) was used as an explanatory variable. The GLMM was also applied to test factors influencing the time interval between captures. In this analysis, the time interval (in sec) between a capture and a subsequent capture was used as a response variable with Gamma distribution (log link), and the explanatory variables were the trial number (1 − 5) and the rank order of the captures during the test period (10 min).
In addition, to evaluate the effect of inking on the predatory behavior of the fish, the Gamma GLMM was employed to evaluate the effect of inking (explanatory variable, inking: 1, not inking: 0) on the time interval between captures (response variable). The effect of experience of the fish encountering an ink mass on the frequency of ink-feeding behavior was also evaluated with the GLMM. In this analysis, whether the fish fed on the ink mass or not is a response variable (binomial distribution with logit link, fed: 1, not fed: 0) and the trial number (1 − 5) was an explanatory variable. In these GLMM analyses, the identity of the fish was included as a random intercept effect.
Results
Experiment 1: antipredator behavior of O. sinensis paralarvae in response to G. punctata
The number of paralarvae eaten by the fish ranged from 2 to 4 individuals in each trial, while the majority of the paralarvae (26 − 28 individuals) were not eaten (Fig. 2A). The proportion of paralarvae eaten by the fishes was non-randomly biased in all trials (P < 0.0001, Table S1). When paralarvae were not eaten by the fishes, all, or almost all, paralarvae were engulfed (captured) by the fishes several times (5 − 71 times in Trial 1) during the 10 min test period (Fig. 2B). Thus, the proportions of paralarvae captured by the fishes was high in all trials compared to the number not captured (binomial tests, P < 0.0009, Table S2). These results indicate that the fishes captured the paralarvae but did not eat them in most instances. This characteristic behavior of the fishes and paralarvae was observed: the paralarvae were rejected immediately after they were captured by the fishes, and then the paralarvae escaped by swimming (flight: see Supplemental Videos SV1 − 2). All the paralarvae rejected by the fishes were alive at the end of the test period.
A Proportion of paralarvae of Octopus sinensis that were eaten or not eaten by the predatory fish Girella punctata. B Proportion of paralarvae that were engulfed (but rejected) or not engulfed by the predatory fish. C Proportion of paralarvae that ejected or did not eject an ink mass. D Proportion of predatory fish that ate the ink mass. Trials were carried out in chronological order. Values on the bars indicate the number of observed individuals. Values above the bars indicate the number of individuals examined
The number of captures (engulfing) by fish on individual paralarvae was large in Trial 1, but decreased markedly after Trial 2 (Fig. 3A, Table S3). There was a significant negative correlation between the number of captures and the trial number (Table 1). In relation to the decrease in the number of captures, the time interval between captures was significantly extended as the trials proceeded (Table 1, Fig. 3B). In addition, the time interval between captures became significantly longer as the rank order of the capture event proceeded (Tables 1, S3 − 8). Taken together, the captures (and rejections) by the predatory fish became less frequent as the experience of the fish capturing the paralarvae increased.
None of the paralarvae exhibited ink ejection before being captured by the fish, and all instances of inking behavior were observed immediately after the paralarvae had been captured and rejected by the fish. The proportion of the paralarvae exhibiting ink release ranged from 36.0 to 60.7% in each trial (Fig. 2C, Supplementary Video S2, S3), and these values were not biased (P > 0.2295, Table S9). The ink masses ejected by the paralarvae were ingested by the fishes in proportions ranging from 30.0 to 88.9% (Fig. 2D, Supplementary Video SV3). The proportion of fishes consuming the ink mass increased significantly as the trials proceeded (Table 1). When a paralarva ejected an ink mass, the time interval between captures increased significantly compared to that between captures that did not result in ink ejection (Table 1, Fig. 4).
Experiment 2: difference in predatory behavior of G. punctata on O. sinensis paralarvae and Artemia
Among 15 test fishes, 11, 1, and 2 fishes consumed 5 (all), 3, and 2 individuals of supplied Artemia, and only 1 fish did not eat any Artemia. Regarding the predatory behavior of fish on the paralarvae, 12 fishes captured the paralarvae, but all paralarvae were rejected (Supplementary Video S4) and no paralarvae were eaten by any of the fishes.
