Abstract
Despite growing evidence that crustaceans produce and detect sounds, the behavioural and biological function of these sounds is still poorly understood. Here, we describe sounds produced by the New Zealand paddle crab, Ovalipes catharus, and provide evidence of intraspecific communication using underwater sound. Acoustic and video analyses of tank-based experiments show that O. catharus produce at least three distinct sounds: the rasp, zip and bass. Notably, two of these sounds, the zip and bass, were directly correlated with post-copulatory mate-guarding and courtship behaviour and produced only by competing adult male crabs in the presence of a receptive female. Rasp sounds were produced by both sexes; the occurrence significantly increased in the presence of food, and play-back experiments of these sounds initiated a foraging-like behaviour. Responses to rasps might have evolved as a result of acoustic spying. Further, we show that both the rasp and bass sounds were produced by an alternative mechanism than stridulation of the chela ridges. This refutes widespread assumptions that Ovalipes crabs use only stridulation of ridges along their chelae to produce rasp-like sounds. Our results suggest that sound production in decapod crustaceans may be more widespread than previously presumed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of sound as a source of communication is common and widespread among vertebrates that live in the aquatic environment. It is well known that large baleen whales use sound to communicate over vast distances (Payne and Webb 1971) and toothed whales use echolocation to hunt for prey (Madsen and Wahlberg 2007). Different species of fish use sound for contact calls (van Oosterom et al. 2016), mate selection (Myrberg et al. 1986), territory defence (Myrberg 1997), and jamming potential competitor’s signals (Mensinger 2013). The acoustic complexity and encoded information in any of these signals can vary between species or within animal groups, as can the target and the purpose (Parmentier and Fine 2016).
In sharp contrast, there are only a few examples of aquatic invertebrates and in particular, brachyurans that have been shown to use sound for communication, with most studies focused on semi-terrestrial crabs (Salmon and Horch 1972; Patek et al. 2009; Staaterman et al. 2010; Favaro et al. 2011). Specifically, ghost and fiddler crabs produce drumming and thumping sounds during courtship displays (Crane 1966; Salmon 1967; Salmon and Atsaides 1968; Horch 1971, 1975; Popper et al. 2001; Clayton 2008). Among the few examples, there is considerable variation in the range of sound types and sound-producing mechanisms. Stridulation structures have been reported in nearly 50 genera of Crustacea, the majority of which are decapods and are structurally similar to the file and pick mechanism commonly found in insects (Gerhardt and Huber 2002).
It has been presumed that all species of Ovalipes are capable of producing sounds via stridulation, specifically by rubbing of hard body parts along ridges on the lower section of the chelae (Stephenson 1969). However, the ability to produce sound is an assumption based primarily on anatomical observations rather than functional evidence. These ridges are enlarged by a modified dactyl of the cheliped, of which 29 different stridulatory repertories are theoretically possible (Stephenson 1969). Males, females, and juveniles all have serrated chela ridges but they tend to be more prominent in adult males (Stephenson 1969). The proposed function for sound production in these species includes mate attraction, aggressive/territorial signals, aggregation signals, courtship and predator warnings (McLay 1988). However, these hypotheses remain to be tested experimentally, as none of the proposed behaviours have yet been observed. Surprisingly, to date, underwater sound production has only been described in a single paddle crab species, O. trimaculatus (Buscaino et al. 2015). These crabs have been shown to produce a wide-frequency multi-pulse signal, presumably to play a role in mate attraction. However, the sound producing mechanism itself was not investigated but hypothesised to be produced via stridulation.
Ovalipes catharus has 20–26 stout striae or ridges on the underside of the propodus, which is the only described sound producing mechanism of the Ovalipes species (McLay 1988). Therefore, the aims of this study were to: (1) record and document any sounds produced by O. catharus; (2) determine the behavioural context of sounds produced; and (3) describe any sound-producing mechanisms. The underlying hypothesis was that sounds produced are not incidental and potentially play an important role in crab behaviour.
Materials and methods
A total of 175 crabs were collected using baited ring pots placed in 3–10 m water depth in Omaha Bay (36° 20′ 04.3″ S 174° 47′ 58.1″ E) and Whangateau estuary (36° 20′ 09.3″ S 174° 45′ 55.2″ E) in 2016. Pots were baited with fish frames and left to fish for 8 h overnight. These crabs were transported to the nearby Leigh Marine Laboratory where they were held in seven identical large indoor rectangular tanks (160 cm × 80 cm and 20 cm of water depth) with flow-through ambient sea-water. Crabs were monitored daily and fed twice a week, and allowed to acclimate for 2 weeks. All experiments were carried out in accordance with the regulations of the University of Auckland Animal Ethics Committee (Approval #001721).
