Abstract
Coral reefs are threatened by multiple stressors that degrade these ecosystems and the ecosystem services they provide. Critical to the recovery of coral reefs after a disturbance is coral recruitment, but there is still little information about the types of benthic habitats that different species of coral larvae require for settlement. Settlement in the presence of different algae and cyanobacteria was tested for three coral species, Acropora palmata, Acropora cervicornis and Pseudodiploria strigosa. The experiments were conducted in larval chambers placed on the reef to ensure that coral larvae were exposed to natural light, seawater temperature and some water flow. Rates of settlement and metamorphosis were assessed by providing these coral larvae with a standard preferred settlement substratum (individuals of the crustose coralline algal species Hydrolithon boergesenii) with an attached treatment of a small piece of live algae or benthic cyanobacteria. The brown algae Dictyota pulchella and D. bartayresiana did not affect the survival or settlement of larvae of A. palmata in 2010, but D. pulchella did reduce larval survival in 2009. Of the cyanobacteria tested, Caldora penicillata decreased A. palmata survival and settlement. For A. cervicornis, neither Dictyota pulchella nor D. bartayresiana reduced survival or settlement in either 2009 or 2010. Algae and cyanobacteria had no effect on Pseudodiploria strigosa larval survival, but there was reduced settlement in the presence of the cyanobacterium Hormothamnion enteromorphoides. These larval experiments show that some macrophytes can reduce coral larval survival and settlement even in the presence of highly preferred substrata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are threatened by multiple stressors and coral recruitment is a critical process for the recovery of degraded reefs. Biological interactions influence larval settlement and can both inhibit and facilitate coral recruitment (Birrell et al. 2008a; Ritson-Williams et al. 2009). Successful settlement and metamorphosis are critical for coral persistence through the early life history stages as this is the stage when planktonic larvae transform from planulae to sessile recruits, and selecting appropriate settlement substrata is a key step for successful recruitment. Multiple factors including substrata complexity, predation and competition can all impact coral settlement and recruitment success (Doropoulos et al. 2016). As marine habitats continue to be degraded it is critical to identify potential inhibitors of coral larval settlement to better manage habitats for future coral recruitment.
Many factors can influence coral larval settlement, including abiotic factors such as water temperature (Nozawa and Harrison 2007; Putnam et al. 2008; Randall and Szmant 2009a, 2009b; Chua et al. 2013; Ross et al. 2013), depth (Babcock and Mundy 1996; Carlon 2002; Baird et al. 2003), UV exposure (Kuffner 2001; Gleason et al. 2006), and water quality (Gleason et al. 2009). Biotic interactions are also important, and some species of crustose coralline algae (CCA) can increase rates of coral settlement (Harrington et al. 2004; Price 2010; Arnold and Steneck 2011; Ritson-Williams et al. 2016a). Conversely, some species of macroalgae and cyanobacteria are known to inhibit settlement (Kuffner and Paul 2004; Arnold et al. 2010; Doropoulos et al. 2014; Webster et al. 2015). As more algal species have been tested, there is evidence for settlement inhibition by some algal species but not others (Kuffner et al. 2006; Birrell et al. 2008a; Diaz-Pulido et al. 2010), suggesting species specific interactions. There is currently little information on how the larvae of different coral species respond to local competition with macroalgae and benthic cyanobacteria (together referred to as macrophytes in this paper).
The experiments that test larval settlement inhibition by benthic organisms have been limited to relatively few coral species. In the Pacific, Acropora spp. are most often tested for their larval settlement in the presence of macroalgae (Birrell et al. 2008b; Morrow et al. 2017), but other experiments tested larvae of Platygyra daedalea (Diaz-Pulido et al. 2010), larvae of Pocillopora damicornis (Maypa and Raymundo 2004; Kuffner and Paul 2004), and larvae of Montipora capitata (Vermeij et al. 2009). In all of these experiments, one or more species of macrophytes reduced coral recruitment. In the Caribbean, effects of macroalgae on settlement behavior have been studied mainly with the larvae of brooding corals. Larvae of Porites astreoides were tested for settlement in the presence of algae and cyanobacteria in Florida (Kuffner et al. 2006; Paul et al. 2011; Olsen et al. 2014, 2016), and Favia fragum larvae were tested with Halimeda opuntia in Curacao (Nugues and Szmant 2006). In all of these experiments there was also recruitment inhibition by macrophytes, except that the experiments with F. fragum showed that Halimeda opuntia could increase the settlement of coral larvae. Overall, these experiments only tested a few coral species and the effect of macroalgae on the recruitment of most coral species remains poorly understood.
