Abstract
Demography and life-history characteristics of reef fishes may vary as a consequence of ecological and environmental factors that lead to changes among populations. We evaluated variation in life-history traits in the leatherjacket Meuschenia scaber (Monacanthidae) through age-based analysis of 2112 fish collected from three locations in New Zealand distributed along an 8° latitudinal and 5 °C SST gradients. Meuschenia scaber showed distinct differences in age structure, growth patterns, maximum size and age, sex ratio and mortality across this latitudinal gradient. At warmer lower latitudes (Hauraki Gulf) the population displayed significantly greater mean adult body size (Lmax) and asymptotic length (L∞) in comparison with the other two locations. Fish from Tasman Bay (mid-range latitude) had a longer life span (Tmax) and a maximum age of 19 years, which represents the longest-lived monacanthid documented to date. Fish from Pegasus Bay (cooler higher latitude) showed a slower initial growth than lower latitude counterparts. The difference in maximum age between the sexes declined clinally from 7 years in the Hauraki Gulf to 3 years in Tasman Bay and 1 year in Pegasus Bay. Meuschenia scaber females tended to display heavier and larger body size than males at all three locations. Sex ratios varied among populations and with depth, suggesting females in the Hauraki Gulf and Tasman Bay may move into deeper water with age. Given the increasing global exploitation of monacanthids in multispecies fisheries and their long-living nature, our results provide valuable age-based demographic information essential for future conservation, monitoring and management programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life-history traits, such as growth and mortality rates, can be influenced by many factors including habitat, reproductive strategy, seasonal variation, and fishing activities. Life-history traits of many reef fishes have been shown to vary along geographic ranges at different spatial scales and across environmental gradients (Conover 1992; Brown 1995; Choat and Robertson 2002). This is thought to reflect the effects of environmental factors such as temperature, food availability and quality, habitat and predation pressure (Jennings and Beverton 1991; DeMartini et al. 2005), and such variation has been demonstrated in both tropical (Gust et al. 2002; Choat et al. 2003; Pears 2005; Berumen et al. 2012) and temperate reef fish species (Caselle et al. 2011; Hamilton et al. 2011; Trip et al. 2014). For example, several studies have shown that reef fish living at higher latitudes grow slower but attain larger adult body sizes, a pattern referred to as the Temperature Size Rule (Atkinson 1994; Arendt 2011), and that is often associated with later sexual maturation and greater longevity (Stearns and Koella 1986). Lastly, variation in life-history traits such as growth rate, size and age structure, longevity and reproductive characteristics has been associated with selective fishing practices (Law 2000; Hawkins and Roberts 2004; Hamilton et al. 2007). Studies of species that were previously believed to be short-lived (e.g. pomacentrids, acanthurids, and lutjanids) revealed extended life spans (20–40 years) with populations dominated by older individuals (Choat and Robertson 2002), and such underestimation can lead to problems with sustainable exploitation of targeted species (Musick 1999). Thus, understanding the processes that drive changes in demography and life history of fish populations is not only relevant to the knowledge of the species’ ecology but also a central goal for managing exploitation.
The present study examines spatial variability in age-based demographic characteristics in one of the most common monacanthid species in southern temperate Australasia, the leatherjacket Meuschenia scaber. This reef-associated species is widely distributed in the waters of both southern Australia (from Cape Naturaliste to Sydney, including Tasmania) and New Zealand, where its geographical range covers 18° of latitude extending from the Kermadec Islands to Stewart Island (Hutchins and Swainston 1986; Francis 1993, 1996). This makes the study species a good candidate to explore variation in life-history traits along a latitudinal gradient, and gaining a better understanding of its ecology will contribute towards sustainable management of this species. Meuschenia scaber is exploited in both Australia and New Zealand and is the main monacanthid species caught in the Australian southeast trawl fishery (Yearsley et al. 1999). According to paleontological studies, leatherjacket has been harvested by Māori since their arrival in New Zealand, and represented the most common taxon in catches, along with jack mackerel (Trachurus sp.) and snapper (Chrysophrys auratus), in northern New Zealand (Anderson 1997; Allen 2014). Meuschenia scaber has been a bycatch component of New Zealand inshore bottom trawl landings since the early 1950s. The management of the species started in 2003 with its introduction in the national quota management system, and its current total allowable commercial catch (TACC) is set to 1461 tonnes per year (Ministry for Primary Industries 2013). However, due to the fact that ageing fish of the family Monacanthidae is challenging due to the dimension, unusual shape and fragility of the sagittal otoliths (Grove-Jones and Burnell 1991; Mancera-Rodríguez and Castro-Hernández 2004; Kim et al. 2016), there is a lack of information on life history. This appears to have hampered the recognition and understanding of separate stocks not only for M. scaber in New Zealand waters but also that of other monacanthid species around the world (Ministry for Primary Industries 2017; Miller et al. 2010). Recent studies carried out in the Hauraki Gulf (New Zealand) have unlocked various aspects of the species’ life-history including its pair spawning behaviour and gonochorism (Visconti et al. 2018a), as well its unexpected longevity (Visconti et al. 2018b). However, the magnitude of demographic variation among populations in response to environmental variation and along geographical latitudes is still to be examined.