Experimental setup
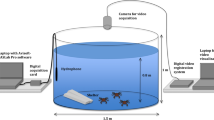
A 1 m diameter (1000 L) circular polyethylene experimental tank was housed in a purpose-built soundproof room and supplied with flow-through ambient seawater. A thin foam rubber mat was glued to the sides and bottom of the tank to reduce sound reflections. A layer of sand was spread evenly across the bottom of the tank to mimic a more natural environment and reduce the noise produced by the crabs walking on the tank floor. Red lights were fitted to the upper rim of the tank to allow video recordings to be undertaken at night. Crustaceans are known to have low sensitivity to red light (Johnson et al. 2002) and therefore, the presence of red light should have little effect on their behaviour.
A CCTV camera (iSpy 64 v6.1.3.0) was mounted 1 m above the water surface centred above the tank for a continuous recording during the trials. In addition, two GoPro Hero cameras (Models 3 and 4, GoPro INC) were placed within the tank looking inwards to allow for a side view of the crab’s behaviour, providing approximately 2 h of recording. A Soundtrap 202 hydrophone (flat frequency response 20 Hz–60 kHz, Ocean Instruments NZ Ltd) with a sampling rate of 96 kHz (Nyquist frequency = 48 kHz), was submerged in the middle of the water column. Minimum background sound level in the experimental tank was calculated from recordings at a regular array of nine points throughout the tank in the absence of crabs. The average background sound level from proximity to the coast, water pumps and nearby electrical equipment was 100 dB re 1 μPa. Clapping on the water surface at the beginning and end of each trial was used to synchronise the audio and video recordings.
Playback experimental setup
This experiment was completed using the same tank set-up as previously described in the sound production experiments. In addition, sound files were played using an mp3 player connected to an amplifier (Sony Xplod, XM-GTX6021) and delivered into the tank via an underwater speaker (UW-30, Underwater Sound Inc., Oklahoma City, OK) suspended midway in the water column, at the margin of the tank projecting inwards. The mp3 player was located outside of the experimental room to allow the sound to be turned on without visual interference.
Sound production trials
Acoustic, locomotor, and agonistic behaviour parameters were extracted from six different experimental conditions to investigate the behaviour and acoustic characteristics of signals produced by O. catharus (Table 1). These six conditions comprised single-sex groups, mixed-sex groups, individual crabs, juveniles, mating events, and feeding events. Additionally, individual crabs were recorded for 24 h to determine if circadian or crepuscular rhythms of sound production existed. To examine the sound-producing mechanisms when a sound was detected, the corresponding video was visually inspected for any crab movements. To avoid any error associated with unsynchronized video and audio time stamps, the video was watched from 2 min before until 2 min after the detection of the acoustic signal.
Crabs were deprived of food 2 days prior to the start of the experimental trials. Crab carapace width (CW) was measured to the nearest 1 mm before being placed in the experimental tank. Individuals under 50 mm CW were classified as juveniles (Osborne 1987).
Crabs were randomly assigned to the different experimental conditions and placed into the experimental tank. Each crab was used only once, to meet the assumption of experimental independence. Experiments began at dusk of the same day as transfer, allowing at least two hours of acclimation. After this acclimation period, the animals appeared calm and sedentary behaviour was observed. At the start of each experiment, the water flow to the tank was turned off to reduce background noise.
The mating behaviours observed in our study were similar to those previously reported in Haddon (1994). Male crabs actively guard and carry female crabs for up to 8 days prior to the female moulting. Some females resisted getting caught by a male crab, but once the male had successfully positioned the female under his body the female became and remained passive (Haddon 1994). To test if there were mating-dependent sounds, two competing males were introduced into the experimental tank of a mating pair (recently moulted female with a male). These mating pairs were obtained by isolating pre-copulatory pairs and waiting for the female to moult. Once the female had moulted and a true mating position was observed (female ventral side up underneath male), they were transferred into the experimental tank and additional competing males were added afterwards. For the purpose of this study a receptive female refers to a recently moulted female. For the feeding trials, food (squid) was placed in a sealed pouch to allow detection but prevent consumption. The pouch was made up of two layers. Food was placed within a fabric mesh, which was enclosed in a fine plastic mesh for added durability. The food pouch was placed in the centre of the tank where it was accessible to the crabs. Some crabs were observed to handle the food pouch, but none were observed accessing the food inside. The crabs were monitored overnight. In total, 930 hours of acoustic and video recordings were collected.
Playback trials
Four different male crabs of similar size (± 10 mm CW) were used in each of the six playback trials. The playback experiment consisted of 30 min of control (recording going but no sounds playing) followed by 10 min of feeding rasps (see supplementary material). The looped 2-min sound file consisted of 56 unique feeding rasps, distributed into 20 s of rasps alternating with 10 s of no rasps. The rasps were obtained from five different trials. The sound level was adjusted by an output control on the amplifier. The playback sounds used for the experiment were played at received levels in the tank ranging from 128.7 to 131.1 dB re 1 μPa (Fig. 1). White noise was played at the similar received levels in the tank ranging from 129.9 to 131.1 dB re 1 μPa to determine if crab movement was evoked from exposure to sound stimuli. No crab movements were observed during white-noise playback.