A series of experiments were conducted to test the effects of macrophytes on the larval settlement of three different coral species, Acropora palmata, A. cervicornis and Pseudodiploria strigosa. All of these species have been studied to determine facilitating settlement cues among CCA species (Ritson-Williams et al. 2016a) but have never been tested for the impact of benthic macrophytes on their larval ecology. Both of the Acropora species were formerly dominant community members on Caribbean reefs and significantly contributed to reef calcification, but are now rare on most reefs; thus they have been listed as threatened under the Endangered Species Act (NMFS 2006). It is critical to understand the marine organisms that facilitate or inhibit the recruitment of these coral species if reefs are to be managed for recovery. In light of the paucity of data for these three coral species we designed a series of experiments to test whether common reef macrophytes inhibit coral recruitment even in the presence of positive settlement cues. These experiments provide novel data for the recruitment ecology of habitat forming coral species, which is critical for managing reefs for persistence into the future.
Materials and methods
Species studied
Acropora palmata, A. cervicornis and Pseudodiploria strigosa are common shallow water corals found throughout the Caribbean basin. All three of these coral species can be found at a range of depths from 1 to 20 m (except A. palmata, which is rarely found below 5 m) and were monitored for spawning on reefs in shallow depths (< 4 m) adjacent to Carrie Bow Cay, Belize, where these experiments were conducted. These three coral species are broadcast spawning hermaphrodites with external fertilization of their gametes. Both A. palmata and A. cervicornis are listed as threatened under the Endangered Species Act (NMFS 2006) due to drastic decreases in their populations across the Caribbean (Aronson and Precht 2001). Pseudodiploria strigosa was selected for these experiments along with the two Acropora spp. since they all are large habitat forming corals and to date they have never been tested for the types of macrophytes that might impact their recruitment ecology.
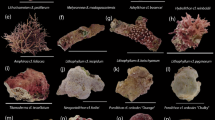
The macrophytes selected were all common on reef flats and fore reefs around the Carrie Bow Cay field station. These common macrophytes have been observed at many locations throughout the Caribbean at depths ranging from 1 to 20 m and were selected due to their prevalence on some reefs and previous literature that showed they compete with corals at other life history stages (Kuffner et al. 2006; Box and Mumby 2007; Rasher et al. 2010). Dictyota pulchella (Fig. 1a) is a common species of brown algae found throughout the Caribbean basin, and it reduced the settlement and metamorphosis of Porites astreoides larvae (Paul et al. 2011). This species can dominate benthic habitats and was collected in Belize from the fore reef below a depth of 15 meters. Dictyota bartayresiana (Fig. 1b) was collected from a shallow (1 m) sandy back reef habitat adjacent to Carrie Bow Cay. The two species of Dictyota had distinct morphological features and distributions that made them easily distinguishable throughout the experiments. Lobophora sp. (Fig. 1c) is another common brown alga that is found throughout the Caribbean basin (Slattery and Lesser 2014; Vieira et al. 2016). This genus of algae is now recognized to consist of multiple species (Sun et al. 2012), but we consistently used the decumbent form found on deeper reefs, which was collected from fore reef habitats at a depth of 15–20 m.
The cyanobacterium Dichothrix sp. (Fig. 1d) often grows in shallow reef waters as small, dark tufts. This species was collected from dead coral on the reef flat adjacent to Carrie Bow Cay at a depth of 1 m. Hormothamnion enteromorphoides (Fig. 1e) is a bright green cyanobacterial species that can be found circumtropically and sometimes forms blooms; it also deters feeding by reef herbivores due to its cyclic peptide secondary metabolites (Pennings et al. 1997). This species was collected from a sandy patch reef on the reef flat at a depth of 2 m. Caldora penicillata (Fig. 1f) is a circumtropical, chemically rich cyanobacterial species that is frequently observed during the summer months in Belize (Engene et al. 2015). This species can be found abundantly at a range of depths (1–25 m) and was collected from the fore reef at a depth of 15 m.
Collection and rearing of coral larvae
For all three coral species, adult colonies were monitored in the field approximately 2–4 h after sunset on nights 2–7 after the full moon in late July or August. If a colony was observed to “set” (shortly before a colony spawns, the gamete bundles are held in the mouth of its polyps), it was covered with a gamete collection net. The net was made of rip stop nylon and served to funnel the positively buoyant gamete bundles into a plastic 0.3 L jar at the top of the net. The nights of spawning for each coral species are listed in Table 1. After the colonies finished releasing their gamete bundles the plastic jars were removed, covered and immediately transported back to the laboratory. As soon as the gamete bundles broke apart, typically within 30 min, the eggs and sperm for each individual colony were passed over 100 µm nitex, which filtered the eggs away from the sperm. Each cup of eggs was then fertilized with conspecific sperm from a different individual. The eggs were held with the sperm for an hour to ensure maximum fertilization, and then the fertilized eggs were rinsed over 100 µm nitex with seawater to remove any excess sperm.