The aim of this study was to assess geographic variation in demography and life-history characteristics of M. scaber in New Zealand waters. The Hauraki Gulf (LEA 1), Tasman Bay/Golden Bay (LEA 2) and Pegasus Bay/Canterbury Bight (LEA 3) represent the three main statistical areas for leatherjacket landings in New Zealand, with almost 80% of the TACC allocated to the central area (LEA 2). Our sampling was designed to reflect this, and M. scaber was collected from each of these three locations, i.e. Hauraki Gulf, Tasman Bay and Pegasus Bay. The reproductive biology and ageing protocol of the species were addressed in previous work (Visconti et al. 2018a, b). The present study examines geographic variation in (1) size and age composition, (2) longevity, (3) growth, (4) length–weight relationship, (5) condition, (6) reproductive seasonality, (7) sex ratio and (8) mortality of M. scaber across the three locations. These parameters were used to test the hypothesis that Meuschenia scaber followed the temperature-size rule (TSR) along a north–south latitudinal gradient in New Zealand waters. With this in mind we further examined whether site-specific variation (i.e. habitat) and temperature influenced reproductive ecology and life-history traits.
Materials and methods
Study areas and sampling procedures
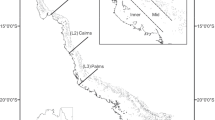
A total of 2112 M. scaber individuals were collected from three areas in New Zealand waters (Fig. 1) between July 2014 and March 2016: the Hauraki Gulf (35.9–36.6°S, 174.7–175.9°E) (n = 658), Tasman Bay (40.5–41.1°S, 173.0–173.4°E) (n = 1003) and Pegasus Bay (43.0–43.4°S, 172.4–173.1°E) (n = 451). The sampling locations differ in mean annual sea surface temperatures (SST), ranging from 17.6 °C for Hauraki Gulf, 14.7 °C for Tasman Bay and 12.6 °C for Pegasus Bay. Sampling methods in the Hauraki Gulf are described in Visconti et al. (2018b), but only data from fish taken by commercial bottom trawlers (n = 152) were used to represent Hauraki Gulf in the present study to allow direct comparison with the other two locations (see below). Monthly samples were obtained from commercial vessels (bottom trawlers) operating in Tasman Bay and supplemented with specimens from the West Coast South Island (WCSI 2015) inshore trawl survey conducted between March and April 2015 (Stevenson and MacGibbon 2015). Pegasus Bay samples were provided as random sub-samples of retained catches by NIWA (National Institute of Water and Atmospheric Research) during the East Coast South Island inshore trawl survey that took place between April and June 2014 (Beentjes et al. 2015). All samples were shipped to the University of Auckland Marine Laboratory for processing—the commercial specimens on ice and survey specimens frozen. The above locations were chosen to reflect the three main fishing areas in New Zealand (LEA 1, LEA 2 and LEA 3).
All fish were measured for standard (SL) and total length (TL) to the nearest mm, and total weight (WT) and gutted weight (WG) were recorded to the nearest 10 g. Samples were sexed considering both external features and macroscopic examination of gonad tissue following Visconti et al. (2018a), and each gonad was weighed to the nearest 0.01 g. Both sagittal otoliths were extracted from each fish, cleaned in 70% ethanol and stored dry in 96-well plates. All otoliths were weighed to the nearest mg and a thin transverse section prepared following Visconti et al. 2018b.
Age, body size, and growth
Mean maximum age Tmax (as a proxy for life span) and mean maximum body size Lmax (as a proxy for mean adult body size) were calculated for each population as the average age (in years) of the 15% oldest individuals, and as the average body size (total length, in mm) of the 15% largest individuals found within each population of M. scaber (Beverton 1992). A bootstrapping technique was used to estimate variance around parameters Tmax and Lmax at each location (Götz et al. 2008; Trip et al. 2008). Age and size data for each population were randomly re-sampled with replication so as to obtain 1000 estimates of Tmax and Lmax. Bias-adjusted 95% percentile confidence intervals (CIs) were calculated from the sorted bootstrap estimates (Haddon 2001; Götz et al. 2008).
The von Bertalanffy growth function (VBGF) was fitted for the sexes combined of M. scaber at each location following the equation:
where Lt is estimated mean size-at-age t, L∞ is mean asymptotic size, K is a curvature parameter and t0 is the age at which the fish have a theoretical size of zero. The VBGF was fitted by constraining the curve to a length-at-settlement of 10 mm TL (Kingsford and Milicich 1987).
Differences in length-at-age among locations were presented using 95% confidence ellipses surrounding the traditional VBGF estimates of parameters L∞ and K (Kimura 1980).