Each crab was marked with white nail polish to help enhance their contrast in the tank. This allowed the tracking software to easily locate and follow individuals. To apply the nail polish, crabs were placed into individual containers. The water level was kept such that the dorsal side of the carapace could be dried while the gills were exposed to the water. Crabs were left in the containers until the nail polish dried completely.
Data analysis
The sounds produced by the crabs were short broadband signals or consisted of a series of pulses. The signals of each sound type were selected manually using Audacity 2.1.2® free software (Mazzoni 2016) to visualise the sound structure in either oscillogram or spectrogram format. To exclude any incidental sounds from contact with another crab or with the Go-Pro equipment, only sounds of a consistent sound signature with more than three pulses were counted. For sound parameter analysis, sounds were required to be absent of any background noise, consistent with previous studies (Buscaino et al. 2015). Recordings in small tanks induce potential distortion to acoustic parameters because of reverberation and tank resonance due to reflection from tank surfaces (Akamatsu et al. 2002). We followed the working protocol for sound recordings in small tanks outlined by Akamatsu et al. (2002). The minimum resonant frequency (F min) of the tank used for sound recordings was 1.6 kHz, and therefore, the rasp (F min < dominant frequency of crab sound), bass and zip (F min > dominant frequency of crab sound) could be recorded in our tank (Akamatsu et al. 2002). The attenuation distance for the zip and bass sound were 0.41 m and 0.36 m, respectively, and the rasp could be detected anywhere in the tank.
Each of the following sound parameters were analysed using Raven Pro 1.4® software (Cornell Lab of Ornithology):
Peak frequency (Hz): The frequency at which Peak Power occurs within the selection.
Duration (s): The time from the start of the signal to the end of the signal.
Pulse number and pulse rate were obtained manually from the oscillogram data.
Pulse number: The number of individual pulses in one sound.
Pulse rate (Hz): The number of pulses divided by the duration of the signal.
Due to the low frequency (< 60 Hz) nature of the bass sound, Raven Pro 1.4® was unable to detect and analyse this sound. Therefore, manual analysis in Audacity was conducted to determine duration, number of pulses and pulse rate. Matlab (MATLAB and Statistics Toolbox Release 2016b, The MathWorks, Inc., Natick, Massachusetts, United States) was used to obtain additional characteristics such as peak frequency and pulse duration.
For the playback experiments, videos were analysed using the commercially available tracking software, EthoVision® XT 12 (Noldus Information Technology Inc.; https://www.noldus.com/animal-behavior-research/products/ethovision-xt). This software was able to obtain data on activity levels (distance travelled (m) and velocity (m s−1)) as well as the distance to sound source. Crab locations and movements were also visualised with heat maps. Crabs that did not move during the trial were assigned an activity score of zero to allow for direct comparisons between trials. Sound recordings were taken during each trial to analyse if there was an acoustic response to the presence of played-back rasps. Individual rasps were counted and analysed using the same method as described above.
Statistical analysis
For each trial, the number of sounds produced was grouped into one-hour bins. From this, the number of sounds was standardized into the average number of sounds per hour per crab and compared using One-Way Analysis of Variance (ANOVA). For each 24-hour trial (n = 5) the average number of sounds produced per hour was calculated. A One-Way ANOVA was performed for each day (0600–1700 h) and night period (1800–0500 h), as well as dusk (1730–1930 h) and dawn (0630–0830 h) periods. Selection of time periods was based on average sunrise and sunset times for Auckland, New Zealand.
Analysis and comparison of sound characteristics were conducted using R-Studio Software (v0.99.903, RStudio INC.). A generalised linear mixed model (GLMM) was fit to the data to determine the difference in acoustic signals between and within trials. Parametric models were calculated to fit means against least significant differences (LSD) between predicted means. To test for significance at the 0.05 level, a One-Way ANOVA was used.
Rasps (n = 119) from nine female and eight male crab single trials were grouped into a CW size category (a = < 50 mm; b = 51–70 mm; c = 71–90 mm; d = 91–110; e = > 111 mm). The correlation between size category and average rasp peak frequency (Hz) was tested using the Pearson Chi square coefficient.
For each playback trial (n = 6), the total number of rasps were grouped into control (speaker and recorder on but not playing any sounds) and treatment groups (playing pre-recorded feeding rasps) and compared using a paired t test. The distances travelled (m) for each trial were compared across groups using a paired t test to test for significance at the 0.05 level.
Results
Types of sounds produced
A total of 930 h of audio and video recordings were analysed. Under our experimental conditions, O. catharus produced three distinct types of sounds. Based on the acoustic characteristics the sounds were classified as (1) the rasp, (2) the zip, and (3) the bass (Tables 2, 3).
Rasps
The rasp is a multi-pulsed broad frequency band sound that was produced by all tested animals (Fig. 2).
