Abstract
The red sea urchin Mesocentrotus franciscanus supports a highly valuable wild fishery along the West Coast of North America, but despite its importance in the ecology of kelp forests and as a harvested species, little is known about how M. franciscanus responds to abiotic stressors associated with ocean warming and acidification during its early development. Here, embryos of M. franciscanus were raised under combinations of two temperatures (13 °C and 17 °C) and two pCO2 levels (475 μatm and 1050 μatm) that represent current and future coastal environments. Elevated pCO2 levels led to a decrease in body size of gastrula stage embryos while temperature had no effect. At the prism stage, both temperature and pCO2 affected body size. The warmer temperature increased the body size of prism stage embryos, offsetting the stunting effect of elevated pCO2 on growth. Thermal tolerance, which was estimated by exposing prism stage embryos to a range of temperatures and estimating the survivorship, was found to be slightly higher in those raised under warmer temperatures. The developmental temperature and pCO2 conditions under which embryos were raised did not have an effect on the metabolic rate as measured by oxygen consumption rate at the prism stage. This study provides important insights into a species of high ecological and economic value. Overall, early development under elevated pCO2 conditions may adversely impact M. franciscanus while moderate warming may improve growth and thermal tolerance. Understanding how fishery species respond to abiotic stressors will facilitate our predictive capacity of how climate change will impact future populations, which links to issues such as sustainability and food security.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon dioxide (CO2) concentrations in Earth’s atmosphere have been on the rise since the Industrial Revolution, and this upward trajectory is predicted to continue (Godbold and Calosi 2013; IPCC 2013) with recent average global atmospheric CO2 levels reaching historic modern-day highs of 411.84 ppm as recorded at Mauna Loa (Dlugokencky and Tans 2016). Ocean warming and ocean acidification are major consequences of increasing atmospheric CO2 that produce considerable alterations in oceanic conditions worldwide. By the end of the twenty-first century, surface ocean temperatures are predicted to rise by 0.73–2.58 °C (IPCC 2019). In addition, prolonged warming events (i.e., marine heatwaves) are predicted to increase in frequency, duration, and intensity (Oliver et al. 2018). The global area affected by marine heatwaves has risen in recent decades (Oliver et al. 2018). Already, marine heatwaves have been associated with large changes in community composition, species range shifts, and mass mortalities (Frölicher and Laufkötter 2018; Smale et al. 2019; Ummenhofer and Meehl 2017). For example, a warming event in 2015–2016 led to bleaching in 91.1% of corals surveyed on the Great Barrier Reef (Hughes et al. 2017). In the northeast Pacific Ocean, warming events associated with “the Blob” occurred between 2013 and 2016 (Gentemann et al. 2017; Hu et al. 2017), leading to northward geographical shifts of several species as well as mass strandings of many marine birds and mammals (Bond et al. 2015; Cavole et al. 2016; Sanford et al. 2019). Ocean warming can also have significant economic impacts if important marine fisheries are negatively impacted by rising temperatures (Bond et al. 2015; Cavole et al. 2016; Frölicher and Laufkötter 2018).
In addition to ocean warming, pH levels are expected to decline by 0.1–0.4 units in this century (IPCC 2013). Decreasing pH conditions can negatively impact marine organisms across a variety of taxa via a reduction in calcium carbonate available for calcification, acid–base imbalance, and a reduction in capacity for oxygen transport (Doney et al. 2009; Fabry et al. 2008). Furthermore, while ocean acidification is the primary process affecting pH in the open ocean, coastal systems can be highly dynamic with much more complex trends in changing pH levels (Doney et al. 2009; Duarte et al. 2013; Fabry et al. 2008; Feely et al. 2010, 2008; Salisbury et al. 2008; Wootton et al. 2008). For example, upwelling processes that bring low pH waters to the surface can cause the pH in certain coastal areas to be highly variable across both time and space (Feely et al. 2008; Gruber et al. 2012; Hofmann et al. 2010). Many studies have focused on how a single environmental stressor impacts marine organisms. However, because factors such as ocean temperature and pH are predicted to change, often simultaneously, this approach may not be adequate to accurately predict organismal responses to continuing ocean warming and acidification, and the dynamic complexity of oceanic habitats (Bockmon et al. 2013; Boyd et al. 2018; Byrne and Przeslawski 2013). It is therefore highly valuable to incorporate multiple stressors into experiments that reflect realistic and ecologically relevant current and future ocean conditions (Frieder et al. 2014; Przeslawski et al. 2015). Such studies are also critical for determining optimal or detrimental conditions for economically valuable species (Munari et al. 2011).
Early developmental stages may be the most vulnerable times during the life history of many marine organisms (Byrne 2011; Dupont and Thorndyke 2009; Gosselin and Qian 1997; Kurihara 2008), and with continuing alterations in the marine environment due to climate change, these stages may act as a bottleneck that determines if a species will be successful in the future (Byrne 2012; Byrne and Przeslawski 2013; Kurihara 2008). Thermal stress and elevated pCO2 levels are expected to affect different life stages to varying degrees, and negative impacts have the potential to carry over into later stages, altering population structure and distribution (Beckerman et al. 2002). Here we examined the early developmental stages of the red sea urchin, Mesocentrotus franciscanus (A. Agassiz, 1863), and assessed how they responded to stressors associated with ocean warming and acidification.
Our focal species in this study, the red sea urchin, is a major ecosystem engineer that feeds upon algal communities, particularly within kelp forest ecosystems (Leighton et al. 1966; Rogers-Bennett 2013). This species is present along the western coast of North America and supports a lucrative wild fishery in the United States. According to data reported by the Pacific Fisheries Information Network (PacFIN), the revenue generated from the red urchin fisheries in Washington, Oregon, and California was estimated to be over $6.4 million USD in 2018 (https://pacfin.psmfc.org; accessed 4 June 2019). Given the large ecological role of M. franciscanus within kelp forest ecosystems (Leighton et al. 1966; Rogers-Bennett 2013) and the economic value of the M. franciscanus fishery (Kalvass 2000; Rogers-Bennett 2013), surprisingly few studies have been conducted to assess how this species responds to environmental stressors. Elevated pCO2 levels have been shown to increase sperm limitation and the potential for polyspermy, and decrease fertilization success in M. franciscanus (Frieder 2014; Reuter et al. 2011). While multistressor studies have focused on some sea urchin species, examination of M. franciscanus development under combined pCO2 and temperature stressors has remained almost entirely absent from climate change biology. In one study, (O'Donnell et al. 2009) found that M. franciscanus larvae raised under elevated pCO2 levels exhibited a decreased ability to respond to acute elevated temperatures, measured by a decrease in expression levels of hsp70. Gaps of knowledge remain regarding how this species responds to elevated temperatures and pCO2 levels, both as singular and as combined stressors. These gaps limit our understanding of how current populations are responding to stress and restrict our predictive capacity of how future populations will fare under continued ocean warming and acidification.
Here we investigated the response of M. franciscanus, a species that has been overlooked in climate change biology despite its sizeable ecological and economic importance (Pearse 2006; Quinn et al. 1993; Rogers-Bennett 2013), to different temperature and pCO2 conditions during its early development. Embryos of M. franciscanus were raised under different combinations of temperature (13 °C and 17 °C) and pCO2 level (475 μatm and 1050 μatm) that represented ecologically relevant ocean conditions. These treatments were selected based on values recorded in kelp-dominated temperate reef ecosystems inhabited by M. francsicanus as well as predicted ocean conditions given projected future atmospheric CO2 levels (Chan et al. 2017; Hofmann and Washburn 2015; IPCC 2013; Kapsenberg and Hofmann 2016; Rivest et al. 2016). After raising M. francisanus embryos under our selected treatment conditions, we examined how temperature and pH stressors affected embryo body size at both the gastrula and prism stages of early development. Lastly, we measured thermal tolerance and metabolic rate at the prism stage to examine how developmental temperature and pCO2 conditions affected embryo physiology. Given previously recorded sensitivity of M. franciscanus to decreased pH levels (Frieder 2014; Reuter et al. 2011), we hypothesized negative effects of elevated pCO2 on embryos, particularly when in combination with elevated temperature (O'Donnell et al. 2009). Although the elevated pCO2 treatment (1050 μatm) did lead to a decrease in body size, this effect was offset by the warmer temperature of the 17 °C treatment, similar to observations made in other urchin species (Byrne et al. 2013a, b, c; Sheppard Brennand et al. 2010). There were surprisingly little effects of either pCO2 or temperature on embryo thermal tolerance or metabolic rate, although development under 17 °C led to a modest increase in embryo thermal tolerance.