The relationship between length and weight of M. scaber females and males was described with the following equation:
where WT is the total weight, a (y-intercept) and b (slope) is the growth coefficient. The above formula was transformed into its linear form as: \({\text{logWT}} = { \log }a + b{ \log }TL.\)
The 95% CI was calculated for both a and b parameters and an analysis of covariance (ANCOVA) was used on the transformed (linear) data to test for differences in the length–weight relationship between the sexes.
Mortality estimates
Total mortality was estimated for M. scaber in each of the three study regions. The total instantaneous mortality estimate (Z) was derived from the generated age distributions using the Chapman and Robson (1960) estimator and the instantaneous natural mortality (M) from Hoeing (1983). In particular, the age structures from survey populations (i.e. Tasman Bay and Pegasus Bay) were derived from age-length-keys based on length frequencies scaled by biomass estimates for each spatial stratum.
Condition, gonado-somatic indices and sex ratio
The relative condition factor was calculated for M. scaber as monthly mean based on Le Cren’s index (1951) and following Griffiths (2002):
where wi is gutted weight (GW) of the ith individual in the jth month; TLi is the total length (mm) of the ith individual; a and b are the constants from the length–weight relationship based on gutted weight (present study); nj is the number of fish sampled in the jth month.
A gonadsomatic index (RGW) was used to represent the proportional investment in reproductive against somatic tissue, and was calculated as RGW (%) = (WGO/WG) × 100 where WGO is the gonad weight and WG is gutted weight (body weight with all of the viscera removed). Mean monthly RGW of mature females and males was used to compare the timing and duration of the spawning season over the 2-year period for the Hauraki Gulf and Tasman Bay samples.
Sex ratios were tested for significant deviations from unity using a Chi-squared test with Yates’ correction factor for all locations and depth variations.
Results
Age, body size, and growth
The maximum size recorded for the Hauraki Gulf was 320 mm TL with a minimum size of 189 mm TL (Fig. 2a), while the largest fish recorded in Tasman Bay was 290 mm TL with a minimum size of 96 mm TL (Fig. 2b). The maximum size recorded in Pegasus Bay was 340 mm TL with a minimum size of 78 mm TL (Fig. 2c).
From the fish examined, the oldest female and male were from Tasman Bay, even though larger fish were recorded in Hauraki Gulf and Pegasus Bay (Fig. 2). The difference in maximum ages between the sexes declined clinally from 7 years in the Hauraki Gulf to 3 years in Tasman Bay and 1 year in Pegasus Bay.
Mean maximum age (Tmax) was highest in Tasman Bay, with Hauraki Gulf the lowest. Conversely, mean maximum body size (Lmax) was highest in Hauraki Gulf and lowest in both Tasman Bay and Pegasus Bay (Table 1).
The best-fit von Bertalanffy growth parameters for the three locations are presented in Table 1. Comparison of the 95% confidence ellipses revealed significant differences across populations in VBGF parameters K and L∞. Pegasus Bay M. scaber reached a significantly smaller asymptotic length than the Hauraki Gulf and Tasman Bay populations, despite its higher growth rates (Fig. 3, Table 1). The growth coefficient K showed some marginal differences across the three locations, with values ranging from 1.05 in Pegasus Bay, 1.23 in Hauraki Gulf and 1.58 in Tasman Bay (Fig. 4). Fish living in the Hauraki Gulf had significantly larger mean asymptotic size L∞, than in Tasman Bay or Pegasus Bay, which displayed similar values of mean adult body sizes (Fig. 4).
Growth of Meuschenia scaber across New Zealand: a Hauraki Gulf, b Tasman Bay and c Pegasus Bay. Observed individual size-at-age estimates (open circles) are fitted with the von Bertalanffy Growth Function (solid line); d ellipses represent the 95% confidence intervals surrounding estimates of, respectively, L∞ and K for Hauraki Gulf (continuous line), Tasman Bay (dashed line) and Pegasus Bay (dotted line)
The relationship between total length (TL) and total weight (WT) was WT = 0.000027*TL2.903492 (r2 = 0.97, n = 659) for the Hauraki Gulf (Fig. 5a), WT = 0.000032*TL2.869788 (r2 = 0.90, n = 1003) for Tasman Bay (Fig. 5b) and WT = 0.000008*TL3.117828 (r2 = 0.98, n = 451) for Pegasus Bay (Fig. 5c). The 95% confidence interval of b ranged between 2.92 and 2.89 for Hauraki Gulf, 2.87 and 2.86 for Tasman Bay, and between 3.14 and 3.09 for Pegasus Bay. The analysis of covariance (ANCOVA) revealed significant difference in the intercept and slope between sexes in all the locations, and it is presented in Table 2.
Mortality estimates
Total mortality rates (Z) were 0.42 year−1 (95% CL 0.39–0.45) for Hauraki Gulf, 0.34 year−1 (95% CL 0.31–0.36) for Tasman Bay, and 0.62 year−1 (95% CL 0.58–0.67) for Pegasus Bay. Using the maximum age (tmax) estimate for each location, Hoenig’s (1983) mortality (M) estimate was 0.24 year−1 for Hauraki Gulf, 0.22 year−1 for Tasman Bay and 0.30 year−1 for Pegasus Bay (Fig. 6).