The peak frequency was negatively correlated (Pearson Correlation: r = − 0.72, p < 0.01) with body size, where smaller animals had higher peak frequencies (e.g., 12.1 kHz for 35 mm CW), while larger animals consistently showed lower peak frequencies (e.g., 2.5 kHz for 135 mm CW) (Fig. 3). The duration of the calls was also sex-dependent (independant of size) (F1,16= 4.94, p < 0.001), where the average female rasp duration was 0.36 ± 0.15 s, and a male’s rasp was 0.56 ± 0.26 s. On the other hand, pulse rate (0.03 ± 0.0006 Hz; F1,16= 1.21, p > 0.05) and number of pulses (18 ± 0.4; F1,16= 1.81, p > 0.05) were similar, regardless of size and sex.
When the rasps (n = 55) from crabs with no food present (NFP) were compared to rasps (n = 46) with food present (FP), the rasp durations of FP crabs (0.158 ± 0.01 s) were significantly (F1,7= 5.74, p < 0.05) shorter than those of NFP crabs (0.376 ± 0.02 s). Within a rasp, the inter-pulse interval of FP crabs (0.008 ± 0.0006 s−1) were significantly (F1,7= 35.26, p <0.001) faster than NFP crabs (0.022 ± 0.001 s−1). The other rasp characteristics, such as peak frequency (F1,7= 0.04, p = 0.84) and number of pulses (F1,7= 1.13, p = 0.32), were not significantly distinct between FP and NFP crabs (Fig. 4).
In contrast to the generally low occurrence of rasp sounds in the absence of food, there was a significant 18-fold increase (F 1, 8= 11.52.96, p < 0.001) in the occurrence of rasps for FP crabs (38.2 ± 10.6 rasps) in comparison to NFP mixed sex crabs (2.1 ± 0.3 rasps) (Fig. 5). There was no significant difference when comparing the number of rasps produced between any of the other experimental conditions (Table 1).
During the feeding-rasp playback experiments, distance travelled by crabs significantly (t5 = − 4.479, p < 0.05) increased with rasp playback (7.99 ± 1.74 m) compared to controls (1.42 ± 0.57 m) (Fig. 6). Movement velocity (t4 = − 0.642, p = 0.556) and distance to sound source (t4 = − 0.032, p = 0.488) were similar between control (1.14 × 10−2 ± 0.2 × 10−2 m s−1 and 31.46 × 10−2 ± 3.9 × 10−2 m, respectively) and playback treatments (1.33 × 10−2 ± 0.3 × 10−2 m s−1 and 31.53 × 10−2 ± 2.0 × 10−2 m, respectively).
a Comparison of average (± SE) distance travelled (m/10 min) for control and feeding rasp playback trials. *Indicates statistical significance p < 0.05. b Example of heat map of a playback trial, showing control (left) and rasp playback (right) conditions. Colours indicate the percentage of time each of the four crabs spent at a given location
Rasp mechanism
The mechanism producing the rasp sound is still unknown. Rasps were produced with no apparent external movements of appendices or mouthparts.
Zip and bass
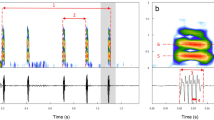
The zip and bass sounds were produced exclusively by adult males based on sound recordings and synchronised movements observed on video recordings. The zip (zip events = 247) was characterised by a peak frequency of 662 ± 12 Hz, consisting of 8 ± 0.1 pulses, which were characterised by an average duration of 0.073 ± 0.001 s. Zips were produced in a series of 39 ± 4 zips to form a zip train (zip train events = 14), with an average duration of 6.6 ± 0.48 s (Fig. 7a, b). The bass (bass events = 82) was characterised by a peak frequency of 45 ± 0.5 Hz, with an average duration of 0.8 ± 0.02 s. Similar to the zip, the bass was produced in a series of 16 ± 1.7 basses, to form a bass train (bass train events = 9) with an average duration of 23.4 ± 1.9 s (Fig. 7c, d).
Example of zip and bass trains produced by a large male (120 mm carapace width). Red rectangles highlight one zip and one bass: a Zip train waveform sound signal (µPa) over time (s); b Zip train spectrogram illustrating frequency (kHz) and power spectral density (dB re 1μ Pa/Hz) over time (s); c Bass train waveform showing sound signal (Pa) over time (s); d Bass spectrogram illustrating the frequency (Hz) and power spectral density (dB re Iμ Pa/Hz) over time (s)
The zip and bass sounds were only produced by a competing male against a mating pair or when there was male competition for a receptive (recently moulted) female (Fig. 8). Specifically, competing male crabs would alternate between producing zip trains and bass trains when exposed to a receptive female. The bass train followed after a zip train 82% of the time. In five out of the seven mating trials, males engaged in fights with other male crabs in the tank, and in two of these trials, females were also injured.
Zip mechanism
The zip was produced by rubbing the plectrum or elbow of the first walking leg along the ventrally located ridges of the chelae (Fig. 9). During this stridulation, the animal was typically moving forward whilst alternating between rubbing the left and right chela against the plectrum.
