Abstract
The Persian Gulf hosts marine mega-herbivores that forage in its coastal habitats. Some areas, mainly along its southern coast, contain abundant benthic plants; however, marine plant resources are limited throughout most of this warm sea, which presents nutritional challenges for large herbivores. We measured curved carapace length (CCL) for 102 green turtles (Chelonia mydas) from foraging grounds with relatively limited plant resources surrounding Qeshm Island at the eastern Persian Gulf. The mean CCL was 41.8 cm (SE = ± 1.3 cm; range = 18.5–99), and 93 turtles (91%) had CCL < 65 cm, indicating the area is primarily a juvenile developmental habitat. To study feeding ecology of green turtles in the area, we analyzed esophageal lavage samples from 36 individuals captured in muddy-bottom (n = 23) and sandy/rocky-bottom (n = 13) habitats. Diet data showed a generalist foraging population with dietary niche variation among individuals that targeted mixtures of macroalgae, seagrasses and mangrove. Green turtles showed a slight preference for green algae (Ulva sp.) at both sites. The mean Fulton’s body condition index for juvenile turtles was 1.14 (SE = ± 0.03; n = 72), which is comparable to values reported elsewhere, and indicates that these turtles were not under-nourished. This is intriguing in light of the paucity of local food resources, and perhaps due to the absence of large sub-adults and adults with higher food demand, and/or individual dietary niche variation among resident turtles, both of which reduce competition among local green turtles for the region’s available resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green turtles, Chelonia mydas, feed almost exclusively on benthic plants once recruiting to neritic foraging areas along tropical and subtropical regions of the world’s oceans, which make them the only herbivorous species among all seven living species of marine turtles (Bjorndal 1997; Hirth 1997). Diet habits of these marine mega-herbivores are largely associated with their ontogeny, which shifts the species’ feeding niche as turtles grow and mature. Hatchling green turtles leave the nesting beach to occupy oceanic habitats where their diet is primarily carnivorous (Reich et al. 2007). After several or more years living in the open ocean, immature green turtles recruit to neritic habitats, where they shift to a more herbivorous diet (Bjorndal 1997; Hirth 1997; Reich et al. 2007). The ontogenic shift in the dietary niche of green turtles significantly affects fundamental demographic aspects such as somatic growth rate, reproductive output, remigration interval, and survivorship (Bjorndal 1982). Therefore, understanding the feeding ecology of foraging green turtles is crucial to develop successful conservation plans for green turtle populations in neritic habitats, which host these long-lived marine reptiles during the longest part of their lifecycle (Bjorndal 1999). The green turtle subpopulation of the Northern Indian Ocean, which includes our study area, is listed as vulnerable by the IUCN Red List due to population decrease (Mancini et al. 2019). Therefore, widespread conservation efforts that integrate local biological information into management strategies can hopefully curb further declines and help this subpopulation reach a positive abundance trend and more optimistic population outlook.
In the Persian Gulf, benthic plant resources have a non-uniform distribution. The region hosts both marine algae and seagrass communities distributed along the shallow margins of this warm sea (Sheppard et al. 2010). Emirati shallow waters in the southern Persian Gulf comprise more than 80% of all seagrass beds in the sea (Erftemeijer and Shuail 2012); however, in many other parts of the Gulf, marine plant resources are sparse and patchy, probably because of its thermally extreme environment and lack of suitable substrates (Phillips 2003; Erftemeijer and Shuail 2012). Nevertheless, the region hosts the world’s second largest dugong (Dugong dugon) population (Al-Abdulrazzak and Pauly 2017), as well as foraging juvenile and adult green turtles (Hasbún et al. 2000). The food shortage in many habitats across the Persian Gulf likely presents nutritional challenges for these resident marine mega-herbivores. These difficult conditions are exacerbated by the fact that plant resources of the Gulf are in decline, even in relatively food-rich habitats, due to large-scale anthropogenic developments (Sheppard et al. 2010). A recent study showed that the Gulf dugong population has already lost about one-fifth of its historical range in the sea, and individuals are now isolated to the seagrass-rich habitats of the southern Gulf (Al-Abdulrazzak and Pauly 2017). However, green turtles, another Gulf mega-herbivore, are distributed throughout the sea, and together with the hawksbill turtle (Eretmochelys imbricata) are the dominant turtle species in the region (Gasperetti et al. 1993; Price et al. 1993; Pilcher et al. 2015; Mobaraki et al. 2020). There are, however, few data on green turtle diet in the area; so far our knowledge is based only on stomach contents of 13 dead-stranded adult specimens from seagrass-rich habitats of the United Arab Emirates (U.A.E.) (Hasbún et al. 2000). Therefore, it is essential to learn the feeding habits of green turtles in the Persian Gulf, particularly in habitats with limited food supplies, and understand how living in food-resource limitation in this marine realm may affect their nutritional status.
The present study investigates the feeding ecology of juvenile green turtles in coastal waters surrounding Qeshm Island in the eastern Persian Gulf, a site with relatively limited marine plant resources. This work used a graphical method for analyzing diet data (Amundsen et al. 1996), which provided insights into the food preference, feeding strategy and niche width of local green turtles. In a broader context, the findings of this study can be used for understanding the responses of foraging green turtles to habitat degradation and climate change. These data will also serve as a baseline with which to compare future studies of green turtle diet in warm seas.
Material and methods
Study area
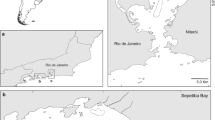
Qeshm Island is known as the largest island in the Persian Gulf with an area of ~ 1490 km2, located at the Strait of Hormuz in the eastern part of the sea (Fig. 1). Capture efforts occurred in two different foraging habitats surrounding Qeshm Island as shown in Fig. 1. The first site was Dokohak Bird’s Wetland (DW; area ~ 21 km2; 26˚59′ N, 56˚12′ E), a muddy-bottom habitat with some small patches of restored gray mangrove, Avicennia marina (Vierh.), lying in the narrow channel between northern Qeshm Island and the Iranian mainland (Fig. 1). The second site was Dolphins’ Bay (DB; area ~ 97 km2; 26˚43′ N, 55˚50′ E), a rocky and sandy-bottom habitat located at the south end of the island.