Fertilized eggs were placed in 3–8 larval containers to allow the larvae to develop. Each larval container consisted of two 4 L plastic buckets nested inside of each other. The top container had its bottom replaced with 180 µm (for both species of Acropora) or 100 µm nitex mesh (P. strigosa) so that fresh seawater could flow through the buckets and over the side of the outer bucket without losing any of the developing embryos. Flowing seawater was constantly supplied to all larval containers, which were cleaned 3–6 times every day and exchanged for clean containers every 2 days. Larvae of both Acropora species were raised for 5–6 days and larvae of P. strigosa were raised for 4 days. All larvae had an elongated body form and were observed probing the bottom (a sign of metamorphic competence) before they were used in the settlement experiments described below.
Larval survival and settlement experiments
Each experiment was deployed on the reef with replicate (n = 8 or 10 per treatment) individual larval chambers, each containing 100 larvae. The chambers were similar to those described in Kuffner et al. (2006) but we used clear acrylic tube that was 5.6 cm inner diameter and 10 cm long. The chambers had nitex mesh glued to one end and on the other end a removable lid with the same size mesh, 180 µm mesh for Acropora spp. and 100 µm mesh for P. strigosa. In this way, the chamber could be partially submerged and the treatments and larvae could be added, and then the lid was attached to contain the larvae for deployment in the field. In each chamber, a 2 × 5 cm piece of the crustose coralline alga Hydrolithon boergesenii was added as positive settlement substrata. Each piece of H. boergesenii was completely covered by CCA on the top surface, and the bottom surface was chipped to create a clean rock surface. H. boergesenii was used because it facilitates the settlement of these spawning coral species (Ritson-Williams et al. 2016a), and it can grow as relatively large individuals making it suitable for large-scale replicated experiments.
Treatment algae and cyanobacteria (described above) were collected 1–2 days before the experiment and maintained in flow-through seawater until their use. Treatment algae and cyanobacteria were attached to the top of the piece of H. boergesenii with a cable tie (Fig. 2a), with only one macrophyte attached to each piece of H. boergesenii. One control for each experiment was a plastic aquarium plant that was attached to the CCA surface in the same way as the macrophytes as a control for space occupation and shading (Fig. 2b). In most experiments there was a cable tie control that served as a positive control without the presence of any macrophyte or plastic plant to ensure that larvae were competent and settlement and metamorphosis occurred. As soon as the chambers were prepared with larvae and a piece of CCA with a treatment or control attached they were placed at a depth of 3 meters on a reef approximately 100 m south of Carrie Bow Cay. Chambers were haphazardly distributed and attached to the benthos with small bungee cords. All of the chambers were placed parallel to the prevailing current to promote water exchange through the chambers. Chambers were checked daily and cleaned of any sediment that accumulated. The chambers were left in the field for 3 days (except for the 2010 A. cervicornis experiment with Dictyota spp., which was left for 4 days), after which they were returned to the laboratory and scored. All of the larvae were counted using a dissecting microscope and were scored as swimming, or settled and metamorphosed on one of 4 substrata; the CCA surface, the rock under the CCA, the chamber itself or the treatments (plastic or live algae). Only settled larvae that had also metamorphosed were counted since settlement alone is a reversible behavior. Very few larvae (≤ 0.2%) ever settled on the plastic or live algae, but they are included in this calculation to show the total settlement and metamorphosis that occurred in each population of coral larvae. The proportion of larvae surviving, which was the total number of swimming larvae plus those that had settled and metamorphosed, and the proportion settled and metamorphosed (a sum of all possible settlement substrata) were calculated as proportions out of 100 (the initial number of larvae added to each chamber), and the means were calculated with each chamber as an individual replicate. Replication was n = 8 or 10 per treatment. In all cases the cable tie control was excluded from the statistical analysis since this was only used as a positive control to ensure that settlement and metamorphosis occurred. The data were arcsine square-root transformed, and appropriate groups were analyzed with a one-way ANOVA. If these data were not normally distributed after transformation (as determined by a Shapiro–Wilk test), the original untransformed data were analyzed with the non-parametric Mann–Whitney Rank Sum test (this was only necessary for the larval settlement data of A. palmata exposed to C. penicillata in 2010). In addition, a Dunnett’s test was conducted on all datasets to determine if there was a difference in survival or settlement and metamorphosis for each treatment macrophyte compared with the plastic plant control.