Bass mechanism
The mechanism producing the bass sound is still unknown. It was accompanied by sideways swaying of the entire body and a digging action of the first walking legs (Fig. 10), while the paddles of the last leg pair moved in a waving or twisting motion. One directional sway to one side corresponded to one bass.
Discussion
The main objective of this study was to improve our current understanding of crab sound production and associated behaviours. We have shown that O. catharus is capable of producing at least three distinct sounds. The first sound, which we called the rasp, was the most common of the three sounds detected. It was produced by adults and juveniles of both sexes, and the occurrence significantly increased in the presence of food and during the post-copulatory phase of mating. Thus, the rasp appears to be a non-sex specific sound, potentially associated with the presence of food or a general state of excitement. Play-back experiments of rasp sounds initiated foraging-like locomotion, suggesting that O. catharus can identify and respond to rasps.
The two other sounds—the zip and the bass—were directly correlated with mating behaviour: they were only produced by adult male crabs in the presence of male competition for a receptive (recently moulted, typically cradled-carried) female. We hypothesize that these two sounds are used for intraspecific communication, possibly between competing males.
The rasp and bass sounds are most likely produced internally, as they occurred without discernible external mechanisms such as stridulation, or movement of appendices. The zip sound, in contrast, is produced by a previously described sound-producing mechanism, which involves stridulation of the chela propodus ridges against the plectra of the stationary first walking legs (Guinot-Dumortier and Dumortier 1960; Stephenson 1969).
Rasps
The mechanism producing the rasp is still undescribed. Through analysis of video recordings, no apparent external movements were observed before, during or after the production of the rasp sound. Buscaino et al. (2015) were also unable to detect any sound-producing mechanisms during the production of a similar rasp-like sound by O. trimaculatus. Our results demonstrate that the rasp was produced by a different mechanism than stridulation of the chela ridges. The lack of detectable movements of the appendices and mouthparts strongly suggests that the rasp was produced internally. Previously, stridulating was assumed to underlie rasp-type sounds in other Ovalipes species (Guinot-Dumortier and Dumortier 1960). Ongoing research in our lab suggests an involvement of the gastric teeth in rasp sound production (Goeritz et al. 2018).
Rasps could convey information about the sender
The sound structure of the rasp consisted of multiple broad pulses (Fig. 2), similar to what was reported to be produced by stridulating crustaceans, such as the paddle crab O. trimaculatus (Buscaino et al. 2015) and the California spiny lobsters, Panulirus interruptus (Patek et al. 2009). There was a relationship between carapace size and peak frequency, with peak frequencies decreasing with increasing animal size. There was also a significant difference between male and female rasps, with a male rasp being on average 0.2 s longer. This is in agreement with Buscaino et al. (2015), who found differences between male and female O. trimaculatus acoustic signals, similar to our described rasp. The rasps thus could potentially convey information regarding the size and sex of the individual to nearby members of the same sex, or to potential mates.
Rasps as a potential “spy phase” cue in the evolution of crustacean sound communication
Rasp rate significantly increased and the rasp characteristics also altered during feeding events compared to non-feeding events, making the feeding rasp distinctly different from the common rasp. During playback experiments of the feeding rasp, crabs initiated locomotion and foraging-like behaviours. When an individual crab detected food, crabs on the far side of the holding tank almost immediately displayed a foraging behaviour in the form of increased locomotion and apparent searching. This quick response suggests that a channel faster than chemical communication, which is limited by the speed of diffusion and water velocity, indicated the presence of food. Thus, it is possible and likely that O. catharus are capable of detecting and processing rasp sounds as an indication of food availability.
Announcing the presence of food has very little benefit to the sender, and it is possible that the rasps are a side effect of another physiological process, for example movement of the gastric mill in anticipation of food (Heinzel 1988; Selverston et al. 2009; Goeritz et al. 2018). In this case, the rasp might not be a specialized communication signal, but a coinciding sound cue indicating the presence of food, on which conspecifics might capitalize. This would be an early step in the evolution of acoustic communication, analogous to the “spy phase” in chemical communication in crustaceans, as postulated by Wyatt (2010), with the gain only to the recipient(s) of the sound, and not the sender.
Interestingly, the frequency of the rasp was outside of what is currently considered the frequency range that decapod crustaceans can perceive (Radford et al. 2016). Radford et al. (2016) only tested statocyst-transmitted hearing thresholds up to 2 kHz; it is still unknown if high-frequency sounds would elicit auditory evoked responses of the statocyst organ. Alternatively, the rasp might be detected by other hearing structures, such as setae cells on the surface of the body (Breithaupt and Tautz 1990; Budelmann 1992) or chordotonal organs associated with joints of the antenna, legs, or other body appendages (Budelmann 1992). Importantly, in the playback experiments we observed a significant increase in crab locomotion in response to sounds above 3 kHz, providing evidence that O. catharus are able to detect these sounds. A poor match between the rasp signal and the sensitivity of sound perception could be another indicator that responses to rasps evolved as a form of acoustic spying, analogous to chemical spying in the evolution of hormonal sex pheromones in teleost fish (Sørensen and Scott 1994).