Turtle capture
Green turtles were collected from arrowhead-fixed fishing traps during 9 days between October 2013 and May 2015. The traps are a type of pound net (locally called moshtah on Qeshm Island) that consists of a fence leader set perpendicular to the shore that acts as a partition to prevent fish from swimming past and thus directing them into an enclosed trap or “pound”. We assumed that all turtles were entrapped on the day of recovery because the local fishermen check the traps daily and release any trapped turtles by removing the seaward wall of the trap. All captured turtles were carried to the nearby beach and kept in a shaded area until processing, and then were returned to the sea nearest their capture sites within 2 h of initial recovery.
Turtle measurement
All captured turtles were measured for curved carapace length (CCL; ± 0.1 cm). To characterize putative maturity status of captured turtles, we used the mean CCL of ca. 99 cm for green turtles nesting in the Persian Gulf (based on the mean CCL of nesting individuals measured in previous studies; e.g., Miller 1989, Al-Merghani et al. 2000, Rees et al. 2013, and Al-Mohanna et al. 2014). All turtles with a notable elongated tail were assumed male, whereas turtles with CCL ≥ 99 cm and lacking a differentiated tail were classified as putative adult females. Green turtles lacking a differentiated tail with CCL < 99 cm were considered undetermined sex and categorized into two groups: juveniles (CCL < 65 cm) and putative sub-adults (CCL = 65–99 cm). Turtles were also weighed to the nearest to 0.1 kg using a spring scale.
Diet sample collection
Recently ingested food items were recovered using the esophageal lavage technique (Forbes and Limpus 1993), and were placed in vials with 70% ethanol and stored at ~ 3 °C until laboratory analysis. In the laboratory, each esophageal lavage sample was spread in a Petri dish, and contents were viewed through a stereoscope. Food items were separated and weighed to the nearest to 0.001 g (wet weight) after being identified to the lowest possible taxonomic level using a combination of available keys for Indian Ocean flora (Børgesen 1939, 1952; Gavino and Trono 1997).
Data analyses
We excluded sub-adult and adult turtles from the body condition and diet analyses, because of their small sample size, and to eliminate the potential effects of adult-specific biological features that affect the body condition and diet (e.g., reproduction and migration).
Fulton’s body condition index was calculated as CI = (Weight/CCL3) × 10,000 (Koch et al. 2007; Labrada-Martagón et al. 2010). Fulton’s CI was also calculated using straight carapace length (SCL, cm) to provide comparability with other studies around the world. For this purpose, SCL was estimated by converting CCL to SCL using a regression equation (SCL = 1.186 + 0.918 CCL) derived from the Persian Gulf green turtles (see Miller 1989).
A graphical analysis, described by Amundsen et al. (1996), was used to define food item importance and feeding strategy. Frequency of occurrence (FOi) for food item i was estimated as the number of esophageal samples in which item i was observed out of all samples. Prey-specific abundance (PAi) for item i was estimated as sample content (wet weight) comprising of item i among all turtles, relative to total sample content among all turtles that had item i. PAiwas plotted against FOion a two-dimensional scatter plot known as a feeding strategy diagram.
Statistical and graphical analyses were performed using Microsoft Office 2007. All data in this study were presented as mean ± standard error (SE).
Results
Turtle size
The mean CCL for 102 examined turtles was 41.8 ± 1.3 cm (range = 18.5–99 cm), of which 93 turtles (91%) were juveniles with CCL < 65 cm. There were also seven putative sub-adults (CCL = 65–95 cm), one adult male, and one putative adult female. Details of turtle size by site and life stage are shown in Table 1. Size class distribution is shown in Fig. 2.
Juvenile weight and body condition index
Mean juvenile body weight was 6.2 ± 0.4 kg (range = 3–26 kg; n = 72). Site-specific mean weights were 6.4 ± 0.5 kg (range = 3–26 kg; n = 51) at DB and 5.7 ± 0.4 kg (range = 4–10 kg; n = 21) at DW. Details of weight and Fulton’s body condition index by site are shown in Table 1. The mean of Fulton’s CI for juveniles from both habitats together (n = 72) was 1.14 based on CCL size data, and 1.33 based on SCL size data.
Juvenile diet composition and feeding strategy
In total, 36 esophageal lavage samples were collected from 58 juveniles. The mean wet weight of the lavage samples was 0.2 g (range = 0.01–1.58 g). In total, 20 food items were found in the diet of juvenile turtles from the both habitats, all of which were vegetative materials (Table 2): 7 green algae (phylum Chlorophyta), 7 red algae (phylum Rhodophyta), 3 brown algae (phylum Ochrophyta), 2 seagrasses (phylum Tracheophyta), and cotyledons of the gray mangrove (Avicennia marina). There were 17 food items identified in DB (green algae = 6, red algae = 6, brown algae = 3, and seagrass = 2), compared to 7 items in DW (green algae = 1, red algae = 2, brown algae = 1, seagrass = 2, and mangrove cotyledon = 1). Diet items found in turtles at both sites (Table 2) included the seagrasses H. ovalis and H. uninervis, one red alga (Hypnea sp.), and one brown alga (Dictyota sp.).
The prey-specific abundance (PAi) and frequency of occurrence (FOi) of food items are plotted in Fig. 3, and depict a generalist community with a dietary niche variation among the individuals. There was no apparent domination in the diet by any single prey item, although a slight preference occurred for the green algae Ulva spp. At DB, there was a slight preference for the green alga U. compressa. Other preferred food items in DB were the seagrasses H. uninervis and H. ovalis, the red algae Hypnea sp. and Laurencia sp., and the green alga Chaetomorpha gracilis. At DW, turtles showed a slight preference for the green alga U. lactuca. Other preferred food items at DW were the seagrass H. ovalis, the red alga Hypnea sp., cotyledons of A. marina, and the brown alga Dictyota sp.