Experiment 3: predatory behavior of G. punctata on immobilized O. sinensis paralarvae
Among nine test fishes, eight fishes captured the immobilized paralarvae at frequencies of 1 − 4 times during the test period. The total number of captures was 13, but only 1 paralarva was eaten by a fish during the test period. A binomial test revealed that the proportion of paralarvae actually eaten among those captured was significantly low (H0 = 0.5, Probability: 0.077, 95% CI: 0.002 − 0.360, P = 0.0034). Thus, the fish captured the immobilized paralarvae but rarely ate them.
Discussion
In Experiment 1, G. punctata exhibited capture behavior on the paralarvae of O. sinensis. However, most fishes did not eat the paralarvae. These results indicate that the fish recognized the paralarvae as prey organisms and attempted to eat them by capturing them over multiple engulfing attacks, but subsequently chose not to eat them. In contrast, the fish successfully ate Artemia concurrently supplied with the paralarvae in Experiment 2. This strongly suggests that the paralarvae possess some kind of countermeasure(s) to avoid being eaten by G. punctata.
The obvious indication that ultimately the fish aborted preying on the paralarvae was rejection of the paralarvae by the fish after first capturing them. Similar behavior was observed with parrot fish Oplegnathus fasciatus Temminck & Schlegel, 1844, which is another predatory fish sympatric with O. sinensis (Dan pers obs). This suggests the presence of some antipredator strategy, because all paralarvae survived and exhibited normal swimming behavior after being captured and rejected by the fish. In addition, the increasing experience of capturing paralarvae by the fish (trial number and capture instance rank order) appears to cause a decrease in the capture incidence by the fish and an increase in the time interval between capture instances, suggesting that the capture experience reduces the fish’s motivation for predatory behavior. It is well known that there are many antipredator strategies in cephalopods after they have been detected by a predator (i.e., secondary defense) such as deimatic and protean behavior, and inking (Hanlon and Messenger 2018). However, a strategy whereby the predator rejects the prey animal has not been reported so far in cephalopods.
In Experiment 3, it was confirmed that immobilized paralarvae, too, were rejected by the fish, indicating that the factor causing rejection might not relate to some active behavior by the paralarva inside the mouth. It is well known that most fishes exhibit suction feeding in which food is once engulfed with water into the oral cavity (mouth) to test the edibility before ingestion (Gerking 1994). Taste bud is known as the primary sense organ which evaluates the edibility and food quality. Indeed, G. punctata has relatively high-density distribution of taste buds (5900) in the oral cavity, indicating that G. punctata test the engulfed paralarvae by the taste buds (Fishelson et al. 2014). There seems to be some chemical (bad taste) or morphological (physical) defense involved. Regarding chemical defense, there is a possibility that the paralarvae secrete some type of repellent, or the fishes already have experience of the (‘bad’) taste of the paralarvae. It is well known that the paralarvae of merobenthic octopuses possess characteristic Kölliker’s organs (KO), which are tufts of chitinous setae within small pockets on the skin surfaces (Boletzky 1973; Villanueva et al. 2021). It has been shown that the paralarvae of O. sinensis, too, possess KOs (Villanueva et al. 2021). Although the functions of KOs are unknown, there is a possibility that the tufts of chitinous setae irritate taste buds or other receptors distributed in the oral cavity of the predatory fish (Gerking 1994; Fishelson et al. 2014). Further research is required to clarify the function of KOs in paralarva ejection behavior by the fish, as well as the potential of chemical defense of the paralarvae.