Zip and bass
The other two sounds produced by O. catharus were the zip and the bass. The low-mid frequency zip and the sub-bass frequency bass consisted of pulses or vibrations and are both within the known sound perception range of O. catharus (Radford et al. 2016). Since low-frequency sounds have considerably longer wavelengths, and produce a much larger near-field region than higher frequency sounds, the bass could be effective over a radius of several meters (Kalmijn 1988).
The zip sound was produced by stridulation of the chela propodus ridges against the first walking leg’s flexed meropodite-carpopodite-joint. During this motion, the chelae were moved back and forth while the first walking legs remained stationary. Whereas the precise mechanism producing the bass remains unknown, it was often accompanied by a swaying motion of the whole body, and a digging action of the first walking legs. Video analysis showed no other appendages were moving; the abdomen was raised off the ground and the walking legs were the only body parts in contact with the ground. While we did not find any reports of bass-type sounds in other decapod crustaceans, the stomatopod Hemisquilla californiensis produces a harmonic, low frequency (20–60 Hz) rumble through vibrating the carapace, possibly playing an agonistic and defensive role (Patek and Caldwell 2006; Staaterman et al. 2011).
Courtship-like behaviour
Zip and bass sounds were only produced by males during male–male competition, when multiple male crabs were in the presence of a recently moulted female. Since the chelae move during zip production, and the bass sound is accompanied by a swaying motion of the entire body, we could visually confirm that only unpartnered males produced these sounds, and not the female or the mating male.
Throughout the production of a zip train, the male crabs actively walk forward and flick both swimming paddles in a twisting motion. This movement may be an integral part of a dance-like courtship display, similar to what has been observed in other portunid crabs and terrestrial arthropods (Salmon and Atsaides 1968; Wood and Derby 1995; Jivoff and Hines 1998a; Sneddon et al. 2003; Elias et al. 2003; Kamio et al. 2008). The paddles of O. catharus are a prominent purple colour, which may aid as a visual cue along with the flicking motion. In addition to a visual signal, the flicking of the paddles may also generate forward water currents that facilitate pheromone dispersal. Although little is known about sex-specific pheromone release by the male, Gleeson (1991) found that male blue crabs Callinectes sapidus attract pubertal females during courtship displays through pheromone release. The zip and bass sounds and the associated paddle and swaying movements in O. catharus may very well be part of a complex courtship behaviour involving sound, chemical communication, and visual cues.
Multimodal communication
Ovalipes catharus lives in shallow sandy habitat but migrates into harbours and muddy estuaries in the winter months for breeding (Wear and Haddon 1987; McLay 1988). Many animals have evolved multimodal communication channels as adaption to low-visibility habitats (Ryan 1990; Hebets and Rundus 2010). In the often-turbid water of the estuaries, the use of sound combined with the visual signals of paddle-waving (and quite possibly chemical signals as well), may be an adaptation of O. catharus to low visibility, analogous to the multimodal courtship signals in other portunid crabs. For example, male blue crabs (C. sapidus) combine visual signals with chemical signal-enhancing stationary paddling, when the female is not accessible (Kamio et al. 2008). While not tested in our study, it would be interesting to observe if the use of sound in O. catharus is similarly context-dependent and increases when the female is not visible. However, it should be pointed out that multimodal signals are not necessarily “back-up signals”, but could also convey multiple messages (e.g., species recognition and mating signals), or could be integrated by the receiver as a multimodal percept (Hebets and Papaj 2005; Partan and Marler 2005; Candolin 2007; Hebets et al. 2016; Halfwerk et al. 2019).
Male-male competition during post-copulatory mate-guarding
Paddle crabs are among the decapod crustacean species that can mate repeatedly and with different males, thus allowing them to be more selective in regards to who fathers their offspring (Rondeau and Sainte-Marie 2001; Bilodeau et al. 2005; Brockerhoff and McLay 2005; Duffy and Thiel 2007). Male-male competition therefore extends into the post-copulatory period, and we found that male O. catharus continued to cradle-carry the female for many hours after mating in the presence of other males, while releasing the female within minutes in the absence of other males. We thus hypothesise that these sounds are examples of male–male competition, where the most dominant male mates with the available females, either by attempting to “steal” the cradle-carried female, or by mating with her after she is released by her current mate.
The purpose of the sounds throughout the post-copulatory cradle-carrying period could either be a courtship signal to the female, or an agonistic signal directed at another competing male, or a combination of both. We hypothesize that these sounds are mostly part of male–male competition, based on the facts that (1) the zip and the bass only occurred in the combination of two males and a receptive female, whether the original male was cradle-carrying the female, or if the post-copulatory female had become separated from the original male (in the presence of just one individual male cradle-carrying a female, no zip and bass sounds were recorded), and (2) the zip and bass were frequently accompanied by aggressive behaviours, where in five out of seven trials, one of the non-mating males engaged in fights with either the cradling pair, or another non-mating male. It is worth noting that a mating female is soft-shelled, and will, if exposed to aggressive behaviour from the male, encounter significant injuries, thereby reducing her fitness for rearing eggs. In fact, this was evident in the death of one female and injury in two others.