Graphical representation of feeding habits of juvenile green turtles in the eastern Persian Gulf. Food items are indicated by squares (Filled square) for turtles from Dolphins’ Bay (DB, the open sandy-rocky bottomed habitat; n = 23) and by triangles (Filled triangle) for turtles from Dokohak Birds’ Wetland (DW, the sheltered muddy-bottomed habitat; n = 13). All food items are identified with a two-letter label: AF Actinotrichia fragilis; AM Avicennia marina; AS Acanthophora spicifera; BP Bryopsis pennatula; CA Cladophora aokii; CG Chaetomorpha gracilis; CT Ceramium tenerrimum; DS1 Dictyota sp.; DS2 Dictyosphaeria sp.; FS Feldmania sp.; GS Gelidium sp.; HO Halophila ovalis; HS Hypnea sp.; HU Halodule uninervis; LJ Leveillea jungermannioides; LS Laurencia sp.; PS Padina sp.; UC1 Ulva compressa; UC2 U. clathrata, and UL U. lactuca. The axes of the explanatory diagram (upper right corner): (1) prey importance (preference), (2) feeding strategy, and (3) the phenotype contribution to the niche width (high between-phenotype component or high within-phenotype component). Isolines represent different food abundance values. Dominant food items are indicated by larger font size
Discussion
Food resources of the area
Seaweeds, the primary dietary constituents for the juvenile green turtles (Fig. 3; Table 2), are not permanently abundant around Qeshm Island (Fig. 1), as they exhibit marked seasonality (Fatemi et al. 2012; Kokabi et al. 2016), which is common for seaweed stocks within the wide-ranging temperatures of the Gulf (see John and George 2003). Seagrasses, another preferred food for green turtles (Fig. 3; Table 2), are present in both DB and DW (UNEP-WCMC and Short 2018), but are sparse and patchily distributed. Studies evaluating marine plant resources of the area recorded no abundant seagrass community at any of the sites (e.g., Behzadi et al. 2011 and Yaghubzadeh et al. 2014). A similar pattern occurs among seagrass communities in many other parts of the Persian Gulf, generally because of the environmental extremes (i.e., high salinities and wide-ranging and high temperatures). However, in many parts of the Iranian coastline (Fig. 1), the paucity of seagrass is because shallow bottoms lack suitable substrate (Phillips 2003; Erftemeijer and Shuail 2012), and abundant seagrass meadows are limited to few isolated, soft-bottomed shallow areas like Nayband Bay (CHM 2017). In contrast, there are vast shallow areas with soft sediments along the southern and western Arabian coasts of the Persian Gulf, where abundant seagrass meadows can be found (Phillips 2003; Erftemeijer and Shuail 2012). For example, shallow waters of U. A. E. (Fig. 1) host roughly 5660 km2 of seagrass beds, which amounts to more than 80% of all seagrass beds of the Persian Gulf (Erftemeijer and Shuail 2012).
The mangrove A. marina was also among the preferred diet items of green turtles from the muddy-bottom site, DW, where, although no natural mangrove habitat is present, there are a few small patches of A. marina planted by the local government. However, there are vast mangrove stands along Iranian coastline of the Persian Gulf, of which the largest is Hara Biosphere Reserve (area ~ 824 km2) lying near DW at the north of Qeshm Island (Zahed et al. 2010). It would be interesting to conduct similar studies in these areas to compare diet strategies between turtles with and without access to significant mangrove resources.
Remarks on the lavage technique
Although the lavage technique is a rapid procedure that can be completed within 10 min for each turtle (Forbes and Limpus 1993), this approach has logistical hurdles (e.g., sufficient time, infrastructure, and personnel) that can limit its application, particularly in rustic field conditions. In the present study, limited time allotments for lavage efforts allowed us to carry out the procedure for an average of five to six turtles per day (a total of 58 juveniles in the 9 days of field working). Additional turtles were captured, yet because we gained access to all entrapped turtles almost at the same time each field day (during spring low tide), it was not possible to conduct measurements and lavage on all turtles within the 2-h time limit prior to release.
Another aspect affecting the utility of lavage is the depth at which water flushing occurs. Both gastric (deeper) and esophageal (shallower) lavage have been applied to the study of wildlife (Silvy 2020); however, sea turtle researchers often prefer esophageal lavage, as this is less invasive (e.g., Seminoff et al. 2002; López-Mendilaharsu et al. 2005, 2008, and Amorocho and Reina 2007). Yet, because consumed foods only remain in the esophagus for a limited time, this approach typically results in smaller sample volumes (Arthur and Balazs 2008) or sometimes no sample, if all food has passed to the stomach. In this study, only the esophagus was flushed, which like other similar studies (e.g., Amorocho and Reina 2007 and Nagaoka et al. 2012) yielded small volumes of diet samples (mean wet weight = 0.2 g). We also conducted lavage on an additional 22 turtles, but did not recover any foods, likely due to the reasons described above. Nevertheless, our results yielded novel diet items and provided important information about the types and relative amounts of prey consumed by local green turtles. We encourage the application of esophageal lavage for future study of green turtle diet, although we urge that such techniques are performed as soon as possible after turtle capture.
Diet composition
In the feeding strategy diagram (Fig. 3), prey importance increases along a positive-slope diagonal line (Amundsen et al. 1996), which means the items with higher PAi and FOi values are more preferred (Table 2). The primary dietary constituents for juvenile green turtles at both of our study sites were green algae of the genus Ulva (Fig. 3; Table 2), which is not surprising considering that Ulvaceae is the most abundant family of green algae along coastal waters of Qeshm Island (Kokabi et al. 2016). Prior to this study, consumption of Ulva sp. by green turtles in the western Indian Ocean had only been reported from an Arabian Sea feeding ground (Ross 1985). Outside the region, Ulva sp. consumption by green turtles is widespread, occurring in areas such as the western Atlantic (Guebert-Bartholo et al. 2011; Coyne 1994) and eastern Pacific (Seminoff et al. 2002; Carrión-Cortez et al. 2010).