Results
When larvae of Acropora cervicornis were tested with Dictyota spp. attached to the CCA there was no effect of either algal species on larval survival in 2009 (Fig. 3a; n = 8, F = 0.51, p = 0.607) and 2010 (Fig. 3b; n = 10, F = 2.31, p = 0.119) or settlement and metamorphosis in 2009 (Fig. 3c; n = 8, F = 0.18, p = 0.836) and 2010 (Fig. 3d; n = 10, F = 2.06, p = 0.148). There were not enough A. cervicornis larvae available to test against the cyanobacteria and Lobophora sp. treatments.
Larval survival in a 2009, and b 2010 and the settlement and metamorphosis in c 2009 and d 2010 of Acropora cervicornis exposed to Dictyota spp. Dotted lines indicate the control and treatments that were statistically analyzed as a group. Black bars are controls, white and gray bars are macroalgae spp
When larvae of Acropora palmata were exposed to either Dictyota pulchella or D. bartayresiana there was a reduction of larval survival in 2009 in the D. pulchella treatment (Fig. 4a; n = 10, F = 3.56, p = 0.042) but there was no significant effect of D. bartayresiana on larval survival in 2009. Neither of these two Dictyota species caused mortality in 2010 (Fig. 4b; n = 10, F = 1.36, p = 0.273). There was no effect of either of these Dictyota species on settlement and metamorphosis in 2009 (Fig. 4c; n = 10, F = 0.92, p = 0.412) or in 2010 (Fig. 4d; n = 10, F = 1.64, p = 0.212).
Larval survival in a 2009 and b 2010 and the settlement and metamorphosis in c 2009 and d 2010 of Acropora palmata exposed to Dictyota spp. Dotted lines indicate the control and treatments that were statistically analyzed as a group. Black bars are controls, white and gray bars are macroalgae spp. Asterisks indicate a treatment that was significantly different from the plastic plant control as determined by a Dunnett’s test
When larvae of A. palmata were tested against cyanobacteria and Lobophora sp. attached to the CCA in 2009, there was an effect on survival (Fig. 5a; n = 8, F = 3.94, p = 0.018), but no significant difference in settlement and metamorphosis among the different treatments (Fig. 5c; n = 8, F = 2.24, p = 0.106). Caldora penicillata significantly reduced both larval survival and settlement and metamorphosis when compared directly to the plastic plant control (Dunnett’s test, D = 2.483). Neither Lobophora sp. nor Dichothrix sp. caused significant larval mortality or significantly reduced settlement and metamorphosis. When a smaller experiment was conducted with just C. penicillata in 2010, it again reduced A. palmata larval survival (Fig. 5b; n = 10, F = 22.48, p < 0.001) and settlement and metamorphosis (Fig. 5d; n = 10, Mann–Whitney Rank Sum: U = 12.0, p = 0.004). These larvae were not tested with Hormothamnion enteromorphoides because this cyanobacterium was not found on the reef when these larvae were available.
Larval survival in a. 2009 and b. 2010 and settlement and metamorphosis in c 2009 and d 2010 of Acropora palmata exposed to live algae and cyanobacteria species. Dotted lines indicate the control and treatments that were statistically analyzed as a group. Black bars are controls, white bars are macroalgae and gray bars are cyanobacteria. Asterisks indicate a treatment that was significantly different from the plastic plant control as determined by a Dunnett’s test
For larvae of Pseudodiploria strigosa there was a difference in survival (Fig. 6a; n = 10, F = 2.85, p = 0.035), and a difference in the amount of settlement and metamorphosis (Fig. 6b; n = 10, F = 4.54, p = 0.004), when tested against the different macrophytes. However, for larval survival a Dunnett’s test did not detect a difference between any of the macrophyte treatments and the plastic algal mimic. Hormothamnion enteromorphoides significantly reduced the larval settlement and metamorphosis of P. strigosa (Dunnett’s test, D = 2.531). None of the other cyanobacteria species or Lobophora sp. significantly affected the settlement and metamorphosis of larvae of P. strigosa. Larvae of P. strigosa were not tested with Dictyota spp. due to a limited number of larvae.