Condition, gonado-somatic indices and sex ratio
The mean monthly relative condition (Kn) calculated for Tasman Bay ranged from 1.04 (October 2014) to 0.99 (March 2015) in females, and 1.04 (October 2014) and 0.97 (March 2015) in males (Fig. 7). Female condition was highest in October, declining until March, and showed two peaks in April and May, immediately following the beginning of the spawning season. The same pattern is observed in male condition for Tasman Bay.
The analyses of gonadosomatic index (RGW) in sexually mature females and males showed a consistent trend in both Hauraki Gulf and Tasman Bay, with clear peaks between August and December (spawning season) (Fig. 8). Mean (± se) ROW and RTW values for Tasman Bay were generally higher than those of Hauraki Gulf, with females reaching 15.6% (± 0.3) in December 2014 and males achieving 0.5% (± 0.03) in October 2015. The RGW values obtained for Pegasus Bay samples were in line with the other two locations in the same period of the year (April–June 2014).
Monthly progression of relative gonad weight of sexually mature female (relative ovary weight, ROW; a) and male (relative testis weight, RTW; b) of Meuschenia scaber in the Hauraki Gulf (solid line) and Tasman Bay (dashed line). ROW and RTW were estimated as a proportion of gutted bodyweight (%) and the mean values of ROW and RTW across individuals sampled each month are presented with standard error (SEM) bars
Sex ratio analyses revealed some significant differences across the three locations; Hauraki Gulf and Pegasus Bay deviated significantly from the hypothetical distribution of 1:1 (see Table 3). However, Tasman Bay samples showed a clear even ratio of 1.09:1 between females and males (\(\chi_{\text{Yates'}}^{2}\) = 1.780, df = 1, p < 0.05, n = 991). Sex ratio also varied with depth in all the locations, with males generally showing higher abundances in 10–25 depth ranges (Table 4). Females were significantly more abundant than males deeper than 40 m in the Hauraki Gulf and Tasman Bay, but less abundant beyond this depth in Pegasus Bay.
Discussion
This study examined the spatial variability in the life history of the leatherjacket M. scaber at three different locations in New Zealand waters. Leatherjacket populations showed several distinct trends in terms of age structure, growth patterns, maximum size and age, sex ratio and mortality. Overall, fish from warmer lower latitudes (Hauraki Gulf) attained a greater mean maximum size in comparison with their counterparts from cooler mid- and higher latitudes (Tasman Bay and Pegasus Bay, respectively). Fish from Tasman Bay had the longest life spans with a maximum age of 19 years. The difference in maximum ages between the sexes declined clinally from 7 years in the Hauraki Gulf to 3 years in Tasman Bay and 1 year in Pegasus Bay. Growth trajectories varied substantially across locations over the latitudinal gradient, with fish collected from Tasman Bay and Hauraki Gulf displaying the fastest growth. Reproductive features of Meuschenia scaber also varied geographically, suggesting a shift in the spawning time.
Length-based metrics (i.e. maximum length, mean adult body size) represent important demographic characteristics that explain variation and consistency among populations (Calder 1984; LaBarbera 1989). Meuschenia scaber size distributions from the three locations revealed that the dominant size classes surveyed were between 200 and 280 mm TL (although the Hauraki Gulf was slightly larger at ̴ 220 to 320 mm). Although Francis (1988) reported that M. scaber can attain 430 mm TL in New Zealand waters, the maximum size recorded in the present study (n = 2112) was 320 mm TL in the Hauraki Gulf and 340 mm TL in Pegasus Bay (ECSI-NIWA). However, Lmax was higher in the Hauraki Gulf, possibly reflecting faster growth and a lower fishing pressure in this area.
Meuschenia scaber females also attained greater weight at length compared to males at all three locations investigated. Additionally, mean monthly female condition followed a similar pattern in both the Hauraki Gulf and Tasman Bay, with the latter having the highest mean monthly values. Both the length–weight relationship and condition results helped to test the hypothesis raised in Visconti et al. (2018a) that M. scaber females tend to invest more towards a larger body size that accommodates larger gonads and thus enhances batch fecundity, whereas males appear to allocate more energy to territory defense and courtship displays over the protracted spawning season.