There were no instances when the paralarvae ejected ink masses before being engulfed by the predatory fish. This indicates that the decision of the paralarva to eject ink does not depend on the detection by the paralarva of an attack by the fish at least for newly hatched paralarvae. This is consistent with the response of squid paralarvae to the predator; it has been reported that the squid paralarvae rarely eject ink mass when they encounter with predator fish (York and Bartol 2016). However, in case of O. sinensis, a certain part (43%) of the paralarvae ejected ink mass immediately after the paralarva was ejected by the fish, suggesting that the stimulus of physical contact of paralarval body with fish mouth and subsequent rejection from the mouth of the fish may be a cue to release ink. This in turn suggests that the shift of antipredator strategy from primary defense (crypsis) to secondary defense (response against the predator) is not active but passive in the paralarva of O. sinensis. This differs from the responses of juveniles and adults of squid and cuttlefish species, which show the “blanch-ink-jet manoeuvre” (Hanlon and Messenger 2018; York and Bartol 2016). Nevertheless, the proportion of paralarvae exhibiting ink release was not markedly high (at 43%), since being ejected did not always induce inking.
The number of ink masses ejected by a paralarva was one or two (see Supplementary Video SV2, SV3). Taking the small somatic size of paralarvae into account, the capacity in terms of ink amount and frequency of ink release are likely to be limited. Further research is needed to understand how the paralarvae decide whether or not to release ink, and any trade-off between the energy cost of inking and its effect on reduction of predatory risk.
The fishes ingested the ink masses ejected by the paralarvae, and the proportion of fish ingesting ink increased as their experience of encountering an ink mass increased. These results suggest that the ink is somehow attractive to the predatory fish perhaps because of “phagomimicry” rather than (or in addition to) visual attraction by a “pseudomorph” (Wood et al. 2010; Hanlon and Messenger 2018). It is known that the ink of octopuses includes various free amino acids that may provide it with phagomimetic potency (Derby et al. 2007). By distracting the fish, the pseudomorph ink mass may allow the paralarvae time to make good their escape before another attack by the fish. This implies that inking may increase the success rate of paralarvae escaping from the predator. At the same time, because the ink may stimulate the appetite of the fish and promote another search for prey, there is an apparent dilemma that inking results in an increase of predation risk for the paralarvae, if the paralarvae release an ink mass before being detected by the predator. The strategy of ink ejection after being rejected seems a reasonable solution to this contradiction.
The results of this study showed a characteristic antipredator strategy of the paralarva of O. sinensis, as summarized in Fig. 5. The paralarvae devote themselves to primary defense as they have transparent musculature and a silvery membranous layer over their opaque viscera even when the predatory fish approaches. If the paralarvae are unfortunate to be detected by the predator, they are engulfed once by the fish but then are rejected. After being rejected, the paralarvae flee by swimming away and/or eject an ink mass. The paralarvae can gain time for escape probably due to the phagomimetic potency of the ink that can attract the predatory fish for a certain period of time. Thus, the paralarvae of merobenthic octopuses have characteristic, effective antipredator strategies although the details of how these strategies are effective requires further investigation.
Data availability
All relevant data are within the paper and its Supporting Information files.