Materials and methods
Animal collection and culturing
Adult M. franciscanus were hand-collected by SCUBA on February 21, 2018 from a site in the Santa Barbara Channel near Ellwood Mesa, Goleta, California, USA (34° 25.065′ N, 119° 54.092′ W) at a depth of 14 m under California Scientific Collection permit SC-1223. The water temperature at the location and time of collection was 13.3 °C, while the water temperature recorded during the three weeks preceding the collection ranged from 12.5 to 15.4 °C with an average daily temperature of 14.6 ± 0.5 °C (Reed 2019). The sea urchins were immediately transported to the Marine Science Institute at the University of California Santa Barbara (UCSB), where they were maintained in flow-through seawater tanks for approximately one week prior to spawning. Adults were spawned via an intracoelomic injection of 0.53 M KCl (Strathmann 1987). Sperm from a single adult male was collected dry and was activated by dilution in 0.35 μm filtered, UV-sterilized seawater (FSW) immediately prior to performing crosses. Activated sperm was visually inspected to verify high motility. Eggs were collected in FSW from five adult females. Eggs were visually inspected for quality and maturity (i.e., approximately symmetrical in shape and lacked large, visible germinal vesicles). A small sample of eggs from each female was fertilized with activated sperm from the male. These test fertilizations were only used to verify adequate sperm quality and high male–female compatibility within each cross, and were not used in the final experiment. An approximately equal number of eggs from each of the five females were gently pooled together to simulate mass spawning events that occur in nature. Dilute, activated sperm from the single male was added to the pooled eggs until approximately 98% fertilization success was reached. The use of a single male to fertilize the eggs resulted in cultures of only full- or half-sibling embryos, limiting potential effects caused by paternal genetic variation and male–female interaction variation.
Approximately 120,000 embryos were placed into each culture vessel (total volume = 12 L) at a concentration of no more than 10 embryos per mL of FSW. Each culture vessel was composed of two, nested 5-gal buckets in which the inner bucket had 12 holes 5.5 cm in diameter that were fitted with 64-μm mesh. This allowed for a continuous flow-through of FSW while preventing the loss of embryos. The temperature was modified using two Delta Star® heat pumps with Nema 4× digital temperature controllers (AquaLogic, San Diego, CA, USA) to maintain the culturing temperatures at either ~ 13 °C or ~ 17 °C. A flow-through CO2-mixing system modified from Fangue et al. (2010) was used to establish pCO2 levels of either ~ 475 μatm (pH ~ 7.66) or ~ 1050 μatm (pH ~ 7.97). Four 5-gallon reservoir tanks were used to establish the target pCO2 levels for each temperature, resulting in four total treatments. These temperature and pCO2 conditions were selected as representative of conditions currently measured in the Santa Barbara Channel and projected conditions given continuing ocean warming and acidification (Chan et al. 2017; Hofmann and Washburn 2015; IPCC 2013; Kapsenberg and Hofmann 2016; Rivest et al. 2016). Specifically, the elevated temperature treatment (17 °C) represents the higher end of temperatures currently experienced in this region as well as temperature conditions that are expected to become more prevalent with continued ocean warming. The combination of the lower temperature treatment (13 °C) and the elevated pCO2 treatment (1050 μatm) is representative of upwelling events that occur regularly in this region (Chan et al. 2017; Hofmann and Washburn 2015; Kapsenberg and Hofmann 2016; Rivest et al. 2016). Lastly, in the absence of upwelling, the elevated temperature treatment (17 °C) in combination with the elevated pCO2 treatment (1050 μatm) represent future conditions given continued ocean acidification and warming. Treated water was transported to each culture vessel at a rate of 6 L/hr. There were three replicate culture vessels for each experimental condition for a total of 12 culture vessels.
Temperature, pH, salinity, and total alkalinity (TA) were recorded daily throughout embryo development. Temperature was measured using a wire thermocouple (Thermolyne PM 20700 / Series 1218). Salinity was measured using a conductivity meter (YSI 3100). The pH was measured following the standard operating procedure (SOP) 6b (Dickson et al. 2007b), using a m-cresol purple (Sigma-Aldrich) indicator dye and a spectrophotometer (Bio Spec-1601, Shimadzu). Water samples for measuring TA were preserved by poisoning FSW with saturated 0.02% mercuric chloride and storing the samples at 4 °C until analyzed. The TA was estimated using SOP 3b (Dickson et al. 2007a). Using the measured temperature, spectrophotometric pH, salinity, and TA, parameters of pCO2 and aragonite saturation state (Ωara) were calculated using the carbonic acid dissociation constants from Mehrbach et al. (1973) refit by Dickson and Millero (1987) using CO2calc software (Robbins et al. 2010).
Sampling
The early gastrula stage (~ 23.5 h post-fertilization (hpf) at 17 °C and ~ 32.5 hpf at 13 °C) and the prism stage (~ 44 hpf at 17 °C and ~ 55.5 hpf at 13 °C) were sampled from each culture bucket. The early gastrula stage was identified by the extension of the archenteron to approximately one-half the body length of the embryo and by the formation of secondary mesenchyme cells. The prism stage was designated by the archenteron becoming tripartite, the development of skeletal rods, and the formation of a pyramid-like body shape. Sampling was accomplished by gently siphoning embryos from each culture vessel onto a submerged, mesh filter (35-μm). Embryos were carefully concentrated onto the mesh filter and transferred to a 15 mL falcon tube using a plastic transfer pipette. The total number of embryos collected from each culture vessel at each stage was estimated by counting three small aliquots of embryos so that a coefficient of variance (CV) of less than 10% was reached. The average of the counts was used to calculate the average number of embryos per mL of FSW.
Morphometrics
Gastrula-stage embryos were preserved for body size analyses using 4% formalin in FSW. The fixative for the prism stage was 4% formalin in 0.01 M phosphate buffered saline (PBS) that was buffered with 100 mM sodium perborate to a pH of 8.7. This buffered formalin was used for the prism stage to prevent dissolution of the skeletal rods. The 4% fixatives were added to an equal volume of seawater with embryos, fixing the samples in a final concentration of 2% formalin in seawater. These samples were stored at 4 °C for no longer than three weeks prior to imaging. Additionally, while egg size should not impact differences in embryo size between treatments because the same pool of eggs was distributed across all cultures and treatments, excess eggs that were not used to culture embryos were preserved and measured using the same methods as in Wong et al. (2019).
At both the gastrula and prism stages, individual embryos (n = 35) from each of the 12 culture vessels (i.e., four treatments with three replicate vessels each) were photographed. Embryos were digitally photographed under bright field DIC illumination using a compound microscope (Olympus BX50) with an attached digital camera (Infinity Lite) and Motic Images Plus software (version 3.0). The gastrula embryos were oriented so that the side profile of their archenteron was visible and aligned with the center of the vegetal plate. For the gastrula stage, the length (i.e., the linear distance from the anterior to posterior end when measured across the center of the archenteron) and 2-dimensional area were measured (Fig. 2). The prism stage was oriented in a ventral view and each individual was measured by the length of the skeletal rod, from the tip of the body rod to the tip of the post-oral rod (Fig. 2). The digital images were calibrated for the 20× objective using ImageJ (National Institutes of Health, USA). Differences in body size measured at the gastrula and prism stages were tested using a two-way ANOVA with temperature and pCO2 set as fixed factors and culture vessel identity set as a random factor. These statistical analyses were performed using JMP Pro software (version 11.2.0).