Two seagrass species, Halodule uninervis and Halophila ovalis, were also among the preferred food items for the turtles (Fig. 3). In the only previous report on green turtle diet in the Persian Gulf, Hasbún et al. (2000) examined 13 dead-stranded adult green turtles (CCL > 89 cm) recovered at a seagrass meadow in Ras Al Khaimah of U.A.E., which is very close to our study region, but on the other side of the Strait of Hormuz. The authors found a specialized foraging strategy with H. uninervis and H. ovalis comprising 99% of stomach contents. Both of these, along with Halophila stipulacea, are the only seagrass species that can tolerate environmental (i.e., water temperature) extremes of the Persian Gulf (Erftemeijer and Shuail 2012). However, H. stipulacea is a rare seagrass in the area (Erftemeijer and Shuail 2012), which may explain its absence in the dietary regime of Gulf green turtles (e.g., Hasbún et al. 2000; this study). Seagrass plays an important role in the diet of green turtles in the Western Indian Ocean, and all accessible reports have recorded seagrass as part of their diet, as either the main food item (i.e., Yemen: Hirth et al. 1973; Seychelles: Frazier 1984, Stokes et al. 2019; and Comoro Archipelago: Frazier 1985, Ballorain et al. 2010) or among preferred food items (i.e., Oman: Ross 1985; Ferreira et al. 2006. and Iran: this study).
The red alga Hypnea sp., which was also a preferred food item at both sites (Fig. 3; Table 2), had previously been reported in the diet of green turtles from the Western Indian Ocean (Oman: Ross 1985; Seychelles: Stokes et al. 2019), although in trace amounts only. Hypnea spp. have been reported as primary food for green turtles at several Pacific foraging habitats (e.g., López-Mendilaharsu et al. 2005; Arthur and Balazs 2008; Arthur et al. 2009; and Carrión-Cortez et al. 2010).
Three preferred food items in this study have not previously been reported in the diet of green turtles in the Western Indian Ocean, including the brown alga Dictyota sp. and cotyledons of the mangrove A. marina in DW, and the red alga Laurencia sp. in DB (Fig. 3; Table 2). However, outside the region, all these three have been reported as primary food items for foraging green turtles (Laurencia spp. [Garnett et al. 1985], Dictyota spp. [Carrión-Cortez et al. 2010; Sampson et al. 2017], and A. marina [Limpus and Limpus 2000]).
In addition to cotyledons, other mangrove plant parts have also been found in green turtle diets, including leaves (Nagaoka et al. 2012; Pendoley and Fitzpatrick 1999; Limpus and Limpus 2000), roots/shoots (Pritchard 1971; Carrión-Cortez et al. 2010), and fruits (Amorocho and Reina 2007). However, here only mangrove cotyledons were encountered. Had more turtles been subjected to esophageal lavage technique, or had lavage efforts occurred more rapidly upon turtle capture, it is possible that other mangrove parts would have been encountered. Nevertheless, our results further underscore the value of mangrove plants as a dietary resource for green turtles, and provide the first example of mangrove consumption in the Western Indian Ocean.
Feeding habits
In the feeding strategy diagram (Fig. 3), a negative-slope diagonal line shows the between- and within-phenotype contributions to niche width: a community with a high between-phenotype component is comprised of different individuals that are specialized on different resource types, whereas a community with a high within-phenotype component contains individuals that mostly utilize many resource types simultaneously (Amundsen et al. 1996). High prey-specific abundance (PAi) and low frequency of occurrence (FOi) of most food items located at the upper left of feeding strategy diagram (Fig. 3) indicate a juvenile green turtle community with a high between-phenotype component. It suggests an individual-level niche variation, meaning that juvenile green turtles have a broad trophic niche (a generalist community) with little or no overlap in resource use among individuals (individual-level niche variation). Although this generalist community adheres to a largely herbivorous diet, our results indicate a greater dietary diversity than previously described for the region (Fig. 3; Table 2). Prior to this study, two other studies also reported diverse diets for foraging green turtles from the western Indian Ocean: Ferreira et al. (2006) reported a mixture of gastropods, seagrass and seaweeds in stomachs of ten dead-stranded adult green turtles from the entrance of the Gulf of Oman; and Ross (1985) reported a mixture of seagrass and seaweeds in stomachs of individuals from an Arabian sea feeding ground. All other studies that examined diet of green turtles from the Western Indian Ocean reported specialized diets dominated by seagrass (e.g., Hirth et al. 1973; Hasbún et al. 2000; and Stokes et al. 2019).
Overcoming nutritional challenges
A well-nourished animal with higher energy reserves, which presumably enhances survival, successful migration, and reproduction, is assumed to be healthy, which is reflected in good body condition (Stevenson and Woods 2006). Our results demonstrate that body condition of juvenile green turtles residing in relatively food-poor habitats surrounding Qeshm Island is generally the same as green turtles of similar size around the world (Table 3). This suggests local turtles are not under-nourished despite the apparent food shortage in the area. Similarly, at a Qatari coastal area at the western Persian Gulf, where previously abundant seagrass meadows had become degraded due to intense anthropogenic coastal development, Pilcher et al. (2015) carried out laparoscopic examinations for juvenile green turtles and reported that the animals were mostly healthy and well-nourished. Data presented here, and by Pilcher et al. (2015), indicate that Gulf juvenile green turtles can somehow overcome the nutritional challenges of limited food resources. We believe their ability to survive and flourish under these adverse conditions might be led by two previously documented ecological features of foraging green turtles.