References
Agnisola C, Castaldo P, Fiorito G (1996) Octopus vulgaris (Mollusca, Cephalopoda) as a model in behavioral pharmacology: a test of handling effect. Physiol Behev 59:729–733. https://doi.org/10.1016/0031-9384(95)02153-1
Alexander RM (1967) Functional design in fishes. Hutchinson, London
Andrews PLR, Tansey EM (1981) The effects of some anaesthetic agents in Octopus vulgaris. Comp Biochem Physiol C Comp Pharmacol 70:241–247. https://doi.org/10.1016/0306-4492(81)90057-5
Andrews PLR, Darmaillacq A-S, Dennison N, Gleadall IG, Hawkins P, Messenger JB, Osorio D, Smith VJ, Smith JA (2013) The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J Exp Mar Biol Ecol 447:46–64. https://doi.org/10.1016/j.jembe.2013.02.010
Bates DM, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects model using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Boletzky SV (1973) Structure and function of the Kölliker organs in young octopods (Mollusca, Cephalopoda). Z Morphol Tiere 75:315–327. https://doi.org/10.1007/bf00288477
Boyle PR, Rodhouse PGK (2005) Cephalopods: ecology and fisheries. Blackwell Science Ltd, Oxford
Crook RJ, Dickson K, Hanlon RT, Walters ET (2014) Nociceptive sensitization reduces predation risk. Curr Biol 24:1121–1125. https://doi.org/10.1016/j.cub.2014.03.043
Dan S, Iwasaki H, Takasugi A, Yamazaki H, Hamasaki K (2018) An upwelling system for culturing common octopus paralarvae and its combined effect with supplying natural zooplankton on paralarval survival and growth. Aquaculture 495:98–105. https://doi.org/10.1016/j.aquaculture.2018.05.036
Dan S, Yamamoto Y, Nishiwaki D, Matsunari H, Kado Y, Yamaki D, Takeshima S, Kamei Y, Hara S, Sakiyama K, Isojima N, Narita A, Hamasaki K (2023) Dietary effects of intensively reared swimming crab Portunus trituberculatus zoeae on survival and growth of East Asian common octopus Octopus sinensis paralarvae. Aquaculture 573:739617. https://doi.org/10.1016/j.aquaculture.2023.739617
Derby CD, Kicklighter CE, Johnson PM, Zhang X (2007) Chemical composition of inks of diverse marine molluscs suggests convergent chemical defenses. J Chem Ecol 33:1105–1113. https://doi.org/10.1007/s10886-007-9279-0
Escánez A, Rubio J, Riera R, Almansa E (2018) Assessment of various anesthetic agents on Octopus vulgaris paralarvae. J World Aquac Soc 49:1019–1025. https://doi.org/10.1111/jwas.12444
Fiorito G, Affuso A, Basil J, Cole A, Girolamo P, D’Angelo L, Dickel L, Gestal C, Grasso F, Kuba M, Mark F, Melillo D, Osorio D, Perkins K, Ponte G, Shasher N, Smith D, Smith J, Andrews PLR (2015) Guidelines for the care and welfare of cephalopods in research -a consensus based on an initiative by CephRes, FELASA and the boyed group. Lab Anim 49:1–90. https://doi.org/10.1177/0023677215580006
Fishelson L, Golani D, Diamant A (2014) SEM study of the oral cavity of members of the Kyphosidae and Girellidae (Pisces, Teleostei), with remarks on Crenidens (Sparidae), focusing on teeth and taste bud numbers and distribution. Zoology 117:122–130. https://doi.org/10.1016/j.zool.2013.10.012
Gerking SD (1994) Mouth and sense organs. In: Gerking SD (ed) Feeding ecology of fish. Academic Press, New York, pp 15–40
Gleadall IG (2013) The effects of prospective anaesthetic substances on cephalopods: summary of original data and a brief review of studies over the last two decades. J Exp Mar Biol Ecol 447:23–30. https://doi.org/10.1016/j.jembe.2013.02.008
Hanlon RT, Messenger JB (1988) Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Philos Trans R Soc Lond B Biol Sci 320:437–487. https://doi.org/10.1098/rstb.1988.0087
Hanlon RT, Messenger JB (2018) Cephalopod behaviour, 2nd edn. Cambridge University Press, Cambridge
Lauder GV (1982) Patterns of evolution in the feeding mechanism of actinopterygian fishes. Am Zool 22:275–285. https://doi.org/10.1093/icb/22.2.275
Messenger JB (2001) Cephalopod chromatophores: neurobiology and natural history. Biol Rev 76:473–528. https://doi.org/10.1017/S1464793101005772