Thermal tolerance
To assess if developmental conditions resulted in physiological differences between the embryos, we measured thermal tolerance using constant, acute temperature exposures. Thermal tolerance was only measured for the prism stage. For each treatment, embryos across replicate culture vessels were pooled together to obtain approximately 7000 embryos per treatment. For each treatment, the embryos were divided equally among seven 20-mL vials so that each vial contained approximately 1000 embryos in 5 mL of FSW (200 embryos mL−1). Two water baths were attached to either end of an aluminum heat block to create a temperature gradient spanning from ~ 16 to ~ 32 °C. Each of the seven vials were distributed across the heat block so that embryos were exposed to different temperatures (~ 16 °C, ~ 21.5 °C, ~ 25 °C, ~ 26.5 °C, ~ 28.5 °C, ~ 30 °C, and ~ 32 °C) for 1 h (Fig. 1). All vials were placed randomly within the heat block while ensuring that each temperature contained a vial from each treatment. Temperatures were recorded using a wire thermocouple (Thermolyne PM 20700 / Series 1218). Following the 1-h exposure, the vials were removed and 100 embryos from each vial were scored as either alive or dead based on the presence or absence of ciliary movement viewed under a light microscope. Hammond and Hofmann (2010) tested the effects of recovery periods of different lengths following a 1-hr temperature treatment of urchin larvae and found that recovery periods did not significantly affect mortality. Therefore, given these past results, it was not deemed necessary to incorporate a recovery period into this experiment. Due to the differences in timing of developmental progression, this procedure was performed separately for embryos raised under the 13 °C treatment and embryos raised under the 17 °C treatment.
Using the binary mortality data (alive or dead) for prism embryos exposed to each temperature, a generalized linear mixed-effects model was used to test for differences in thermal tolerance across treatments. The model included temperature treatment and pCO2 treatment as fixed factors and vial identity as a random factor. Lethal temperature (LT) values were calculated via a logistic regression for each treatment. While LT50 values are the standard metric used when assessing temperature sensitivity (de Vries et al. 2008), LT10 and LT25 values were also calculated because these smaller reductions in survival may still have biologically meaningful consequences (Collin and Chan 2016). The statistical analyses for thermal tolerance were performed using the lme4 (Bates et al. 2015), MASS (Venables and Ripley 2002), and base packages in R (version 3.4.4).
Respirometry
To examine metabolic differences in embryos raised under different temperature and pCO2 conditions, oxygen consumption rates were measured in embryos from each treatment following Marsh and Manahan (1999), with some modifications. Metabolic rate was only measured for the prism stage. For each culture vessel replicate per treatment (n = 12 cultures), embryos were placed into five glass respirometry chamber vials (684–795 μL) at a range of concentrations (approximately 50, 100, 200, 400, and 500 individuals per vial). The embryos were placed in FSW that matched their respective pCO2 treatment, and all vials were incubated at a single, intermediate temperature of 15° C. A single incubation temperature was chosen in an attempt to avoid differences in development and growth rates that would otherwise occur across treatments as a result of using two different temperatures during the incubation period. One “blank” vial for each of the three culture replicates per treatment was filled with only FSW and used as a control to account for any possible background respiration. Oxygen concentrations were measured following a 5- to 6-h incubation of “blank” vials and vials containing a range of densities of embryos (n = 34–534). At the end of each incubation, the seawater was transferred using a gas-tight syringe (Hamilton Company, USA) to an optode containing a fiber-optic oxygen meter (Micro TX3; PreSens, Germany) that had been calibrated using sodium sulfite (Na2SO3) and FSW. A standard curve was generated from which the rate of oxygen consumption per individual (in pmol O2 h−1 individual−1) was estimated. Differences in respiration rates across treatments were tested using a two-way ANOVA using JMP Pro software (version 11.2.0), with temperature and pCO2 set as fixed factors and culture vessel identity set as a random factor.
Results
Eggs and culturing of embryos
The average diameter of the eggs was 0.1272 ± 0.0039 mm and the average 2D area of the eggs was 0.0128 ± 0.0008 mm2. Additionally, the diameter and 2D area of the eggs were highly correlated to one another (Linear regression, r2 = 0.99, F (1,173) = 21,138, p < 0.001). The early development of the M. franciscanus embryos was normal, with few observed morphological abnormalities and low mortality rates. In addition, target seawater conditions for each of the four treatments were reached and remained largely stable throughout the culturing period (Table 1) across all 12 culture vessels (three replicate cultures per treatment). The average salinity was 33.3 ± 0.05 and the average TA was 2222 ± 3 μmol kgFSW−1.
Body size
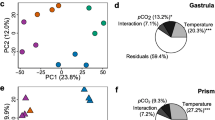
At the gastrula stage, length and 2D area were measured for 35 individual embryos from each of the 12 culture vessels (Table 2). All morphometric data were tested to verify that they met the assumptions of an ANOVA (i.e., approximate normality and homogeneity of variance). We also measured the length of the archenteron to calculate the ratio of the archenteron length to the full body length of the embryo, and found that the archenteron was on average extended 52% the length of the embryo upon sampling for the gastrula stage (see Supplementary Material). Additionally, there was no significant effect of temperature (ANOVA, F (1,9) = 0.0717, p = 0.3308) or pCO2 treatment (ANOVA, F (1,9) = 0.0167, p = 0.9003) on the ratio of the archenteron to the embryo body length, verifying that all embryos were at the same stage of developmental progression at the time of sampling regardless of the temperature or pCO2 treatment. For the gastrula stage, there was no significant effect of the temperature treatment on either gastrula length (ANOVA, F (1,9) = 4.7359, p = 0.0575), or 2D area (ANOVA, F (1,9) = 2.3969, p = 0.1560) (Fig. 2a, b). There was, however, a significant effect of the pCO2 treatment on both gastrula length (ANOVA, F (1,9) = 44.6841, p < 0.0001) and 2D area (ANOVA, F (1,9) = 43.6387, p < 0.0001). Gastrula raised under low pCO2 conditions (475 μatm) were on average 4.83% longer and 8.62% greater in area than those raised under high pCO2 conditions (1050 μatm). There was no significant interaction between temperature and pCO2 treatment conditions for the gastrula stage (p > 0.05).
Body size measurements of embryos raised under different temperature and pCO2 treatment conditions, including a the length of the gastrula stage, b the 2D area of the gastrula stage, and c the length of the prism stage. Bars show standard error. Only ANOVA results with significant p-values are displayed on each graph
Prism length was measured for 35 individual embryos from each of the 12 culture vessels (Table 2). Unlike the gastrula stage, at the prism stage there were treatment effects for both temperature and pCO2. There was a significant effect of temperature treatment on prism length (ANOVA, F (1,9) = 160.5712, p < 0.0001), in which embryos raised under high temperature conditions (17 °C) were on average 20.81% greater in length than those raised under low temperature conditions (13 °C) (Fig. 2C). There was also a significant effect of pCO2 treatment on prism length (ANOVA, F (1,9) = 7.2205, p = 0.0249), in which embryos raised under low pCO2 conditions (475 μatm) were on average 4.08% greater in length than those raised under high pCO2 conditions (1050 μatm). There was no significant interaction between temperature and pCO2 treatment conditions for the prism stage (p > 0.05).