Interestingly, based on observations it appears that the female has little choice over the male they mated with as males generally hold onto a female prior to her moulting for several days. In this scenario, there appears to be little benefit for a male to invest energy into producing sounds directed at a female that has minimal control over escape and mate choice. However, this may not be the case as even a cradle-carried female might still exert mate-choice by encouraging larger or more aggressive males to challenge the male cradling her, or by encouraging them to remain in the area for repeated mating after she has been released (Jivoff and Hines 1998b). Additionally, Sneddon et al. (2003) reported that female Carcinus maenas can provoke male–male competition by pheromone release, especially when held by small crabs. In a similar manner, although not directly investigated in this study, cradle-carried receptive female O. catharus might provoke single males to produce sounds, and the perception of these sounds may be one of multiple ways to assess quality of future mates.
In conclusion, this study described sounds produced by O. catharus. Of the three sound types, the rasp was produced by all individuals independent of sex and age, while the zip and bass were only produced by adult competing males in the presence of receptive females. The sound characteristics of the rasp varied with sex and size and were significantly altered during feeding events. Playback experiments of feeding rasps lead to increased exploratory movement, suggesting that the rasp may be used in a form of acoustic spying, triggering foraging behaviour in conspecifics. The rasp is a well-documented sound in stridulating decapod crustaceans. However, we show here that the mechanism producing this sound is not, as often assumed, stridulation of the chela ridges, but instead produced by an internal mechanism. The zip and bass sequences and their associated movements combine to create an elaborate, multi-modal signal. This study provides further evidence that crustacean sound production is not limited to external mechanisms, and that sound production in decapod crustaceans may be more common than previously thought. Our results highlight the importance of behavioural experiments in addition to anatomic cues when considering the potential role of a crustacean species in underwater soundscape ecology.
References
Akamatsu T, Okumura T, Novarini N, Yan HY (2002) Empirical refinements applicable to the recording of fish sounds in small tanks. J Acoust Soc Am 112(6):3073–3082. https://doi.org/10.1121/1.1515799
Bilodeau AL, Felder DL, Neigel JE (2005) Multiple paternity in the thalassinidean ghost shrimp, Callichirus islagrande (Crustacea: Decapoda: Callianassidae). Mar Biol 146(2):381–385. https://doi.org/10.1007/s00227-004-1444-1
Breithaupt T, Tautz J (1990) The sensitivity of crayfish mechanoreceptors to hydrodynamic and acoustic stimuli. In: Wiese K, Krenz WD, Tautz J, Reichert H, Mulloney B (eds) Frontiers in crustacean neurobiology. Advances in life sciences. Birkhäuser, Basel. pp 114–120. https://doi.org/10.1007/978-3-0348-5689-8_12
Brockerhoff A, McLay C (2005) Mating behaviour, female receptivity and male–male competition in the intertidal crab Hemigrapsus sexdentatus (Brachyura: Grapsidae). Mar Ecol Prog Ser 290:179–191. https://doi.org/10.3354/meps290179
Budelmann BU (1992) Hearing in Crustacea. In: Webster DB, Popper AN, Fay RR (eds) The evolutionary biology of hearing. Springer, New York, pp 131–139. https://doi.org/10.1007/978-1-4612-2784-7_9
Buscaino G, Gavio A, Galvan D, Filiciotto F, Maccarrone V, de Vincenzi G, Mazzola S, Orensanz JM (2015) Acoustic signals and behaviour of Ovalipes trimaculatus in the context of reproduction. Aquat Biol 24:61–73. https://doi.org/10.3354/ab00636
Candolin U (2007) The use of multiple cues in mate choice. Biol Rev 78(4):575–595. https://doi.org/10.1017/S1464793103006158
Clayton D (2008) Singing and dancing in the ghost crab Ocypode platytarsus (Crustacea, Decapoda, Ocypodidae). J Nat Hist 42(3–4):141–155. https://doi.org/10.1080/00222930701840530
Crane J (1966) Combat, display and ritualization in fiddler crabs (Ocypodidae, genus Uca). Philos T R Soc B, Biol Sci 251(772):459–472. https://doi.org/10.1098/rstb.1966.0035
Duffy JE, Thiel M (2007) Sexual and Social Behavior of Crustacea—a Way Forward. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems: crustaceans as model organisms. Oxford University Press on Demand, New York, pp 461–474. https://doi.org/10.1093/acprof:oso/9780195179927.001.0001