Although body size information can provide valuable insight into the biology of fish species, the present study demonstrates that age-based analysis of M. scaber revealed specific demographic characteristics and considerable life-history variation among populations. The age composition of M. scaber varied over the spatial scale of the study along with the mean maximum age Tmax (life span) ranging between 7.8 ± 0.15 years (Hauraki Gulf) and 11.28 ± 0.07 years (Tasman Bay), and substantial differences in the initial growth patterns were found. Overall, these results indicate that M. scaber has a reduced mean maximum age at warmer lower latitudes (Hauraki Gulf ̴ 36°S), maximum age at Tasman Bay (̴ 41°S) and slower growth at cooler higher latitudes (Pegasus Bay ̴ 43°S). This is suggestive of some aspects of the temperature-size rule (TSR) developed by Atkinson (1994) and Arendt (2011), which predicts that ectotherms growing up in warmer environments (lower latitudes) have a shorter life span and faster initial growth than individuals living in colder environments (higher latitudes). In line with this theory, sea surface temperature (SST) records show that yearly averaged temperatures are 2.9–5.0 °C higher in the Hauraki Gulf than in Tasman Bay and Pegasus Bay, respectively. However, the two southern M. scaber populations (i.e. Tasman Bay and Pegasus Bay) attained smaller L∞ (i.e. opposite to the predictions of the TSR model), indicating that in this case the TSR model could be outweighed by variation associated with habitat and nutrition and not just temperature, as previously documented for many other species (Yamahira and Conover 2002; Floeter et al. 2005; Robertson et al. 2005; Behrens and Lafferty 2007, 2012; Trip et al. 2014). More generally, our findings on age composition are consistent with those reported for other monacanthids in Miller and Stewart (2009) with the exception of the oldest fish recorded here. The maximum age recorded for M. scaber in this study was 19 years, which is much longer than the previous lifespan estimates of 5 years (Poynter 1980), and represents the greatest age reported for the family Monacanthidae to date.
Meuschenia scaber reproduction also seemed to be influenced by a north–south temperature gradient. Spawning was recorded between August and December each year in both Hauraki Gulf and Tasman Bay. However, the maximum peak of monthly relative ovary weight (ROW) in Tasman Bay was delayed by ca. 2 months compared to Hauraki Gulf females. The difference in peak gonad weight between the two locations could be associated with differences in mean sea surface temperature (Hauraki Gulf mean annual SST 17.6 °C cf. Tasman Bay mean SST 14.7 °C), as temperature has been shown to be the factor that triggers leatherjacket reproduction (Visconti et al. 2018a). RGW values obtained for Pegasus Bay between April and May were in line with those found in the Hauraki Gulf and Tasman Bay during the same season, suggesting that spawning happens in the same seasonal window.
Importantly, the von Bertalanffy growth function fitted for the three populations surveyed unequivocally confirmed the pattern of rapid initial growth for M. scaber in New Zealand waters, along with a significant difference in the growth trajectories across locations. Hauraki Gulf fishes attained greater size-at-age than other southern populations and a faster early growth rate than the Pegasus Bay population. These findings might reflect ecological differences among the locations sampled in this study. In particular, there is some evidence that deeper continental shelf habitats, e.g. sand and mud bottoms associated with the bottom trawl fisheries of Pegasus Bay area, do not provide the same availability and quality of food resources as those in the Hauraki Gulf. Meuschenia scaber feeds selectively on various sponge species, mainly Ecionemia alata and Polymastia fusca, and ascidians like Synoicum kuranui (Ayling 1981; Russell 1983), a degree of feeding selectivity that is similar to that seen in other monacanthid species (Randall and Hartman 1968; Wulff 1994). None of these major dietary sponge or ascidian species occur in Pegasus Bay (Kelly 2015; Page and Kelly 2016). This may force M. scaber to target less suitable prey species, possibly resulting in reduced growth and higher mortality rates in comparison with the Hauraki Gulf and Tasman Bay. Meuschenia scaber from Tasman Bay may benefit from the presence of E. alata and other ascidians (i.e. Clavelina sp.), and this may explain the similarity of growth trajectory with that in the Hauraki Gulf. In addition, M. scaber from Hauraki Gulf have been also documented to feed on salps (Russell 1983; Kingsford and Milicich, 1987), which represent an important source of protein for many reef fishes (Janssen and Harbison 1981; Arkhipkin and Laptikhovsky 2013) and are particularly abundant in the Hauraki Gulf in early spring to early summer (Zeldis and Willis 2015). This further supports our hypothesis that M. scaber growth patterns might be influenced by the presence or absence of particular habitat features that not only provide protection but also food resources (e.g. sponges). However, the lack of data on the diet of the Pegasus Bay population prevents us from drawing firm conclusions on the role that diet may play in the different growth trajectories observed. Future studies should assess the diet of M. scaber at regional scales to better evaluate possible dietary constraints with increasing latitude.