Thermal tolerance
Unfortunately, due to the loss of embryos during the sampling process, we were unable to include embryos from two culture vessels (one replicate of 17 °C, 1050 μatm and one replicate of 17 °C, 475 μatm). Therefore, for these two treatments, only representatives from two of the three replicate culture vessels were pooled together for the thermal tolerance assay. The survivorship of prism stage embryos was assessed across a range of temperatures (16.1–32.2 °C) to generate performance curves for each treatment (Fig. 3). A generalized linear mixed-effects model found that there was an effect of temperature treatment on the survivorship of embryos in response to temperature (p = 0.0389). However, pCO2 treatment had no such effect (p = 0.8431). The calculated LT10, LT25, and LT50 values of embryos raised under the high temperature treatment (17 °C) were greater than those of embryos raised under the low temperature treatment (13 °C) (Table 3). Although the effect of temperature treatment on thermal tolerance was statistically significant, the difference in LT50 values was relatively small. Specifically, the average LT50 of embryos raised at 17 °C was only 0.3 °C higher than the average LT50 of embryos raised at 13 °C.
Respiration rate
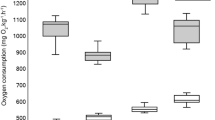
The respirometry data was tested to verify it met the assumptions of an ANOVA (i.e., approximate normality and homogeneity of variance). There was no effect of temperature treatment (ANOVA, F (1,9) = 1.9819, p = 0.1927) or pCO2 treatment (ANOVA, F (1,9) = 0.9042, p = 0.3665) on the respiration rate of prism embryos (Fig. 4). Additionally, there was no evident relationship between the rate of oxygen consumption and embryo body size (i.e., the average length of prism embryos from each culture vessel) (p > 0.056).
Respiration rates (expressed as pmol O2 h−1 individual−1) for prism embryos raised under different temperature and pCO2 treatment conditions. Error bars show standard error. For each culture vessel (n = 12), oxygen consumption was measured using glass respirometry chamber vials (n = 5) that contained a range of different numbers of individual embryos per vial. All respiration measurements were performed at an intermediate temperature of 15 °C
Discussion
Increasing temperatures and pCO2 levels are anticipated to impact the early developmental stages of many marine organisms (Byrne 2011; Byrne and Przeslawski 2013; Kroeker et al. 2013). However, there remains limited knowledge regarding how M. franciscanus will be impacted by these environmental stressors despite its considerable economic and ecological value as a fishery species that inhabits kelp forest ecosystems. In this study, we investigated how development under elevated temperature and pCO2 conditions affected the morphometry and physiology of M. franciscanus embryos. There were four salient findings: (1) at the gastrula stage, only pCO2 had an effect on embryo body size, (2) at the prism stage, temperature and pCO2 had an antagonistic effect on body size, although temperature appeared to be the dominant factor, (3) temperature had a modest effect on thermal tolerance of the prism stage, and (4) being raised under different pCO2 or temperature conditions did not drive significant differences in prism metabolic rate.
It should be noted that this study implemented an experimental design in which eggs from five adult females were pooled together to simulate mass spawning events that occur in nature. However, in an effort to somewhat limit the genetic diversity of the embryos as well as to minimize male–female pairing effects, the pooled eggs were fertilized using sperm from a single adult male to ensure only full- or half-sibling embryos were used in this study. We acknowledge the caveat that this approach results in psuedoreplication. Future studies may opt to include multiple male–female crosses per experiment treatment. An experiment with multiple crosses will exhibit greater genetic diversity, and may require additional culturing space and resources to ensure adequate replication. It is possible that the results of this study are influenced by paternal effects, and that the patterns observed here are unique to the quality of the male selected. While we are unable to separate potential paternal effects due to the use of a single male, visual inspection of high sperm motility and high fertilization success (> 98%) indicate suitable sperm quality and male–female compatibility. Another important caveat of the reported results is that this study represents a subset of individuals from a single population. Therefore, we recommend additional investigations involving different individuals from within this population and across different populations to determine if the observations reported in this study remain consistent within the species. Nonetheless, this study represents an important first step in understanding the physiological responses of M. franciscanus to different environmental conditions.
Only pCO2 impacts gastrula size
The effects of temperature and pCO2 on embryo body size differed by developmental stage. Temperature had no effect on body size of gastrula embryos, while elevated pCO2 conditions decreased both gastrula length and 2D area. Some studies have shown that elevated pCO2 levels can decrease gastrulation success and survival in some echinoderm species (Ericson et al. 2010; Foo et al. 2014; Martin et al. 2011; Nguyen et al. 2012). In the sea urchins Strongylocentrotus purpuratus and Heliocidaris erythrogramma, however, high pCO2 was found to have no effect on developmental progression or morphological abnormality in gastrula embryos (Byrne et al. 2009; Padilla-Gamiño et al. 2013). In this study, abnormality and mortality were not directly measured. There was, however, an observed effect on morphometry.
Most ocean acidification studies have not examined the effects of pCO2 on embryo size, instead focusing primarily on the body size of later, calcifying stages (e.g., pluteus larvae). However, elevated pCO2 levels can impact processes other than calcification via alterations in acid–base regulation physiology (Fabry et al. 2008). Similar to what was observed in this study, low pH levels have been shown to decrease gastrula embryo length in the Antarctic urchin, Sterechinus neumayeri (Ericson et al. 2010; Stuck 2014). Stuck (2014) measured the extracellular pH (pHe) in S. neumayeri gastrula embryos and found that external pH treatments did not affect the pHe in either the gastrula blastocoel or blastoderm. Therefore, the observed reduction in size of the gastrula embryos may be due to the energetic demands associated with maintaining the pHe levels within the embryo despite lower pH conditions in its environment (Pecorino et al. 2014). This metabolic demand could leave less energy for the embryos to allocate towards growth (Stumpp et al. 2011).
In contrast, in an investigation of S. purpuratus, a species whose habitat overlaps with that of M. franciscanus, we found no effect of pCO2 stress on gastrula body size (Wong et al. 2019). The pCO2 stress response may be influenced by differences in maternal provisioning because, unlike the current study in which adults were spawned shortly after being collected from the field, in Wong et al. (2019), adults were provided high quality kelp feed ad libitum throughout gametogenesis. The decrease in body size observed here may have significant biological consequences for M. franciscanus, as larger body sizes during early development have been linked to higher fitness (Smith and Fretwell 1974). Organisms that are larger during early development may exhibit greater performance (i.e. greater growth, survivorship, and reproductive output) at later life stages (Marshall et al. 2003; Moran and Emlet 2001). Thus, a decrease in body size, even at a single stage during early development, may have adverse long-term effects on an organism’s fitness, particularly when the stressor occurs during an essential developmental process such as gastrulation.
Temperature and pCO2 impact prism size
Developing under different temperature and pCO2 conditions had an effect on body size at the prism stage. Prism development is immediately prior to the formation of the early pluteus larval stage and includes initial calcification processes for skeletal rod formation. Here, temperature appears to be the dominant factor, although an effect of pCO2 is still evident. Although a statistically significant interaction between stressors was not detected, temperature and pCO2 appear to have an antagonistic effect on prism body size, in which elevated temperature appears to increase body size and offset the reduction in body size due to elevated pCO2 levels. In contrast, pCO2 but not temperature affect larval body size in S. purpuratus (Padilla-Gamiño et al. 2013), despite this species sharing similar habitats with M. franciscanus. Nevertheless, the pattern reported here has been documented in several other sea urchins species from tropical, temperate, and polar regions in which temperature can mitigate the negative effects of low pH on early developmental stages undergoing calcification (Byrne et al. 2013a, b, c; Sheppard Brennand et al. 2010). Therefore, as ocean warming and ocean acidification progress, moderate warming may provide a compensatory effect as ocean pH levels continue to decline. With the increasing frequency of marine heatwave events (Frölicher and Laufkötter 2018; Ummenhofer and Meehl 2017), temperature may be a major factor influencing M. franciscanus during early development.