The first is size-based habitat segregation led by differentiation in food resources between the habitats; this happens when large sub-adult and adult green turtles with higher demand for food avoid food-limited habitats and aggregate in areas with abundant vegetative resources (seeLópez-Mendilaharsu et al. 2005; Amorocho and Reina 2007; Koch et al. 2007; Ballorain et al. 2010). Available data confirm this pattern for Persian Gulf green turtles as well. For example, in the shallow waters of U.A.E. with plentiful marine plant resources, about 95% (135 out of 142) of all green turtles collected by Hasbún et al. (2000), and about 93% (13 of 14) of those collected by Al-Ghais et al. (1998) were large sub-adults and adults (CCL > 60 cm CCL). Further, in one of the few isolated abundant seagrass meadows along the Iranian coast of the Persian Gulf (CHM 2017), 17 green turtles collected by Mobaraki et al. (2020) had a CCL mean of about 70 cm. On the other hand, about 91% (93 of 102; Fig. 2) of all foraging green turtles collected in our relatively food-limited study area, and about 97% (73 of 75) of all individuals collected by Pilcher et al. (2015) from the degraded seagrass meadows of Qatar, were small juveniles (CCL < 60 cm). Moreover, 108 green turtles collected by Mobaraki et al (2020) from Islands located at the strait of Hormuz, the deepest part of the Persian Gulf, with lack of shallow areas and abundant seagrasses (UNEP-WCMC and Short 2018), had a mean CCL smaller than 60 cm, suggesting they too were juveniles. These habitats, with many smaller turtles and only a few sub-adults and putative adults, are considered juvenile development foraging grounds. The absence of the large turtles probably gives juvenile turtles access to greater food resources.
The second noteworthy ecological feature of green turtles is the ability of post-pelagic individuals to occupy a variety of dietary niches while recruiting from offshore pelagic habitats to neritic juvenile development habitats (Bjorndal 1980). The variability in green turtle diet once recruiting to near shore areas is probably possible because their gut microflora, which aid in digesting vegetative foods (Bjorndal 1980), adapt to vegetative resources they encounter in the new neritic habitats (Price et al. 2017). Such dietary niche variation has been widely reported among foraging green turtle communities (e.g., Bjorndal 1980; Brand-Gardner et al. 1999; López-Mendilaharsu et al. 2008; Reisser et al. 2013; Nagaoka et al. 2012; Thomson et al. 2018; and Martins et al. 2020). However, data from animal-borne video and stable isotope analysis revealed that dietary niche may vary not only at the population-level, but also at the individual level (Burkholder et al. 2011; Vander Zanden et al. 2013; Thomson et al. 2018), which is also demonstrated by the graphical analyses of diet data in this study (Fig. 3). In addition to segregation from adults, the dietary niche variation observed among juvenile green turtles in this study likely increases feeding efficiency by reducing the number of turtles sharing or competing for the same resources.
Conclusions and conservation implications
We found that coastal waters surrounding Qeshm Island at the eastern Persian Gulf is primarily a developmental habitat for juvenile green turtles, where individuals occupy various dietary niches and collectively forage on different plant resources, including seagrass, seaweeds and mangrove parts. Despite perspectives that suggest the area has relatively limited benthic plant resources, body condition data demonstrate that the juvenile turtles are healthy and well nourished. This food sufficiency may be related to the absence of large sub-adult and adult turtles in the area, as well as diet variation among juvenile individuals, which allows the population to more evenly distribute foraging pressure on available foods in the area. Even so, the fact that green turtles are able to persist and thrive in putative low-quality habitats indicates their resiliency to suboptimal environmental conditions. The intrinsic ability of green turtles to adjust dietary intake based on food availability has been reported elsewhere (Russell and Balazs 2009; Gama et al. 2016) and likely facilitates their ability to live in conditions such as those present in the Persian Gulf. Furthermore, the encounters with juvenile green turtles with good body condition in our study highlights the fact that in the Persian Gulf, even habitats with lower amounts of benthic primary producers play a vital role in the lifecycle of green turtles, and should, therefore, be considered as critical habitats for conservation programs. Hence, we suggest that before conducting any proposed coastal development project in the Persian Gulf, Environmental Impact Assessments (EIA) clearly address how the projects will limit and mitigate impacts to benthic communities, including seagrasses and seaweeds, to preserve food resources for marine mega-herbivores.