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Sauer WHH, Gleadall IG, Downey-Breedt N, Doubleday Z, Gillespie G, Haimovici M, Ibáñez CM, Katugin ON, Leporati S, Lipinski MR, Markaida U, Ramos JE, Rosa R, Villanueva R, Arguelles J, Briceño FA, Carrasco SA, Che LJ, Chen CS, Cisneros R, Conners E, Crespi-Abril AC, Kulik VV, Drobyazin EN, Emery T, Fernández-Álvarez FA, Furuya H, González LW, Gough C, Krishnan P, Kumar B, Leite T, Lu CC, Mohamed KS, Nabhitabhata J, Noro K, Petchkamnerd J, Putra D, Rocliffe S, Sajikumar KK, Sakaguchi H, Samuel D, Sasikumar G, Wada T, Zheng X, Tian Y, Pang Y, Yamrungrueng A, Pecl G (2021) World octopus fisheries. Rev Fish Sci Aquac 29:279–429. https://doi.org/10.1080/23308249.2019.1680603
Villanueva R, Norman MD (2008) Biology of the planktonic stages of benthic octopuses. Oceanogr Mar Biol 46:105–202. https://doi.org/10.1201/9781420065756.ch4
Villanueva R, Coll-Lladó M, Bonnaud-Ponticelli L, Carrasco SA, Escolar O, Fernández-Álvarez FÁ, Gleadall IG, Nabhitabhata J, Ortiz N, Rosas C, Sánchez P, Voight JR, Swoger J (2021) Born with bristles: new insights on the Kölliker’s organs of octopus skin. Front Mar Sci 8:645738. https://doi.org/10.3389/fmars.2021.645738
Wood JB, Maynard AE, Lawlor AG, Sawyer EK, Simmons DM, Pennoyer KE, Derby CD (2010) Caribbean reef squid, Sepioteuthis sepioidea, use ink as a defense against predatory French grunts, Haemulon flavolineatum. J Mar Biol Ecol 388:20–27. https://doi.org/10.1016/j.jembe.2010.03.010
Yagishita N, Nakabo T (2003) Evolutionary trend in feeding habits of Girella (Perciformes: Girellidae). Ichthyol Res 50:358–366. https://doi.org/10.1007/s10228-003-0180-8
Yasuda F (1960) The types of food habits of fishes assured by stomach contents examination. Bull Japan Soc Sci Fish 26:653–662. https://doi.org/10.2331/suisan.26.653 (in Japanese with English abstract)
York CA, Bartol IK (2016) Anti-predator behavior of squid throughout ontogeny. J Exp Mar Biol 480:26–35. https://doi.org/10.1016/j.jembe.2016.03.011
Young RE, Harman RF (1988) “Larva”, “paralarva” and “subadult” in cephalopod terminology. Malacologia 29(1):201–207
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer publishing, New York
Acknowledgements
We thank Kei Yamamoto, Takanari Iwata, Saki Fujiwara, and Zen Yamada for helping with sample collection, and Ian G. Gleadall (AiCeph LLC, Sendai) for assistance in preparing the manuscript and valuable advice. We are also grateful to the editors and anonymous reviewers for valuable comments and suggestions, which have improved the manuscript.
Funding
This research was supported by the research program on development of innovative technology grants from the Project of the Bio-oriented Technology Research Advancement Institution (BRAIN) under Grant Number JPJ007097.
Author information
Authors and Affiliations
Contributions
Conceptualization: SD, KH, methodology: SD, MY, formal analysis and investigation: SD, MY, KH, writing—original draft preparation: SD, writing—review and editing: KH, YK, KS, resources: YK, KS, SD, MY.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest directly relevant to the content of this article.
Ethical approval
This study was approved by the Committee on the Ethics of Animal Experiments at Tokyo University of Marine Science and Technology (permission number: R2-S1), where the experiments were carried out.
Additional information
Responsible Editor: H.-J. Hoving .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 97220 KB)
Supplementary file3 (MP4 75479 KB)
Supplementary file4 (MP4 3800 KB)
Supplementary file5 (MP4 5718 KB)
Supplementary file6 (MP4 1772 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dan, S., Yamaji, M., Kamei, Y. et al. Antipredator strategy of paralarvae of East Asian common octopus Octopus sinensis d’Orbigny, 1841: causing rejection after engulfing by a fish, and subsequent ink release for distraction during escape. Mar Biol 170, 109 (2023). https://doi.org/10.1007/s00227-023-04250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04250-z