In the absence of increased temperatures, elevated pCO2 levels stunt prism growth. This was observed in prism embryos raised under the 13 °C, 1050 μatm pCO2 treatment (Fig. 2). The temperature and pCO2 level of this treatment are characteristic of water conditions that occur during upwelling events in the Santa Barbara Channel (Hofmann and Washburn 2015; Kapsenberg and Hofmann 2016; Rivest et al. 2016). Upwelling events off the West Coast of North America are caused by a strengthening of northwesterly winds that typically begin in the early spring season (Fabry et al. 2008; Pennington and Chavez 2000). The timing of these events are highly variable, but they can coincide with the peak spawning period of M. franciscanus, which typically occurs in the spring and early summer (Bennett and Giese 1995; Kato and Schroeter 1985; Strathmann 1987). It is therefore possible that M. franciscanus embryos experience these combined low temperature and low pH conditions in nature when spawning occurs during or immediately prior to an upwelling event. Additionally, the intensity and duration of upwelling events are expected to increase in the future (Diffenbaugh et al. 2004). Size reduction during early development may lead to increased mortality due to predation, as there is some evidence of predator preference for smaller echinoderm embryos and larvae (Allen 2008). Furthermore, a reduction in body size can hamper feeding success and further growth once the prism embryos develop into pluteus larvae (Chan et al. 2011; Hart 1995; Hart and Strathmann 1994). Lastly, the rate of development was influenced by temperature in which development occurred more slowly under the lower temperature condition of 13 °C. Here, embryos reached the prism stage after ~ 55.5 h post-fertilization (hpf) when raised at 13 °C in comparison to ~ 44 hpf at 17 °C. Therefore, slower development caused by decreased temperatures may increase the period during which vulnerable developmental stages are exposed to stressful pH conditions.
Temperature conditions during development have a modest effect on thermal tolerance
In addition to body size, we also investigated how developing under the different temperature and pCO2 treatments impacted the physiological performance of M. franciscanus at the prism stage. Although the embryos were raised under a multistressor environment, acute temperature exposures were used to assess thermal tolerance. These acute temperature exposures were not designed to be ecologically relevant (i.e., represent events likely to occur in their natural environment), but were instead used to assess if physiological differences in thermal tolerance existed among the embryos as a result of having developed under different multistressor conditions. Temperature (i.e. 17 °C versus 13 °C), but not pCO2 (i.e., 1050 μatm versus 475 μatm), affected thermal tolerance. This result is in agreement with findings by Karelitz et al. (2017), in which pH conditions did not alter thermal tolerance during early development, a result that was consistent across five echinoderm species despite differences in their ecology and environments (i.e., tropical, temperate, and polar). However, O'Donnell et al. (2009) found elevated pCO2 conditions appeared to affect the thermal stress response of M. franciscanus larvae raised at 15 °C. Specifically, M. franciscanus raised under elevated pCO2 levels exhibited lower levels of expression of the molecular chaperone hsp70, a gene affiliated with thermal defense, which the authors suggested meant larvae exposed to low pH conditions were more vulnerable to heat stress. Additionally, O'Donnell et al. (2009) found that following a 1-hr temperature exposure to 31 °C, cultures displayed over 90% survivorship regardless of pCO2 treatment. In contrast, during this study, less than 16% survivorship was observed across all cultures following a 1-h temperature exposure to 30 °C, and the calculated LT50 values, the temperature at which 50% mortality occurs (de Vries et al. 2008), across all treatments were around 29.1–29.4 °C. The observed disparities between this study and O'Donnell et al. (2009) could be due to the slight difference in developmental stage (i.e., prism versus pluteus larvae), alterations in culturing temperatures (i.e. 15 °C versus 13 or 17 °C), genetic variance, or transgenerational effects caused by dissimilarities in adult environmental history and quality.
The effect of temperature on thermal tolerance was relatively modest. Nonetheless, LT10, LT25, and LT50 values were consistently higher for embryos raised under the higher temperature of 17 °C. Even small differences in temperature can have biologically meaningful consequences. The results of this study differ from observations made in S. purpuratus, in which embryos raised under different temperatures that matched sites where the adults were collected (i.e., sites that varied across the species biogeographic range) were found not to differ in their thermotolerance (Hammond and Hofmann 2010). Additionally, the LT50 values reported here for M. franciscanus are slightly below those recorded for S. purpuratus, which are between 29.7 and 31.0 °C during early development (Hammond and Hofmann 2010). Other sea urchin species have exhibited a potential for thermal acclimation and adaptation, with offspring of adults from warmer regions displaying increased thermal tolerance during early development (Byrne et al. 2011; Pecorino et al. 2013). In this study, all of the embryos across treatments were from the same parents collected together from the same site. Therefore, we do not anticipate that variation in parental experience affected the differences in thermal tolerance observed here (i.e., transgenerational effects). Rather, the difference in thermal tolerance, while relatively small, is likely due to rapid acclimation and developmental plasticity within embryos raised under the warmer temperature.
Metabolic rate is unaffected by experiment treatments
We also examined how developing under different temperature and pCO2 conditions influenced the metabolic rate of M. franciscanus embryos. In general, metabolic rates have been shown to increase with temperature (Arnberg et al. 2013; Manríquez et al. 2017; McElroy et al. 2012; Pimentel et al. 2012). Low pH levels, however, can have varying effects on metabolism. For instance, low pH may decrease metabolism via extracellular acid–base imbalances and direct hypercapnic suppression, or it may increase metabolism due to higher costs associated with maintaining calcification and cellular homeostasis (Hoshijima et al. 2017; Pörtner 2008; Stumpp et al. 2011; Widdicombe and Spicer 2008). In this study, we found no effect of either stressor on the rate of oxygen consumption at the prism stage. This differs from observations by Padilla-Gamiño et al. (2013), in which simultaneous exposure to a warmer temperature and higher pCO2 level depressed respiration rates of S. purpuratus pluteus larvae.
These discordant results between studies could be due to species-level variances or differences in respirometry methods. Here, respiration rates were measured at a single, intermediate temperature (15 °C) for the purpose of removing the confounding factor of increased developmental rate under warmer temperatures. It is possible that embryos raised at 13 °C increased their oxygen consumption while embryos raised at 17 °C decreased their oxygen consumption upon encountering the temperature change associated with entering the respirometry vials. This may have masked the effects of developmental temperature on metabolism. Otherwise, respiration rates may have increased predictably with temperature in accordance with the Arrhenius equation (Alcaraz et al. 2013). Alternatively, if the temperature treatment was maintained throughout respiration measurements, we may have detected metabolic depression due to simultaneous high temperature and pCO2 exposure, similar to what was observed in S. purpuratus (Padilla-Gamiño et al. 2013). The lack of metabolic differences in this study could also be due to the developmental stage that was examined. For instance, Stumpp et al. (2011) found that there was an effect of pCO2 on metabolic rate only once pluteus larvae began feeding. At earlier embryonic stages, metabolic rate did not vary by pCO2 treatment. Therefore, the effects of temperature and pCO2 on metabolic rates in M. franciscanus may only be visible later in development.
Conclusions
The goal of this study was to provide much needed information regarding a species of significant ecological and economic value by examining its capacity to respond to stressors related to climate change within an ecologically relevant context. Here, we observed a decrease in gastrula body size due to high pCO2 levels. This leads to two points for consideration regarding studies of this nature. First, even though they may not be actively undergoing calcification, earlier embryological stages should not be overlooked in ocean acidification studies. Low pH can influence important processes other than calcification, and negative impacts during early embryological development can have detrimental carry-over effects and/or act as a major bottleneck for populations (Byrne 2011). Second, while most studies report the percent success of gastrulation, few studies report how stressors impact gastrula body size. Sublethal effects, however, may still have significant biological consequences (Przeslawski et al. 2015).