Meuschenia scaber is an indeterminate serial spawning gonochorist that appears to be a paired spawner (Visconti et al. 2018a), and as such an equal number of male and females might be expected. However, sex ratio varied among both locations and depth; in two out of three locations the sex ratio deviated significantly from the hypothetical distribution of 1:1 in favour of males, compromising the overall ratio. Nonetheless, the hypothesis of an even ratio could be supported by two main findings of this study. First, the overall sex ratio recorded for the Tasman Bay was significantly close to the unit (1.09:1). Second, the analysis of sex ratio in relation to depth showed that in most cases the proportion of females was higher at depths > 25 meters. A possible advantage to the population with a skew towards more males represents a potential strategy allowing females to spawn more than once per day with different male partners, consistent with the proposed strategy of many baskets with few eggs proposed in Visconti et al. (2018a). Although this finding highlights an important key demographic feature of this species, our results partially also support the hypothesis that M. scaber might undergo short vertical movements that could be associated with either spawning dynamics or feeding habits. Similar trends have been observed in many gonochoristic lutjanids (Guerra Campos and Bashirullah 1975; Reshetnikov and Claro 1976; Grimes and Huntsman 1980; Grimes 1987; Everson et al. 1989) and in the ocean leatherjacket Nelusetta ayraudi (Lindholm 1984; Miller and Stewart 2009), although actual movement patterns have not been observed. Alternatively, this pattern reflects a possibility that males are more susceptible to trawling. However, further studies are needed to determine specific movement patterns in M. scaber, and thus identify specific spawning grounds and their associated substrate type across the latitudinal range of the species.
In summary, the information presented herein revealed substantial variations in life history traits across a latitudinal range of 8° latitude and 5 °C SST gradients for one of the most common monacanthid species in Australasian waters. The results from this study highlight the importance of age-based demographic studies for species distributed along a temperature gradient, as they provide estimates of the degree of plasticity in growth parameters, life span and reproductive characteristics. However, such information remains limited not only for many reef taxa but especially for the diverse family Monacanthidae. While data on movement patterns and site-specific diets are necessary to clarify the ecology of M. scaber, the variation reported between populations in the present study indicates that region-specific demographic data are required to monitor and optimally manage the resource.
References
Allen MS (2014) Variability is in the mesh-size of the sorter: Harataonga Beach and spatio-temporal patterning in northern Maori fisheries. J Pac Archaeol 5:21–38
Anderson A (1997) Uniformity and regional variation in marine fish catches from prehistoric New Zealand. Asian Perspect 36(1):1–26
Arendt JD (2011) Size-fecundity relationships, growth trajectories, and the temperature-size rule for ectotherms. Evolution 65:43–51
Arkhipkin A, Laptikhovsky V (2013) From gelatinous to muscle food chain: rock cod Patagonotothen ramsayi recycles coelenterate and tunicate resources on the Patagonian Shelf. J Fish Biol 83:1210–1220
Atkinson D (1994) Temperature and organism size—a biological law for ectotherms? Adv Ecol Res 25:1–58
Ayling A (1981) The role of biological disturbance in temperate subtidal encrusting communities. Ecology 62:830–847
Beentjes M, MacGibbon D, Lyon W (2015) Inshore trawl survey of Canterbury Bight and Pegasus Bay, April–June 2014 (KAH1402). N Z Fish Assess Rep 2015(14):136
Behrens MD, Lafferty KD (2007) Temperature and diet effects on omnivorous fish performance: implications for the latitudinal diversity gradient in herbivorous fishes. Can J Fish Aquat Sci 64(6):867–873
Behrens MD, Lafferty KD (2012) Geographic variation in the diet of Opaleye (Girella nigricans) with respect to temperature and habitat. PLoS One 7(9):e45901
Berumen ML, Trip E, Pratchett MS, Choat JH (2012) Differences in demographic traits of four butterflyfish species between two reefs of the Great Barrier Reef separated by 1,200 km. Coral Reefs 31:169–177
Beverton RJH (1992) Patterns of reproductive strategy parameters in some marine teleost fishes. J Fish Biol 41:137–160
Brown JH (1995) Macroecology. University of Chicago Press, USA
Calder WA (1984) Size, function, and life history. Courier Corporation, USA
Caselle JE, Hamilton SL, Schroeder DM, Love MS, Standish JD, Rosales-Casian JA, Sosa-Nishizaki O (2011) Geographic variation in density, demography, and life history traits of a harvested, sex-changing, temperate reef fish. Can J Fish Aquat Sci 68:288–303
Chapman DG, Robson DS (1960) The analysis of a catch curve. Biometrics 16:354–368