Larval a survival and b settlement and metamorphosis of Pseudodiploria strigosa exposed to live algae and cyanobacteria species. Dotted lines indicate the control and treatments that were statistically analyzed as a group. Black bars are controls, white bars are macroalgae and gray bars are cyanobacteria. Asterisks indicate a treatment that was significantly different from the plastic plant control as determined by a Dunnett’s test
Discussion
In this series of experiments, there were variable effects of macrophytes on the patterns of coral larval survival and settlement depending on the coral and macrophyte species tested (Table 2). The larvae of A. cervicornis were not impacted by the Dictyota spp. tested. The larvae of A. palmata had reduced survival and settlement and metamorphosis in the presence of C. penicillata and had increased mortality in the presence of D. pulchella in 2009, but not in 2010. P. strigosa had reduced settlement and metamorphosis in the presence of Hormothamnion enteromorphoides, but was not affected by the other species of macrophytes. These results suggest that reefs that have extensive cover of diverse macrophytes are more likely to inhibit the recruitment of multiple coral species even when facilitating species of CCA are present.
Dictyota spp. were previously found to inhibit the settlement and metamorphosis of Porites astreoides (Kuffner et al. 2006), but they had variable effects on larval mortality of Acropora palmata in the experiments presented here. Further work testing extracts of Dictyota with larvae of Porites astreoides found settlement inhibition to be driven to some extent by the algal compounds, possibly including secondary metabolites (terpenes) found in Dictyota spp. (Paul et al. 2011). It may be that live algae have a few compounds on their surface (Lane et al. 2009), but recent experiments showed no difference in the effect of whole algal extracts compared to algal surface extracts on fragments of adult corals (Rasher et al. 2011; Longo and Hay 2017). It may be that the reduced survival of larvae of A. palmata in the presence of D. pulchella was variable between years due to different concentrations of secondary metabolites, or a difference in the coral larvae themselves. There are a variety of abiotic and biotic conditions (different abiotic stress regimes, maternal effects, differential response among genotypes) that might cause annual variation in larval susceptibility to macroalgae.
The brown alga Lobophora variegata has also shown conflicting results when tested for its impact on coral recruitment. Lobophora was recently found to be a species complex with molecular markers showing extensive genetic diversity in the genus (Sun et al. 2012; Vieira et al. 2017), which may explain some of these variable results (Vieira 2019). Both Acropora palifera and Stylophora pistillata had reduced larval metamorphosis in the presence of L. variegata (Baird and Morse 2004), and larvae of A. millepora and Porites astreoides had reduced settlement in the presence of this alga (Kuffner et al. 2006; Morrow et al. 2017). In contrast, larvae of Acropora millepora had increased settlement when exposed to L. variegata (Birrell et al. 2008b). However, there was no effect of live Lobophora sp. on larvae of either A. palmata or P. strigosa when the alga was in the presence of the positive settlement substratum Hydrolithon boergesenii. Some of these published experiments were conducted with very different methods than in the present study including low replication with live algae (n = 2), live algae soaked in still seawater for 90 min, and the use of lyophilized algae. These methodological differences might be responsible for the different observed impacts on settlement, especially between experiments using the same coral species. Alternatively, it may be that this alga has variable traits across its range. Some, but not all species of Lobophora had allelopathic activity against adult corals (Vieira et al. 2016). Without distinguishing among these species of Lobophora it will be challenging to understand the diversity of impacts that different secondary metabolites might have on coral larval settlement. Alternatively, it may be that the larvae of the Caribbean coral species tested in our experiments are resistant to Lobophora sp. while larvae from other coral species are not.
In the experiments presented here the cyanobacterium Caldora penicillata consistently caused mortality and inhibited the settlement of A. palmata. Additionally, Hormothamnion inhibited the settlement of P. strigosa. Multiple experiments have shown that cyanobacteria can reduce the larval settlement of Porites astreoides (Kuffner et al. 2006; Ritson-Williams et al. 2016b) and Pocillopora damicornis (Kuffner and Paul 2004). Cyanobacteria produce a rich variety of secondary metabolites and one study showed that the isolated compound microcolin A can inhibit coral recruitment (Ritson-Williams et al. 2016b). Cyanobacterial compounds can also stress and kill adult corals (Titlyanov et al. 2007). During benthic surveys, cyanobacteria are often lumped with turf algae or macroalgae and thus their current abundance on coral reefs is probably underestimated. Additionally, with higher seawater temperatures it seems likely that harmful blooms of cyanobacteria will increase in the future (Paul 2008; Paerl and Paul 2012; Ford et al. 2018). Given the experimental results presented here and in other studies, increased frequency and extent of cyanobacteria blooms should be considered a serious threat to future coral recruitment.