Moderate ocean warming may be beneficial to M. franciscanus during early development, helping to offset the negative effects of ocean acidification or acute warming events. We observed evidence of this at the prism stage. This will not occur, however, during periods of upwelling, in which organisms will experience elevated pCO2 levels without simultaneous exposure to elevated temperatures. It is also important to note that while elevated temperatures can increase embryo growth and thermal tolerance, there may be unanticipated biological consequences to ocean warming. This is particularly pertinent because marine heatwaves are anticipated to increase in frequency and intensity (Frölicher and Laufkötter 2018; Ummenhofer and Meehl 2017). Importantly, understanding how this fishery species will be affected by climate change interacts heavily with the role of fisheries management. For example, following an extreme marine heatwave event along the coast of Western Australia, early detection of declines in abundance and growth of several marine invertebrate fisheries (e.g., abalone, scallops, prawns, and crabs) was quickly followed by responsive changes in harvest strategies, allowing for protection and possible recovery of spawning stocks (Caputi et al. 2016). Effective fisheries management relies on studies such as the one presented here that provide important insight into the biology of the organisms and help enable predictions regarding future population dynamics. Failure to adequately consider the biology of a species can lead to its mismanagement. For example, Teck et al. (2018) identified a mismatch between the phenology of M. franciscanus and fishing regulations in which the seasonal reproductive cycle of southern California populations did not align with harvest period restrictions. Ideally, informed management decisions should protect the fishery species and bolster ecosystem resilience while also maintaining a sustainable fishery that people rely on as a source of food and revenue.
As climate change continues, it will be necessary to enact adaptive management strategies that can keep pace with affected M. franciscanus populations so as to decrease the vulnerability of the fishery. Here, we identified negative consequences of elevated pCO2 on embryological body size, particularly in the absence of simultaneous warming. Declines in the quality and/or quantity of recruits due to adverse environmental effects that occur during early development may not be immediately evident to the fishery, as there will be a lag between poor recruitment and low abundance of mature adults available for harvest. Thus, proactive fisheries management may be necessary in order to curb the effects of climate change on early life stages that may lead to substandard recruitment (Caputi et al. 2016). In contrast, passive management systems that respond reactively to declines in population quality and size will be improperly suited to cope with the negative impacts of climate change (Wilson et al. 2018). Growing concerns of climate change effects on the red urchin fishery have already led to suggestions of replacing or supplementing M. franciscanus with more resilient and climate change-tolerant urchin species, such as the pink sea urchin, Strongylocentrotus fragilis (Sato et al. 2018). Alternatively, the establishment of marine protected areas (MPAs) have been shown to lead to increased biomass of M. franciscanus (Teck et al. 2017). Overall, a deeper understanding of how climate change stressors affect M. franciscanus will facilitate the implementation of effective fisheries management strategies.
Future studies would benefit from examining the effects of prolonged exposure to high temperatures, particularly as M. franciscanus begin feeding as pluteus larvae and metamorphose into juveniles. There may be negative carry-over effects of elevated temperatures, and what may be beneficial at the prism stage could be detrimental at other life history stages. For instance, warming can decrease gonad quality and increase disease susceptibility in adult sea urchins (Tajima et al. 1997; Uthicke et al. 2014). Additionally, quicker development rates under elevated temperatures may shorten the planktonic larval duration (i.e., the length of time embryos and larvae are in the plankton prior to metamorphosis and settlement), which can affect dispersal, recruitment and gene flow dynamics (Byrne et al. 2011). Overall, more work is required to understand how positive or negative effects of ocean warming will interact with ocean acidification, and to accurately predict how they will impact populations of M. franciscanus.
Data availability
All data associated with this article are available online as electronic supplementary material.
References
Alcaraz M et al (2013) Effects of temperature on the metabolic stoichiometry of Arctic zooplankton. Biogeosciences 10:689–697. https://doi.org/10.5194/bg-10-689-2013
Allen JD (2008) Size-specific predation on marine invertebrate larvae. Biol Bull 214:42–49
Arnberg M, Calosi P, Spicer J, Tandberg A, Nilsen M, Westerlund S, Bechmann R (2013) Elevated temperature elicits greater effects than decreased pH on the development, feeding and metabolism of northern shrimp (Pandalus borealis) larvae. Mar Biol 160:2037–2048. https://doi.org/10.1007/s00227-012-2072-9
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beckerman A, Benton T, Ranta E, Kaitala V, Lundberg P (2002) Population dynamic consequences of delayed life-history effects. Trends Ecol Evol 17:263–269
Bennett J, Giese AC (1995) The annual reproductive and nutritional cycles in two western sea urchins. Mar Biol Lab 109:226–237
Bockmon E, Frieder C, Navarro M, White-Kershek L, Dickson A (2013) Technical note: controlled experimental aquarium system for multi-stressor investigation of carbonate chemistry, oxygen saturation, and temperature. Biogeosciences 10:5967–5975
Bond NA, Cronin MF, Freeland H, Mantua N (2015) Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys Res Lett 43:3414–3420. https://doi.org/10.1002/2015GL063306
Boyd PW et al (2018) Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—a review. Glob Chang Biol 24:2239–2261. https://doi.org/10.1111/gcb.14102
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Byrne M (2012) Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar Environ Res 76:3–15
Byrne M, Przeslawski R (2013) Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr Comp Biol 53:582–596. https://doi.org/10.1093/icb/ict049
Byrne M, Ho M, Selvakumaraswamy P, Nguyen H, Dworjanyn S, Davis A (2009) Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc R Soc B 276:1883–1888. https://doi.org/10.1098/rspb.2008.1935
Byrne M, Selvakumaraswamy P, Ho MA, Woolsey E, Nguyen HD (2011) Sea urchin development in a global change hotspot, potential for southerly migration of thermotolerant propagules. Deep Sea Res Part 2(58):712–719
Byrne M, Foo SA, Soars NA, Wolfe KDL, Nguyen HD, Hardy N, Dworjanyn SA (2013a) Ocean warming will mitigate the effects of acidification on calcifying sea urchin larvae (Heliocidaris tuberculata) from the Australian global warming hot spot. J Exp Mar Biol Ecol 448:250–257. https://doi.org/10.1016/j.jembe.2013.07.016
Byrne M et al (2013b) Vulnerability of the calcifying larval stage of the Antarctic sea urchin Sterechinus neumayeri to near-future ocean acidification and warming. Glob Chang Biol 19:2264–2275. https://doi.org/10.1111/gcb.12190
Byrne M, Lamare M, Winter D, Dworjanyn SA, Uthicke S (2013c) The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2012.0439
Caputi N, Kangas M, Denham A, Feng M, Pearce A, Hetzel Y, Chandrapavan A (2016) Management adaptation of invertebrate fisheries to an extreme marine heat wave event at a global warming hot spot. Ecol Evol 6:3583–3593. https://doi.org/10.1002/ece3.2137
Cavole LM et al (2016) Biological impacts of the 2013–2015 warm-water anomaly in the Northeast Pacific: winners, losers, and the future. Oceanogr 29:273–285. https://doi.org/10.5670/oceanog.2016.32
Chan KYK, Grünbaum D, O’Donnell MJ (2011) Effects of ocean-acidification-induced morphological changes on larval swimming and feeding. J Exp Biol 214:3857–3867. https://doi.org/10.1242/jeb.054809
Chan F et al (2017) Persistent spatial structuring of coastal ocean acidification in the California current system. Sci Rep. https://doi.org/10.1038/s41598-017-02777-y
Collin R, Chan KYK (2016) The sea urchin Lytechinus variegatus lives close to the upper thermal limit for early development in a tropical lagoon. Ecol Evol 6:5623–5634. https://doi.org/10.1002/ece3.2317
de Vries P, Tamis JE, Murk AJ, Smit MGD (2008) Development and application of a species sensitivity distribution for temperature-induced mortality in the aquatic environment. Environ Toxicol Chem 27:2591–2598
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res Pt I 34:1773
Dickson AG, Sabine CL, Christian JR (2007) SOP 3b. Determination of total alkalinity in seawater using an open-cell titration, Ver. 3 01 2008
Dickson AG, Sabine CL, Christian JR (2007b) SOP 6b. Determination of the pH of seawater using the indicator dye m-cresol purple. Ver. 3.01. Jan 28, 2009.