Data availability
Data are provided as open access under CC BY 4.0 License and are assessable from the link: https://doi.org/10.6084/m9.figshare.13203875.v2
References
Al-Abdulrazzak D, Pauly D (2017) Reconstructing historical baselines for the Persian/Arabian Gulf Dugong, Dugong dugon (Mammalia: Sirena). Zool Middle East 63:95–102. https://doi.org/10.1080/09397140.2017.1315853
Al-Ghais SM, Balazs GH, Hasbún CR (1998) Preliminary observations on green turtles, Chelonia mydas, in foraging pastures of the United Arab Emirates. Mar Turt Newsl 80:8–9
Al-Merghani M, Miller JD, Pilcher NJ, Al-Mansi A (2000) The green and hawksbill turtles in the Kingdom of Saudi Arabia: synopsis of nesting studies 1986–1997. Fauna Arabia 18:369–384
Al-Mohanna SY, Al-Zaidan ASY, George P (2014) Green turtles (Chelonia mydas) of the north-western Arabian Gulf, Kuwait: the need for conservation. Aquatic Conserv Mar Freshw Ecosyst 24:166–178. https://doi.org/10.1002/aqc.2371
Amorocho DF, Reina RD (2007) Feeding ecology of the east pacific green sea turtle, Chelonia mydas agassizii, at Gorgona National Park, Colombia. Endang Spec Res 3:43–51. https://doi.org/10.3354/esr003043
Amundsen PA, Gabler HM, Staldvik FJ (1996) A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. J Fish Biol 48:607–614. https://doi.org/10.1111/j.1095-8649.1996.tb01455.x
Arthur KE, Balazs GH (2008) A comparison of immature green turtle (Chelonia mydas) diets among seven sites in the main Hawaiian Islands. Pac Sci 62(2):205–217. https://doi.org/10.2984/1534-6188(2008)62[205:ACOIGT]2.0.CO;2
Arthur KE, McMahon KM, Limpus CJ, Dennison WC (2009) Feeding ecology of green turtles (Chelonia mydas) from Shoalwater Bay, Australia. Mar Turt Newsl 123:6–12
Ballorain K, Ciccione S, Bourjea J, Grizel H, Enstipp M, Georges J (2010) Habitat use of a multispecific seagrass meadow by green turtles Chelonia mydas at Mayotte Island. Mar Biol 157:2581–2590. https://doi.org/10.1007/s00227-010-1520-7
Behzadi S, Salarpouri A, Darvishi M, Daghoghi B, Mortazavi MS (2011) Study of biotic communities for artificial reef placement in Hormuzgan Province waters, the Persian Gulf. Iran Sci Fish J 20:9–16 ([in Persian])
Bjorndal KA (1980) Nutrition and grazing behavior of the green turtle Chelonia mydas. Mar Biol 56:147–154. https://doi.org/10.1007/BF00397131
Bjorndal KA (1982) The consequences of herbivory for the life history pattern of the Caribbean green turtle, Chelonia mydas. In: Bjorndal KA (ed) Biology and Conservation of Sea Turtles. Smithsonian Institution Press, Washington DC, pp 111–116
Bjorndal KA (1997) Foraging ecology and nutrition of sea turtles. In: Lutz P, Musick J (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 199–232
Bjorndal KA (1999) Priorities for research in foraging habitats. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication No 4, pp 1–3
Børgesen F (1939) Marine algae from the Iranian Gulf especially from the innermost part near Bushire and the Island Kharg. In: Jesse K, Sparck R (eds) Danish Scientific Investigations in Iran, Part 1. Munksgaard, Copenhagen, pp 42–141
Børgesen F (1952) Some marine algae from Mauritius. Additions to the parts previously published, 4rd edn. Kongelige Danske Videnskabernes Selskab, Biologiske Meddelelser 18: 1–72
Brand-Gardner SJ, Lanyon JM, Limpus CJ (1999) Diet selection by immature green turtles, Chelonia mydas, in subtropical Moreton bay, southeast Queensland. Aust J Zool 47:181–191. https://doi.org/10.1071/ZO98065
Burkholder DA, Heithaus MR, Thomson JA, Fourqurean JW (2011) Diversity in trophic interactions of green sea turtles Chelonia mydason a relatively pristine coastal foraging ground. Mar Ecol Prog Ser 439:277–293. https://doi.org/10.3354/meps09313
Carrión-Cortez JA, Zárate P, Seminoff JA (2010) Feeding ecology of the green turtle (Chelonia mydas) in the Galapagos Islands. J Mar Biol Assoc UK 90:1005–1013. https://doi.org/10.1017/S0025315410000226
CHM, Clearing-House Mechanism of the Convention on Biological Diversity (2017) Ecologically or biologically significant areas: Nayband bay. Convention on Biological Diversity. https://chm.cbd.int/database/record?documentID=237778. Accessed 25 Aug 2020
Coyne MS (1994) Feeding ecology of sub-adult green sea turtles in south Texas waters. MSc thesis, Texas A&M University, Texas
Erftemeijer PLA, Shuail DA (2012) Seagrass habitats in the Arabian Gulf: distribution, tolerance thresholds and threats. Aquat Ecosyst Health 15:73–83. https://doi.org/10.1080/14634988.2012.668479
Fatemi S, Ghavam MP, Rafiee F, Taheri MS (2012) The study of seaweeds biomass from intertidal rocky shores of Qeshm Island, Persian Gulf. Int J Mar Sci Eng 2:101–106
Ferreira B, Garcia M, Jupp BP, Al-Kiyumi A (2006) Diet of the green turtle (Chelonia mydas) at Ra’s Al Hadd, Sultanate of Oman. Chelonian Conserv Biol 5:141–146. https://doi.org/10.2744/1071-8443(2006)5[141:DOTGTC]2.0.CO;2
Forbes GA, Limpus CJ (1993) A non-lethal method for retrieving stomach contents from sea turtles. Wildl Res 20:339–343. https://doi.org/10.1071/WR9930339
Frazier J (1984) Marine turtles in the Seychelles and adjacent territories. In: Stoddart DR (ed) Biogeography and Ecology of the Seychelles Islands. Springer, Netherlands, pp 417–468
Frazier J (1985) Marine Turtles in the Comoro Archipelago. Royal Academy of Netherlands. (84)
Gama LR, Domit C, Broadhurst MK, Fuentes MM, Millar RB (2016) Green turtle Chelonia mydas foraging ecology at 25°S in the western Atlantic: evidence to support a feeding model driven by intrinsic and extrinsic variability. Mar Ecol Prog Ser 542:209–219. https://doi.org/10.3354/meps11576