Choat JH, Robertson DR (2002) Age-based studies Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 57–80
Choat JH, Robertson DR, Ackerman J, Posada JM (2003) An age-based demographic analysis of the Caribbean stoplight parrotfish Sparisoma viride. Mar Ecol Prog Ser 246:265–277
Conover D (1992) Seasonality and the scheduling of life history at different latitudes. J Fish Biol 41:161–178
DeMartini EE, Friedlander AM, Holzwarth SR (2005) Size at sex change in protogynous labroids, prey body size distributions, and apex predator densities at NW Hawaiian atolls. Mar Ecol Prog Ser 297:259–271
Everson AR, Williams H, Ito B (1989) Maturation and reproduction in two Hawaiian eteline snappers, uku, Aprion virescens, and onaga, Etelis coruscans. Fish Bull 87:877–888
Floeter SR, Behrens MD, Ferreira CEL, Paddack MJ, Horn MH (2005) Geographical gradients of marine herbivorous fishes: patterns and processes. Mar Biol 147(6):1435–1447
Francis R (1988) Are growth parameters estimated from tagging and age–length data comparable? Can J Fish Aquat Sci 45:936–942
Francis MP (1993) Checklist of the coastal fishes of Lord Howe, Norfolk, and Kermadec Islands, southwest Pacific Ocean. Pac Sci 47:136–170
Francis MP (1996) Geographic distribution of marine reef fishes in the New Zealand region. N Z J Mar Freshw Res 30:35–55
Götz A, Kerwath SE, Attwood CG, Sauer WH (2008) Effects of fishing on population structure and life history of roman Chrysoblephus laticeps (Sparidae). Mar Ecol Prog Ser 362:245–259
Griffiths MH (2002) Life history of South African snoek, Thyrsites atun (Pisces: Gempylidae): a pelagic predator of the Benguela ecosystem. Fish Bull 100(4):690–710
Grimes CB (1987) Reproductive biology of the Lutjanidae: a review. In: Polovina JJ, Ralston S (eds) Tropical snappers and groupers. Biology and fisheries management. Westview Press, Boulder and London
Grimes CB, Huntsman GR (1980) Reproductive biology of the vermilion snapper, Rhomboplites aurorubens, from North Carolina and South Carolina. Fish Bull 78:137–146
Grove-Jones RP, Burnell AF (1991) Fisheries biology of the ocean jacket (Monacanthidae: Nelusetta Ayraudi) in the Eastern Waters of the Great Australian Bight, South Australia: Final Report to the Fishing Industry Research and Development Council, Grant No. DFS01Z: South Australian Department of Fisheries
Guerra Campos A, Bashirullah A (1975) Biología del pargo Lutjanus griseus (Linn.) de la Isla de Cubagua, Venezuela, 2: Maduración sexual y fecundidad. Bol Inst Oceanogr Venezuela 14:109–116
Gust N, Choat J, Ackerman J (2002) Demographic plasticity in tropical reef fishes. Mar Biol 140(5):1039–1051
Haddon M (2001) Modelling and quantitative methods in fisheries. Chapman and Hall/CRC, Boca Raton
Hamilton S, Caselle J, Standish J, Schroeder D, Love M, Rosales-Casian J, Sosa-Nishizaki O (2007) Size-selective harvesting alters life histories of a temperate sex-changing fish. Ecol Appl 17:2268–2280
Hamilton SL, Caselle JE, Lantz CA, Egloff TL, Kondo E, Newsome SD, Loke-Smith K, Pondella DJ II, Young KA, Lowe CG (2011) Extensive geographic and ontogenetic variation characterizes the trophic ecology of a temperate reef fish on southern California (USA) rocky reefs. Mar Ecol Prog Ser 429:227–244
Hawkins JP, Roberts CM (2004) Effects of fishing on sex-changing Caribbean parrotfishes. Biol Conserv 115:213–226
Hoenig JM (1983) Empirical use of longevity data to estimate mortality rates. Fish Bull 82:898–903
Hutchins JB, Swainston R (1986) Sea fishes of southern Australia: complete field guide for anglers and divers. Swainston, Perth
Janssen J, Harbison G (1981) Fish in Salps: the association of Squaretails (Tetragonurus Spp.) With Pelagic Tunicates1. J Mar Biol Assoc UK 61:917–927
Jennings S, Beverton R (1991) Intraspecific variation in the life history tactics of Atlantic herring (Clupea harengus L.) stocks. ICES J Mar Sci 48:117–125
Kelly M (2015) Splendid sponges: a guide to the sponges of New Zealand. NIWA, Wellington
Kim A, Bae H, Kim H, Oh C (2016) Age and growth of filefish, Thamnaconus modestus (Günther, 1877) off the Jeju Island of Korea. Ocean Sci J 51:355–362
Kimura DK (1980) Likelihood methods for the von Bertalanffy growth curve. Fish Bull 77:765–776
Kingsford M, Milicich M (1987) Presettlement phase of Parika scaber (Pisces: Monacanthidae): a temperate reef fish. Mar Ecol Prog Ser 36:65–79
LaBarbera M (1989) Analyzing body size as a factor in ecology and evolution. Annu Rev Ecol Syst 20:97–117
Law R (2000) Fishing, selection, and phenotypic evolution. ICES J Mar Sci 57:659–668
Le Cren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol 20(2):201–219
Lindholm R (1984) Observations on the chinaman leatherjacket Nelusetta ayraudi (Quoy and Gaimard) in the Great Australian Bight. Mar Freshw Res 35:597–599
Mancera-Rodríguez NJ, Castro-Hernández JJ (2004) Age and growth of Stephanolepis hispidus (Linnaeus, 1766) (Pisces: Monacanthidae), in the Canary Islands area. Fish Res 66:381–386