These experiments were designed to test whether macrophytes can inhibit coral recruitment even in the presence of substrata that facilitate the settlement of Caribbean broadcast spawning coral species. Importantly, H. boergesenii is known as a settlement facilitator for these broadcast spawning coral species (Ritson-Williams et al. 2016a), although it is not common on many modern Caribbean reefs. While its abundance might vary among locations, surveys in Belize found only 3 individual H. boergesenii on 120 m of transects, for a total of 0.075 percent cover (Ritson-Williams et al. 2014). Settlement facilitation and inhibition probably interact, but studies that test the relative importance of these processes for coral larvae are rare. It may be that facilitation is much more important for new coral recruits to select settlement habitat, especially since macrophyte abundance can vary over relatively short time scales (Carpenter 1988). A few other experiments have tested coral larval responses to macrophytes using a settlement facilitating CCA species as the settlement substrata (Birrell et al. 2008b; Diaz-Pulido et al. 2010). Both of these studies showed that at least one species of macroalgae could inhibit coral settlement even in the presence of a positive settlement cue. Further work should aim to establish the relative importance of facilitation and inhibition for coral recruitment.
The experiments presented in this manuscript highlight the species-specific nature of competition on coral reefs, not only variation in the impact of different macrophyte species to coral larvae, but also variability in the susceptibility of larvae from different coral species. There has been extensive experimental work with larvae of Porites astreoides in the Florida Keys, and settlement of these larvae can be negatively affected by Dictyota spp. (Kuffner et al. 2006; Olsen et al. 2014). However, in all of those experiments the larvae were offered conditioned settlement tiles that had complex communities of biofilms and CCA on their surface. Due to the recent recruitment of CCA on those tiles (less than 6 weeks old) it was impossible to determine if they had individuals of Hydrolithon boergesenii growing on them. Additionally, brooding corals are just as responsive to biofilms as they are to facilitating CCA species (Ritson-Williams et al. 2016a), suggesting that there may be more suitable substrata available on present day reefs for brooding corals than for spawners.
Coral recruitment is a critical process for coral persistence and there is evidence that multiple stressors can reduce coral settlement. The experiments presented here only tested one potential stressor, competition with benthic macrophytes. However, it may be that these algae are causing sublethal stress to coral larvae, and when combined with elevated temperatures, both together may interact to further reduce coral larval survival (Ritson-Williams et al. 2016b). Even though there is a pressing need to increase coral recruitment on reefs, there remains a paucity of information about what types of benthic habitat can increase coral settlement and recruitment. Some benthic competitors inhibit larval survival and settlement even in the presence of facilitating CCA species. This research highlights the importance of understanding both competition and facilitation for successful coral recruitment on modern reefs.
Data availability
The datasets generated and analyzed during the current study are available in the Zenodo repository, https://doi.org/10.5281/zenodo.3579577
References
Arnold S, Steneck R (2011) Settling into an increasingly hostile world: the rapidly closing “recruitment window” for corals. PLoS One 6:e28681
Arnold SN, Steneck RS, Mumby PJ (2010) Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91–105. https://doi.org/10.3354/meps08724
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38. https://doi.org/10.1023/a:1013103928980
Babcock R, Mundy C (1996) Coral recruitment: Consequences of settlement choice for early growth and survivorship in two scleractinians. J Exp Mar Biol Ecol 206:179–201. https://doi.org/10.1016/s0022-0981(96)02622-6
Baird AH, Morse ANC (2004) Induction of metamorphosis in larvae of the brooding corals Acropora palifera and Stylophora pistillata. Mar Freshw Res 55:469–472
Baird AH, Babcock RC, Mundy CP (2003) Habitat selection by larvae influences the depth distribution of six common coral species. Mar Ecol Prog Ser 252:289–293
Birrell CL, McCook LJ, Willis BL, Diaz-Pulido G (2008a) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol 46:25–63
Birrell CL, McCook LJ, Willis BL, Harrington L (2008b) Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora. Mar Ecol Prog Ser 362:129–137