Garnett ST, Price IR, Scott FJ (1985) The diet of the green turtle, Chelonia mydas (L.). Torres Strait. Aust Wildl Res 12(1):103–112. https://doi.org/10.1071/WR9850103
Gasperetti J, Stimson A, Miller J, Ross P, Gasperetti P (1993) Turtles of Arabia. In: Buttiker W, Krupp F (eds) Fauna of Saudi Arabia, vol 13. National Commission for Wildlife Conservation and Development, Riyadh, and Pro Entomolgia. Natural History Museum, Basle, pp 170–367
Gavino C, Trono JR (1997) Field guide and atlas of the seaweed resources of the Philippines. Bookmark, Makati
Guebert-Bartholo FM, Barletta M, Costa MF, Monteiro-Filho ELA (2011) Using gut contents to access foraging patterns of juvenile green turtles Chelonia mydas in the Paranaguá Estuary, Brazil. Endang Spec Res 13:131–143. https://doi.org/10.3354/esr00320
Hasbún CR, Lawrence AJ, Samour JH, Al-Ghais SM (2000) Preliminary observations on the biology of green turtles, Chelonia mydas, from the United Arab Emirates. Aquatic Conserv: Mar Freshw Ecosyst 10:311–322. https://doi.org/10.1002/1099-0755(200009/10)10:5%3c311::AID-AQC414%3e3.0.CO;2-Z
Hirth HF (1997) Synopsis of the biological data on the green turtle, Chelonia mydas (Linnaeus 1758). US Fish Wildl Serv Biol Rep 97(1), Washington DC 120pp
Hirth HF, Klikoff LG, Harper KT (1973) Sea grasses at Khor Umaira, People’s Democratic Republic of Yemen with reference to their role in the diet of the green turtle, Chelonia mydas. Fish Bull 71:1093–1097
John DM, George JD (2003) Coral death and seasonal seawater temperature regime: their influence on the marine algae of Abu Dhabi (UAE) in the Arabian Gulf. In: Proceedings of the International Seaweed Symposium, vol 17. Oxford University Press, Oxford and New York, pp 341–348
Koch V, Brooks LB, Nichols WJ (2007) Population ecology of the green/black turtle (Chelonia mydas) in Bahía Magdalena, Mexico. Mar Biol 153:35–46. https://doi.org/10.1007/s00227-007-0782-1
Kokabi M, Yousefzadi M, Razaghi M, Feghhi MA (2016) Zonation patterns, composition and diversity of macroalgal communities in the eastern coasts of Qeshm Island, Persian Gulf. Iran Mar Biodivers Rec 9:96. https://doi.org/10.1186/s41200-016-0096-4
Labrada-Martagón VL, Méndez-Rodríguez LC, Gardner SC, Cruz-Escalona VH, Zenteno-Savín T (2010) Health indices of the green turtle (Chelonia mydas) along the Pacific coast of Baja California Sur, Mexico. II Body condition index. Chelonian Conserv Biol 9:173–183. https://doi.org/10.2744/CCB-0807.1
Limpus CJ, Limpus DJ (2000) Mangroves in the diet of Chelonia mydas in Queensland, Australia. Mar Turt Newsl 89:13–15
López-Mendilaharsu M, Gardner SC, Seminoff JA, Riosmena-Rodriguez R (2005) Identifying critical foraging habitats of the green turtle (Chelonia mydas) along the Pacific coast of the Baja California peninsula, Mexico. Aquat Conserv Mar Freshw Ecosyst 15:259–269. https://doi.org/10.1002/aqc.676
López-Mendilaharsu M, Gardner SC, Riosmena-Rodriguez R, Seminoff JA (2008) Diet selection by immature green turtles (Chelonia mydas) at Bahía Magdalena foraging ground in the Pacific Coast of the Baja California Peninsula, México. J Mar Biol Assoc UK 88:641–647. https://doi.org/10.1017/S0025315408001057
Mancini A, Phillott AD, Rees AF (2019) Chelonia mydas (North Indian Ocean subpopulation). The IUCN Red List of Threatened Species 2019: e.T142121108A154845002. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T142121108A154845002.en. Downloaded on 18 July 2020.
Martins RF, Andrades R, Nagaoka SM, Martins AS, Longo LL, Ferreira JS, Bastos KV, Joyeux JC, Santos RG (2020) Niche partitioning between sea turtles in waters of a protected tropical island. Regional Stud Marine Sci 27:101439. https://doi.org/10.1016/j.rsma.2020.101439
Miller JD (1989) Marine turtles Vol 1: an assessment of the conservation status of marine turtles in the Kingdom of Saudi Arabia. MEPA Coastal and Marine Management Series, Report No 9, Ministry of Defense and Aviation, Kingdom of Saudi Arabia, 209 p
Mobaraki A, RastegarPouyani E, Kami HG, Khorasani N (2020) Population study of foraging Green sea turtles (Chelonia mydas) in the Northern Persian Gulf and Oman Sea Iran. Reg Stud Mar Sci 26:101433. https://doi.org/10.1016/j.rsma.2020.101433
Nagaoka SM, Martins AS, dos Santos RG, Tognella MMP, de Oliveira Filho EC, Seminoff JA (2012) Diet of juvenile green turtles (Chelonia mydas) associating with artisanal fishing traps in a subtropical estuary in Brazil. Mar Biol 159:573–581. https://doi.org/10.1007/s00227-011-1836-y
Pendoley K, Fitzpatrick J (1999) Browsing on mangroves by green turtles in Western Australia. Mar Turt Newsl 84:10
Phillips RC (2003) The seagrasses of the Arabian Gulf and Arabian region. In: Green EP, Short FT (eds) World atlas of seagrasses. University of California Press, Berkeley, UNEP-WCMC, pp 74–81
Pilcher NJ, Al-Maslamani I, Williams J, Gasang R, Chikhi A (2015) Population structure of marine turtles in coastal waters of Qatar. Endang Spec Res 28:163–174. https://doi.org/10.3354/esr00688
Price ARG, Sheppard CRC, Roberts CM (1993) The gulf: its biological setting. Mar Pollut Bull 27:9–15. https://doi.org/10.1016/0025-326X(93)90004-4
Price JT, Paladino FV, Lamont MM, Witherington BE, Bates ST, Soule T (2017) Characterization of the juvenile green turtle (Chelonia mydas) microbiome throughout an ontogenetic shift from pelagic to neritic habitats. PLoS ONE 12:e0177642. https://doi.org/10.1371/journal.pone.0177642
Pritchard PCH (1971) Galapagos sea turtles: preliminary findings. J Herpetol 5:1–9. https://doi.org/10.2307/1562836