Miller M, Stewart J (2009) The commercial fishery for ocean leatherjackets (Nelusetta ayraudi, Monacanthidae) in New South Wales, Australia. Asian Fish Sci 22:257–264
Miller M, Stewart J, West RJ (2010) Using otoliths to estimate age and growth of a large Australian endemic monocanthid, Nelusetta ayraudi (Quoy and Gaimard, 1824). Environ Biol Fish 88:263–271
Ministry for Primary Industries (2013) Fisheries assessment plenary, May 2013: stock assessments and yield estimates, vol 3. Compiled by the Fisheries Science Group, Ministry for Primary Industries, pp 1–1357
Ministry for Primary Industries (2017) Leatherjacket (LEA), pp 660–669, In: Fisheries assessment plenary, May 2017: stock assessments and stock status. Compiled by the Fisheries Science Group, Ministry for Primary Industries, Wellington, New Zealand, p 1596
Musick JA (1999) Criteria to define extinction risk in marine fishes: the American Fisheries Society initiative. Fisheries 24:6–14
Page M, Kelly M (2016) Awesome ascidians: a guide to the sea squirts of New Zealand. NIWA, Wellington
Pears RJ (2005) Comparative demography and assemblage structure of serranid fishes: implications for conservation and fisheries management. PhD Dissertation, James Cook University
Poynter MR (1980) The distribution and abundance of a temperate reef fish Parika scaber (Monacanthidae) with emphasis on recruitment, plus aspects of feeding ecology and growth. PhD Thesis, University of Auckland, Auckland, New Zealand
Randall JE, Hartman W (1968) Sponge-feeding fishes of the West Indies. Mar Biol 1:216–225
Reshetnikov YS, Claro R (1976) Cycles of biological processes in tropical fishes with reference to Lutjanus synagris. J Ichthyol 16:711–723
Robertson DR, Ackerman J, Choat JH, Posada JM, Pitt J (2005) Ocean surgeonfish Acanthurus bahianus. I. The geography of demography. Mar Ecol Prog Ser 295:229–244
Russell BC (1983) The food and feeding habits of rocky reef fish of north- eastern New Zealand. N Z J Mar Freshw Res 17:121–145
Stearns SC, Koella JC (1986) The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution 40:893–913
Stevenson ML, MacGibbon DJ (2015) Inshore trawl survey of the west coast South Island and Tasman and Golden Bays, March–April 2015 (KAH1503) New Zealand Fisheries Assessment Report 2015/67. 94 p
Trip E, Choat JH, Wilson DT, Robertson DR (2008) Inter-oceanic analysis of demographic variation in a widely distributed Indo-Pacific coral reef fish. Mar Ecol Prog Ser 373:97–109
Trip EL, Clements KD, Raubenheimer D, Choat JH (2014) Temperature-related variation in growth rate, size, maturation and life span in a marine herbivorous fish over a latitudinal gradient. J Anim Ecol 83:866–875
Visconti V, Trip ED, Griffiths MH, Clements KD (2018a) Reproductive biology of the leatherjacket, Meuschenia scaber (Monacanthidae) (Forster 1801) in the Hauraki Gulf, New Zealand. N Z J Mar Freshw Res 52(1):82–99
Visconti V, Trip ED, Griffiths MH, Clements KD (2018b) Life-history traits of the leatherjacket Meuschenia scaber, a long-lived monacanthid. J Fish Biol 92(2):470–486
Wulff JL (1994) Sponge feeding by Caribbean angelfishes, trunkfishes, and filefishes. In: van Soest RWM, van Kempen TMG, Braekman JC (eds) Sponges in time and space. Balkema, Rotterdam, pp 265–271
Yamahira K, Conover DO (2002) Intra-vs. interspecific latitudinal variation in growth: adaptation to temperature or seasonality? Ecology 83:1252–1262
Yearsley GK, Ward RD, Last PR (1999) Australian seafood handbook: an identification guide to domestic species. CSIRO, Australia
Zeldis JR, Willis KJ (2015) Biogeographic and trophic drivers of mesozooplankton distribution on the northeast continental shelf and in Hauraki Gulf, New Zealand. N Z J Mar Freshw Res 49(1):69–86
Acknowledgements
The authors would like to thank P. Caiger, P. Browne and B. Doak for their assistance in the field and J.H. Choat for his comments on the manuscript. This study was supported by the Ministry for Primary Industries and University of Auckland Partnership Postgraduate Scholarships Fund. Any opinions expressed in this paper are those of the authors and not MPI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This study was supported by the Ministry for Primary Industries and University of Auckland Partnership postgraduate Scholarships Fund. Any opinions expressed in this paper are those of the authors and not MPI.
Ethical approval
All applicable international, national, and/or institutional guidelines for sampling were followed in the current study. All fish samples were collected in accordance with the University of Auckland Animal Ethics Committee approvals 001047 and 001676.
Additional information
Responsible Editor: D. Goulet.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Visconti, V., Trip, E.D.L., Griffiths, M.H. et al. Geographic variation in life-history traits of the long-lived monacanthid Meuschenia scaber (Monacanthidae). Mar Biol 167, 18 (2020). https://doi.org/10.1007/s00227-019-3628-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3628-8