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149
Carlon DB (2002) Production and supply of larvae as determinants of zonation in a brooding tropical coral. J Exp Mar Biol Ecol 268:33–46. https://doi.org/10.1016/s0022-0981(01)00369-0
Carpenter RC (1988) Mass mortality of a Caribbean sea urchin: immediate effects on community metabolism and other herbivores. Proc Natl Acad Sci 85:511–514
Chua CM, Leggat W, Moya A, Baird AH (2013) Temperature affects the early life history stages of corals more than near future ocean acidification. Mar Ecol Prog Ser 475:85–92. https://doi.org/10.3354/meps10077
Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O (2010) The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs 29:203–208. https://doi.org/10.1007/s00338-009-0573-x
Doropoulos C, Roff G, Zupan M, Nestor V, Isechal AL, Mumby PJ (2014) Reef-scale failure of coral settlement following typhoon disturbance and macroalgal bloom in Palau, Western Pacific. Coral Reefs 33:613–623. https://doi.org/10.1007/s00338-014-1149-y
Doropoulos C, Roff G, Bozec YM, Zupan M, Werminghausen J, Mumby PJ (2016) Characterizing the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr 86:20–44. https://doi.org/10.1890/15-0668.1
Engene N, Tronholm A, Salvador-Reyes LA, Luesch H, Paul VJ (2015) Caldora penicillata gen. nov., comb. nov. (Cyanobacteria), a pantropical marine species with biomedical relevance. J Phycol 51:670–681
Ford AK, Bejarano S, Nugues MM, Visser PM, Albert S, Ferse SCA (2018) Reefs under siege-the rise, putative drivers, and consequences of benthic cyanobacterial mats. Front Mar Sci 5:18
Gleason DF, Edmunds PJ, Gates RD (2006) Ultraviolet radiation effects on the behavior and recruitment of larvae from the reef coral Porites astreoides. Mar Biol 148:503–512. https://doi.org/10.1007/s00227-005-0098-y
Gleason DF, Danilowicz BS, Nolan CJ (2009) Reef waters stimulate substratum exploration in planulae from brooding Caribbean corals. Coral Reefs 28:549–554. https://doi.org/10.1007/s00338-009-0480-1
Harrington L, Fabricius K, De'ath G, Negri A (2004) Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85:3428–3437
Kuffner IB (2001) Effects of ultraviolet (UV) radiation on larval settlement of the reef coral Pocillopora damicornis. Mar Ecol Prog Ser 217:251–261. https://doi.org/10.3354/meps217251
Kuffner IB, Paul VJ (2004) Effects of the benthic cyanobacterium Lyngbya majuscula on the larval settlement of the reef corals Acropora surculosa and Pocillopora damicornis. Coral Reefs 23:455–458
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach K (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Lane AL, Nyadong L, Galhena AS, Shearer TL, Stout EP, Parry RM, Kwasnik M, Wang MD, Hay ME, Fernandez FM, Kubanek J (2009) Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc Natl Acad Sci 106:7314–7319. https://doi.org/10.1073/pnas.0812020106
Longo GO, Hay ME (2017) Seaweed allelopathy to corals: are active compounds on, or in, seaweeds? Coral Reefs 36:247–253. https://doi.org/10.1007/s00338-016-1526-9
Maypa AP, Raymundo LJ (2004) Algae-coral interactions mediation of coral settlement, early survival and growth by macroalgae. Silliman J 45:76–95
Morrow KM, Bromhall K, Motti CA, Munn CB, Bourne DG (2017) Allelochemicals produced by brown macroalgae of the Lobophora genus are active against coral larvae and associated bacteria, supporting pathogenic shifts to Vibrio dominance. Appl Environ Microbiol 83:e02391–e12316. https://doi.org/10.1128/aem.02391-16
NMFS (2006) Endangered and threatened species: final listing determinations for Elkhorn coral and Staghorn coral. Fed Reg 71:26852–26861
Nozawa Y, Harrison PL (2007) Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis. Mar Biol 152:1181–1185. https://doi.org/10.1007/s00227-007-0765-2
Nugues MM, Szmant AM (2006) Coral settlement onto Halimeda opuntia: a fatal attraction to an ephemeral substrate? Coral Reefs 25:585–591
Olsen K, Ritson-Williams R, Paul VJ, Ross C (2014) Combined effects of macroalgal presence and elevated temperature on the early life-history stages of a common Caribbean coral. Mar Ecol Prog Ser 509:181–191. https://doi.org/10.3354/meps10880
Olsen K, Sneed JM, Paul VJ (2016) Differential larval settlement responses of Porites astreoides and Acropora palmata in the presence of the green alga Halimeda opuntia. Coral Reefs 35:521–525. https://doi.org/10.1007/s00338-015-1394-8
Paerl H, Paul VJ (2012) Climate change: Links to global expansion of harmful cyanobacteria. Water Res 46:1349–1363
Paul VJ (2008) Global warming and cyanobacterial harmful algal blooms. In: Hundnell H (ed) Cyanobacterial harmful algal blooms: state of the science and research needs. Springer, New York, pp 239–257