Rees AF, Al-Hafez A, Lloyd JR, Papathansopoulou N, Godley BJ (2013) Green turtles, Chelonia mydas, in Kuwait: nesting and movements. Chelonian Conserv Biol 12:157–163. https://doi.org/10.2744/CCB-1030.1
Reich KJ, Bjorndal KA, Bolten AB (2007) The lost years of green turtles: using stable isotopes to study cryptic life stages. Biol Lett 3:712–714. https://doi.org/10.1098/rsbl.2007.0394
Reisser J, Proietti M, Sazima I, Kinas P, Horta P, Secchi E (2013) Feeding ecology of the green turtle (Chelonia mydas) at rocky reefs in Western South Atlantic. Mar Biol 160:3169–3179. https://doi.org/10.1007/s00227-013-2304-7
Ross JP (1985) Biology of green turtle, Chelonia mydas, on an Arabian feeding ground. J Herpetol 19:459–468. https://doi.org/10.2307/1564198
Russell DJ, Balazs GH (2009) Dietary shifts by green turtles (Chelonia mydas) in the Kaneohe Bay Region of the Hawaiian Islands: A 28-Year Study. Pac Sci 63:181–192. https://doi.org/10.2984/049.063.0202
Sampson L, Giraldo A, Payan LF, Amorocho DF, Ramos MA, Seminoff JA (2017) Trophic ecology of green turtle Chelonia mydas juveniles in the Colombian Pacific. J Mar Biol Assoc UK. https://doi.org/10.1017/S0025315417001400
Seminoff JA, Resendiz A, Nichols WJ (2002) Diet of the east Pacific green turtle, Chelonia mydas, in the central Gulf of California, México. J Herpetol 36:447–453. https://doi.org/10.2307/1566189
Sheppard CRC, Al-Husiani M, Al-Jamali F, Al-Yamani F, Baldwin R, Bishop J, Benzoni F, Dutrieux E, Dulvy NK, Durvasula SRV, Jones DA, Loughland R, Medio D, Nithyanandan M, Pilling GM, Polikarpov I, Price ARG, Purkis S, Riegl B, Saburova M, Namin KS, Taylor O, Wilson S, Zainal K (2010) The Gulf: A young sea in decline. Mar Pollut Bull 60:13–38. https://doi.org/10.1016/j.marpolbul.2009.10.017
Silvy NJ ed (2020) The Wildlife Techniques Manual: Volume 1: Research. Volume 2: Management. JHU Press. 485pp
Stevenson RD, Woods WA (2006) Condition indices for conservation: new uses for evolving tools. Integr Comp Biol 46:1169–1190. https://doi.org/10.1093/icb/icl052
Stokes HJ, Mortimer JA, Hays GC, Unsworth RKF, Laloë J, Esteban N (2019) Green turtle diet is dominated by seagrass in the Western Indian Ocean except amongst gravid females. Mar Biol 166:135. https://doi.org/10.1007/s00227-019-3584-3
Thomson JA, Whitman ER, Garcia-Rojas MI, Bellgrove A, Ekins M, Hays GC, Heithaus MR (2018) Individual specialization in a migratory grazer reflects long-term diet selectivity on a foraging ground: implications for isotope-based tracking. Oecologia 188:429–439. https://doi.org/10.1007/s00442-018-4218-z
UNEP-WCMC, Short FT (2018) Global distribution of seagrasses, ver 6. Sixth update to the data layer used in Green and Short (2003). Cambridge (UK): UN Environment World Conservation Monitoring Centre. http://data.unep-wcmc.org/datasets/7. Accessed 25 Aug 2020
Vander Zanden HB, Bjorndal KA, Bolten AB (2013) Temporal consistency and individual specialization in resource use by green turtles in successive life stages. Oecologia 173:767–777. https://doi.org/10.1007/s00442-013-2655-2
Yaghubzadeh M, Danehkar A, Amiri BJ, Ashrafi S (2014) Environmental sensitivity of ecosystems in Hormozgan Province. Iran J Nat Resour 67:121–134 ([in Persian])
Zahed MA, Rouhani F, Mohajeri S, Bateni F, Mohajeri L (2010) An overview of Iranian mangrove ecosystems, northern part of the Persian Gulf and Oman Sea. Acta Ecol Sin 30:240–244. https://doi.org/10.1016/j.chnaes.2010.03.013
Acknowledgements
We are grateful to the Environmental Management Office of Qeshm Free Area Organization for logistical support. Nicolas Pilcher (Marine Research Foundation, Sabah, Malaysia) and one anonymous reviewer are gratefully acknowledged for their valuable comments and constructive criticism on the submitted manuscript. We warmly thank David John (The Natural History Museum, London, UK) for providing us insight into the ecology and distribution of seaweeds in the Persian Gulf. We also thank Jeff Miller (Biological Research and Education Consultants, MT, USA) and Jack Frazier (Smithsonian Institute, USA) for providing historic and critical references on green turtles from the NW Indian Ocean. We are indebted to many volunteers for their useful and timely field assistance, far too many to list individually. All local fishermen, who allowed us to use their fishing traps, are also warmly appreciated.
Author information
Authors and Affiliations
Contributions
MRA conceived and designed the study under supervision of JAS. MRA led and participated in all the fieldwork. PG participated in the fieldwork. FI identified food items. MRA analyzed the data. MRA and JAS led the writing with contribution of the other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All the procedures followed animal use and care protocols established by the Herpetological Animal Care and Use Committee of the American Society of Ichthyologists and Herpetologists, which is assessable from the link: https://asih.org/sites/default/files/2018-05/guidelines_herps_research_2004.pdf
Additional information
Reponsible Editor: L Avens.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by N. Pilcher and an undisclosed expert.
Rights and permissions
About this article
Cite this article
Rezaie-Atagholipour, M., Imani, F., Ghezellou, P. et al. Feeding ecology of juvenile green turtles in food-poor habitats of the Persian Gulf. Mar Biol 168, 4 (2021). https://doi.org/10.1007/s00227-020-03809-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03809-4