Diffenbaugh N, Snyder M, Sloan L (2004) Could CO2-induced land-cover feedbacks alter near-shore upwelling regimes? PNAS 101:27–32
Dlugokencky E, Tans P (2016) Trends in atmospheric carbon dioxide. NOAA/ESRL. www.esrl.noaa.gov/gmd/ccgg/trends/
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192. https://doi.org/10.1146/annurev.marine.010908.163834
Duarte C et al (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36:221–236
Dupont S, Thorndyke M (2009) Impact of CO2-driven ocean acidification on invertebrates early life-history—what we know, what we need to know and what we can do. Biogeosciences 6:3109–3131
Ericson JA, Lamare MD, Morley SA, Barker MF (2010) The response of two ecologically important Antarctic invertebrates (Sterechinus neumayeri and Parborlasia corrugatus) to reduced seawater pH: effects on fertilisation and embryonic development. Mar Biol 157:2689–2702. https://doi.org/10.1007/s00227-010-1529-y
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Fangue NA, O'Donnell MJ, Sewell MA, Matson PG, MacPherson AC, Hofmann GE (2010) A laboratory-based, experimental system for the study of ocean acidification effects on marine invertebrate larvae. Limnol Oceanogr Methods 8:441–452
Feely R, Sabine C, Hernandez-Ayon J, Ianson D, Hales B (2008) Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320:1490–1492
Feely R et al (2010) The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar Coast Shelf Sci 88:442–449
Foo SA, Dworjanyn SA, Khatkar MS, Poore AGB, Byrne M (2014) Increased temperature, but not acidification, enhances fertilization and development in a tropical urchin: potential for adaptation to a tropicalized eastern Australia. Evol Appl 7:1226–1237. https://doi.org/10.1111/eva.12218
Frieder CA (2014) Present-day nearshore pH differentially depresses fertilization in congeneric sea urchins. Biol Bull 226:1–7
Frieder C, Gonzales J, Bockmon E, Navarro M, Levin L (2014) Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Glob Chang Biol 20:754–764
Frölicher TL, Laufkötter C (2018) Emerging risks from marine heat waves. Nat Commun. https://doi.org/10.1038/s41467-018-03163-6
Gentemann CL, Fewings MR, García-Reyes M (2017) Satellite sea surface temperatures along the West Coast of the United States during the 2014–2016 northeast Pacific marine heat wave. Geophys Res Lett 44:312–319. https://doi.org/10.1002/2016GL071039
Godbold J, Calosi P (2013) Ocean acidification and climate change: advances in ecology and evolution. Philos Trans R Soc Lond B Biol Sci 368:20120448
Gosselin L, Qian P-Y (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282
Gruber N, Hauri C, Lachkar Z, Loher D, Frölicher T, Plattner G-K (2012) Rapid progression of ocean acidification in the California Current System. Science 337:220–223
Hammond LM, Hofmann GE (2010) Thermal tolerance of Strongylocentrotus purpuratus early life history stages: mortality, stress-induced gene expression and biogeographic patterns. Mar Biol 157:2677–2687. https://doi.org/10.1007/s00227-010-1528-z
Hart MW (1995) What are the costs of small egg size for a marine invertebrate with feeding plaktonic larvae? Am Nat 146:415–426
Hart MW, Strathmann RR (1994) Functional consequences of phenotypic plasticity in echinoid larvae. Biol Bull 186:291–299
IPCC (2019) Summary for Policymakers. In: Pörtner H-O, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Nicolai M, Okem A, Petzold J, Rama B, Weyer N (eds) IPCC Special Report on the Ocean and Cryosphere in a Changing Climate
Hofmann G, Washburn L (2015) SBC LTER: ocean: time-series: mid-water SeaFET and CO2 system chemistry at Mohawk Reef (MKO), ongoing since 2012-01-11. Santa Barbara Coastal LTER. https://doi.org/10.6073/pasta/826b170f29458104621aa9f0e36c8901
Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA (2010) The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu Rev Ecol Evol Syst 41:127–147
Hoshijima U, Wong JM, Hofmann GE (2017) Additive effects of pCO2 and temperature on respiration rates of the Antarctic pteropod Limacina helicina antarctica. Conserv Physiol 5:cox064. https://doi.org/10.1093/conphys/cox064
Hu Z-Z, Kumar A, Jha B, Zhu J, Huang B (2017) Persistence and predictions of the remarkable warm anomaly in the northeastern Pacific Ocean during 2014–16. J Clim 30:689–702. https://doi.org/10.1175/JCLI-D-16-0348.1
Hughes TP et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Kalvass PE (2000) Riding the rollercoaster: boom and decline in the California red sea urchin fishery. J Shellfish Res 19:621–622
Kapsenberg L, Hofmann GE (2016) Ocean pH time-series and drivers of variability along the northern Channel Islands, California, USA. Limnol Oceanogr 61:953–968. https://doi.org/10.1002/lno.10264
Karelitz SE, Uthicke S, Foo SA, Barker MF, Byrne M, Pecorino D, Lamare MD (2017) Ocean acidification has little effect on developmental thermal windows of echinoderms from Antarctica to the tropics. Glob Chang Biol 23:657–672
Kato S, Schroeter SC (1985) Biology of the red sea urchin, Strongylocentrotus franciscanus, and its fishery in California. Mar Fish Rev 47:1–20
Kroeker K et al (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol 19:1884–1896. https://doi.org/10.1111/gcb.12179
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284
Leighton D, Jones L, North W (1966) Ecological relationships between giant kelp and sea urchins in southern California. In: Young E, Maclachlan J (eds) Proceedings of the 5th international seaweed symposium. Pergamon Press, UK, pp 141–153.