Paul VJ, Kuffner IB, Walters LJ, Ritson-Williams R, Beach K, Becerro MA (2011) Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar Ecol Prog Ser 426:161–170
Pennings SC, Pablo SR, Paul VJ (1997) Chemical defenses of the tropical, benthic marine cyanobacterium Hormothamnion enteromorphoides: Diverse consumers and synergisms. Limnol Oceanogr 42:911–917
Price N (2010) Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163:747–758. https://doi.org/10.1007/s00442-010-1578-4
Putnam HM, Edmunds PJ, Fan TY (2008) Effect of temperature on the settlement choice and photophysiology of larvae from the reef coral Stylophora pistillata. Biol Bull 215:135–142
Randall CJ, Szmant AM (2009a) Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816). Biol Bull 217:269–282
Randall CJ, Szmant AM (2009b) Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper). Coral Reefs 28:537–545. https://doi.org/10.1007/s00338-009-0482-z
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci 107:9683–9688
Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME (2011) Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci 108:17726–17731
Ritson-Williams R, Arnold S, Fogarty ND, Steneck RS, Vermeij MJA, Paul VJ (2009) New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci 38:437-457
Ritson-Williams R, Arnold SN, Paul VJ, Steneck RS (2014) Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs 33:59–66. https://doi.org/10.1007/s00338-013-1113-2
Ritson-Williams R, Arnold SN, Paul VJ (2016a) Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar Ecol Prog Ser 548:127–138. https://doi.org/10.3354/meps11688
Ritson-Williams R, Ross C, Paul VJ (2016b) Elevated temperature and allelopathy impact coral recruitment. PLoS One 11:e0166581. https://doi.org/10.1371/journal.pone.0166581
Ross C, Ritson-Williams R, Olsen K, Paul VJ (2013) Short-term and latent post-settlement effects associated with elevated temperature and oxidative stress on larvae from the coral Porites astreoides. Coral Reefs 32:71–79. https://doi.org/10.1007/s00338-012-0956-2
Slattery M, Lesser MP (2014) Allelopathy in the tropical alga Lobophora variegata (Phaeophyceae): mechanistic basis for a phase shift on mesophotic coral reefs? J Phycology 50:493–505
Sun Z, Hanyuda T, Lim P, Tanaka J, Gurgel C, Kawai H (2012) Taxonomic revision of the genus Lobophora (Dictyotales, Phaeophyceae) based on morphological evidence and analyses rbcL and cox3 gene sequences. Phycologia 51:500–512
Titlyanov E, Yakovleva I, Titlyanova T (2007) Interaction between benthic algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites lutea in direct contact. J Exp Mar Biol Ecol 342:282–291
Vermeij MJA, Smith JE, Smith CM, Vega Thurber R, Sandin SA (2009) Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159(2):325–336
Vieira C (2019) Lobophora–coral interactions and phase shifts: summary of current knowledge and future directions. Aquat Ecol. https://doi.org/10.1007/s10452-019-09723-2 (in press)
Vieira C, Thomas O, Culioli G, Genta-Jouve G, Houlbreque F, Gaubert J, De Clerck O, Payri C (2016) Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci Rep 6:18637
Vieira C, Gaubert J, De Clerck O, Payri C, Gulioli G, Thomas O (2017) Biological activities associated to the chemodiversity of the brown algae belonging to genus Lobophora (Dictyotales, Phaeophyceae). Phytochem Rev 16:1–17
Webster FJ, Babcock RC, Van Keulen M, Loneragan NR (2015) Macroalgae inhibits larval settlement and increases recruit mortality at Ningaloo Reef, western Australia. PLoS One 10:e0124162. https://doi.org/10.1371/journal.pone.0124162
Acknowledgements
Special thanks to the many people who helped with coral spawning and counted thousands of larvae with us, including Scott Jones, Laura Diederick, Kathy Morrow, Sherry Reed and Nikki Fogarty. Thanks to Scott Jones and Zach Foltz who consistently facilitated our research on Carrie Bow Cay. Permits for research were provided by the Belize Fisheries Department. This is contribution 1131 of the Smithsonian Marine Station at Fort Pierce and 1035 of the Caribbean Coral Reef Ecosystems Program.
Funding
This work was funded by the Smithsonian Marine Science Network, the Hunterdon Oceanographic Endowment and the Caribbean Coral Reef Ecosystems (CCRE) program at the Smithsonian Institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: D. Gochfeld.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by J. Olson and undisclosed experts
Rights and permissions
About this article
Cite this article
Ritson-Williams, R., Arnold, S.N. & Paul, V.J. The impact of macroalgae and cyanobacteria on larval survival and settlement of the scleractinian corals Acropora palmata, A. cervicornis and Pseudodiploria strigosa. Mar Biol 167, 31 (2020). https://doi.org/10.1007/s00227-019-3639-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3639-5