Manríquez PH et al (2017) Effects of ocean warming and acidification on the early benthic ontogeny of an ecologically and economically important echinoderm. Mar Ecol Prog Ser 563:169–184. https://doi.org/10.3354/meps11973
Marsh AG, Manahan DT (1999) A method for accurate measurements of the respiration rates of marine invertebrate embryos and larvae. Mar Ecol Prog Ser 184:1–10
Marshall DJ, Bolton TF, Keough MJ (2003) Offspring size affects the post-metamorphic performance of a colonial marine invertebrate. Ecology 84:3131–3137
Martin S et al (2011) Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J Exp Biol 214:1357–1368. https://doi.org/10.1242/jeb.051169
McElroy DJ, Nguyen HD, Byrne M (2012) Respiratory response of the intertidal seastar Parvulastra exigua to contemporary and near-future pulses of warming and hypercapnia. J Exp Mar Biol Ecol 416–417:1–7. https://doi.org/10.1016/j.jembe.2012.02.003
Mehrbach C, Culberson C, Hawley J, Pytkowicz R (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Moran A, Emlet R (2001) Offspring size and performance in variable environments: field studies on a marine snail. Ecology 82:1597–1612
Munari M, Matozzo V, Marin M (2011) Combined effects of temperature and salinity on functional responses of haemocytes and survival in air of the clam Ruditapes philippinarum. Fish Shellfish Immunol 30:1024–1030
Nguyen H, Doo S, Soars N, Byrne M (2012) Noncalcifying larvae in a changing ocean: warming, not acidification/hypercapnia, is the dominant stressor on development of the sea star Meridiastra calcar. Glob Chang Biol 18:2466–2476. https://doi.org/10.1111/j.1365-2486.2012.02714.x
O'Donnell M, Hammond L, Hofmann G (2009) Predicted impact of ocean acidification on a marine invertebrate: elevated CO2 alters response to thermal stress in sea urchin larvae. Mar Biol 156:439–446
Oliver ECJ et al (2018) Longer and more frequent marine heatwaves over the past century. Nat Commun. https://doi.org/10.1038/s41467-018-03732-9
Padilla-Gamiño J, Kelley M, Evans T, Hofmann G (2013) Temperature and CO2 additively regulate physiology, morphology and genomic responses of larval sea urchins Strongylocentrotus purpuratus. Proc R Soc B. https://doi.org/10.1098/rspb.2013.0155
Pearse J (2006) Ecological role of purple sea urchins. Science 314:940–941. https://doi.org/10.1126/science.1131888
Pecorino D, Lamare MD, Barker MF, Byrne M (2013) How does embryonic and larval thermal tolerance contribute to the distribution of the sea urchin Centrostephanus rodgersii (Diadematidae) in New Zealand? J Exp Mar Biol Ecol 445:120–128
Pecorino D, Barker MF, Dworjanyn SA, Byrne M, Lamare MD (2014) Impacts of near future sea surface pH and temperature conditions on fertilisation and embryonic development in Centrostephanus rodgersii from northern New Zealand and northern New South Wales, Australia. Mar Biol 161:101–110. https://doi.org/10.1007/s00227-013-2318-1
Pennington JT, Chavez FP (2000) Seasonal fluctuations of temperature, salinity, nitrate, chlorophyll and primary production at station H3/M1 over 1989–1996 in Monterey Bay California. Deep Sea Res Part 2 47:947–973
Pimentel MS, Trübenbach K, Faleiro F, Boavida-Portugal J, Repolho T, Rosa R (2012) Impact of ocean warming on the early ontogeny of cephalopods: a metabolic approach. Mar Biol 159:2051–2059. https://doi.org/10.1007/s00227-012-1991-9
Pörtner H-O (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217. https://doi.org/10.3354/meps07768
Przeslawski R, Byrne M, Mellin C (2015) A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Chang Biol 21:2122–2140. https://doi.org/10.1111/gcb.12833
Quinn JF, Wing SR, Botsford LW (1993) Harvest refugia in marine invertebrate fisheries: models and applications to the red sea urchin, Strongylocentrotus franciscanus. Am Zool 33:537–550
Reed D (2019) SBC LTER: reef: bottom temperature: continuous water temperature, ongoing since 2000. Environmental Data Initiative. https://doi.org/10.6073/pasta/0bf90bc3af15ed6b27161c97f4c92dab
Reuter KE, Lotterhos KE, Crim RN, Thompson CA, Harley CDG (2011) Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Glob Chang Biol 17:163–171. https://doi.org/10.1111/j.1365-2486.2010.02216.x
Rivest EB, O'Brien M, Kapsenberg L, Gotschalk CC, Blanchette CA, Hoshijima U, Hofmann GE (2016) Beyond the benchtop and the benthos: Dataset management planning and design for time series of ocean carbonate chemistry associated with Durafet®-based pH sensors. Ecol Inform 36:209–220. https://doi.org/10.1016/j.ecoinf.2016.08.005
Robbins L, Hansen M, Kleypas J, Meylan S (2010) CO2calc—a user-friendly seawater carbon calculator for Windows, Max OS X, and iOS (iPhone). US Geological Survey Open-File Report:17
Rogers-Bennett L (2013) Strongylocentrotus franciscanus and Strongylocentrotus purpuratus. In: Lawrence JM (ed) Sea Urchins: biology and ecology, vol 38. Developments in aquaculture and fisheries science. pp 413–435
Salisbury J, Green M, Hunt C, Campbell J (2008) Coastal acidification by rivers: a threat to shellfish? Eos 89:513–528
Sanford E, Sones JL, García-Reyes M, Goddard JHR, Largier JL (2019) Widespread shifts in the coastal biota of northern California during the 2014–2016 marine heatwaves. Sci Rep 9(1):4216. https://doi.org/10.1038/s41598-019-40784-3
Sato KN, Powell J, Rudie D, Levin LA (2018) Evaluating the promise and pitfalls of a potential climate change-tolerant sea urchin fishery in southern California. ICES J Mar Sci 75:1029–1041. https://doi.org/10.1093/icesjms/fsx225
Sheppard Brennand H, Soars N, Dworjanyn S, Davis A, Byrne M (2010) Impact of ocean warming and ocean acidification on larval development and calcification in the sea urchin Tripneustes gratilla. PLoS ONE. https://doi.org/10.1371/journal.pone.0011372
Smale DA, Wernberg T, Oliver ECJ, Thomsen M, Harvey BP, Straub SC, Burrows MT, Alexander LV, Benthuysen JA, Donat MG, Feng M, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Sen Gupta A, Payne BL, Moore PJ (2019) Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat Clim Chang 9(4):306–312. https://doi.org/10.1038/s41558-019-0412-1
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Strathmann MF (1987) Reproduction and development of marine invertebrates of the Northern Pacific Coast. University of Washington Press, USA
Stuck E (2014) Extracellular pH changes in echinoderms as a consequence of ocean acidification. Thesis, University of Otago
Stumpp M, Wren J, Melzner F, Thorndyke M, Dupont S (2011) CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comp Biochem Physiol A Mol Integr Physiol 160:331–340
Tajima K, Hirano T, Shimizu M, Ezura Y (1997) Isolation and pathogenicity of the causative bacterium of spotting disease of sea urchin Strongylocentrotus intermedius. Fish Sci 63:249–252
Teck SJ et al (2017) Disentangling the effects of fishing and environmental forcing on demographic variation in an exploited species. Biol Conserv 209:488–498. https://doi.org/10.1016/j.biocon.2017.03.014
Teck SJ et al (2018) Quality of a fished resource: assessing spatial and temporal dynamics. PLoS ONE 13:e0196864. https://doi.org/10.1371/journal.pone.0196864
IPCC (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Stocker TF, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)
Ummenhofer CC, Meehl GA (2017) Extreme weather and climate events with ecological relevance: a review. Philos Trans R Soc B 372:20160135. https://doi.org/10.1098/rstb.2016.0135
Uthicke S, Liddy M, Nguyen HD, Byrne M (2014) Interactive effects of near-future temperature increase and ocean acidification on physiology and gonad development in adult Pacific sea urchin, Echinometra sp. A. Coral Reefs 33:831–845
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Biol Ecol 366:187–197. https://doi.org/10.1016/j.jembe.2008.07.024
Wilson JR et al (2018) Adaptive comanagement to achieve climate-ready fisheries. Conserv Lett 11:e12452. https://doi.org/10.1111/conl.12452
Wong JM, Kozal LC, Leach TS, Hoshijima U, Hofmann GE (2019) Transgenerational effects in an ecological context: conditioning of adult sea urchins to upwelling conditions alters maternal provisioning and progeny phenotype. J Exp Mar Biol Ecol 571:65–77. https://doi.org/10.1016/j.jembe.2019.04.006
Wootton J, Pfister C, Forester J (2008) Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci 105:18848–18853
Acknowledgements
This research was supported by funds from the UC Climate Champion award from the University of California to G.E.H. This work was also supported by resources from the Santa Barbara Coastal Long-Term Ecological Research program (NSF award OCE-1232779). During this project. J.M.W. was supported by a U.S. National Science Foundation (NSF) Graduate Research Fellowship under Grant No. 1650114. Analytical and writing phases of the project were supported by NSF award IOS-1656262 to G.E.H. Specimens were collected in the Santa Barbara Channel under a California Scientific Collecting Permit to G.E.H. (SC-1223). We would like to thank Christoph Pierre, Director of Marine Operations at UC Santa Barbara, for assistance with boating and collections. We also wish to thank Logan Kozal, Terence Leach, Maddie Housh, Jannine Chamorro, Dr. Marie Strader, and Dr. Umihiko Hoshijima for their assistance during the experiment, and Cailan Sugano for his guidance with temperature data. Lastly, we would like to thank the editor and reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: M. Byrne.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wong, J.M., Hofmann, G.E. The effects of temperature and pCO2 on the size, thermal tolerance and metabolic rate of the red sea urchin (Mesocentrotus franciscanus) during early development. Mar Biol 167, 33 (2020). https://doi.org/10.1007/s00227-019-3633-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3633-y