Elias DO, Mason AC, Maddison WP, Hoy RR (2003) Seismic signals in a courting male jumping spider (Araneae: Salticidae). J Exp Biol 206:4029–4039. https://doi.org/10.1242/jeb.00634
Favaro L, Tirelli T, Gamba M, Pessani D (2011) Sound production in the red swamp crayfish Procambarus clarkii (Decapoda: Cambaridae). Zoo Anz 250(2):143–150. https://doi.org/10.1016/j.jcz.2011.01.002
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago, pp 1–509
Gleeson RA (1991) Intrinsic factors mediating pheromone communication in the blue crab, Callinectes sapidus. In: Bauer RT, Martin JW (eds) Crustacean sexual biology. Columbia, New York Chichester, West Sussex. pp 17–32. https://doi.org/10.7312/baue90796-003
Goeritz ML, Flood AS, Radford CA (2018) Sound production in decapod crustaceans: Behavioral contexts and a newly found role for the circuits of the stomatogastric nervous system. In: International congress of neuroethology 2018 Brisbane, Australia. International Society for Neuroethology, pp 31–32
Guinot-Dumortier D, Dumortier B (1960) La stridulation chez les crabes. Crustaceana 1(2):117–155
Haddon M (1994) Size-fecundity relationships, mating behaviour, and larval release in the New Zealand paddle crab, Ovalipes catharus (White 1843) (Brachyura: Portunidae). New Zeal J Mar Fresh 28(4):329–334. https://doi.org/10.1080/00288330.1994.9516622
Halfwerk W, Varkevisser J, Simon R, Mendoza M, Scharff C, Riebel K (2019) Toward testing for multimodal perception of mating signals. Front Ecol Evol 7:124. https://doi.org/10.3389/fevo.2019.00124
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57(3):197–214. https://doi.org/10.1007/s00265-004-0865-7
Hebets EA, Rundus A (2010) Chemical communication in a multimodal context. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 335–354. https://doi.org/10.1007/978-0-387-77101-4_17
Hebets EA, Barron AB, Balakrishnan CN, Hauber ME, Mason PH, Hoke KL (2016) A systems approach to animal communication. Proc R Soc London, Ser 283(1826):20152889. https://doi.org/10.1098/rspb.2015.2889
Heinzel HG (1988) Gastric mill activity in the lobster. I. Spontaneous modes of chewing. J Neurophysiol 59(2):528–550. https://doi.org/10.1152/jn.1988.59.2.528
Horch K (1971) An organ for hearing and vibration sense in the ghost crab Ocypode. Vergl Physiol 73(1):1–21. https://doi.org/10.1007/BF00297698
Horch K (1975) The acoustic behavior of the ghost crab Ocypode cordimana Latreille, 1818 (Decapoda, Brachyura). Crustaceana 29(2):193–205
Jivoff P, Hines AH (1998a) Effect of female molt stage and sex ratio on courtship behavior of the blue crab Callinectes sapidus. Mar Biol 131:533–542. https://doi.org/10.1007/s002270050345
Jivoff P, Hines AH (1998b) Female behaviour, sexual competition and mate guarding in the blue crab, Callinectes sapidus. Anim Behav 55(3):589–603. https://doi.org/10.1006/anbe.1997.0646
Johnson ML, Gaten E, Shelton PM (2002) Spectral sensitivities of five marine decapod crustaceans and a review of spectral sensitivity variation in relation to habitat. J Mar Biol Assoc UK 82(5):835–842. https://doi.org/10.1017/S0025315402006203
Kalmijn AJ (1988) Hydrodynamic and acoustic field detection. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 83–130. https://doi.org/10.1007/978-1-4612-3714-3_4
Kamio M, Reidenbach MA, Derby CD (2008) To paddle or not: context dependent courtship display by male blue crabs, Callinectes sapidus. J Exp Biol 211:1243–1248. https://doi.org/10.1242/jeb.014977
Madsen PT, Wahlberg M (2007) Recording and quantification of ultrasonic echolocation clicks from free-ranging toothed whales. Deep Sea Res Part I 54(8):1421–1444. https://doi.org/10.1016/j.dsr.2007.04.020
Mazzoni D (2016) AUDACITY 2.1.2. Available from: https://www.audacityteam.org/download/
McLay CL (1988) Crabs of New Zealand. Leigh Marine Laboratory Bulletin. University of Auckland, Auckland
Mensinger AF (2013) Disruptive communication: stealth signaling in the toadfish. J Exp Biol 217(3):344–350. https://doi.org/10.1242/jeb.090316
Myrberg AA (1997) Sound production by a coral reef fish (Pomacentrus partitus): evidence for a vocal, territorial “keep-out” signal. Bull Mar Sci 60:1017–1025
Myrberg AA Jr, Mohler M, Catala JD (1986) Sound production by males of a coral reef fish (Pomacentrus partitus): its significance to females. Anim Behav 34(3):913–923. https://doi.org/10.1016/S0003-3472(86)80077-X
Osborne TA (1987) Life history and population biology of the paddle crab, Ovalipes catharus. Dissertation, University of Canterbury, Christchurch, New Zealand
Parmentier E, Fine ML (2016) Fish sound production: insights. In: Suthers R, Fitch W, Fay R, Popper A (eds) Vertebrate sound production and acoustic communication@ Springer Handbook of Auditory Research, vol 53. Springer, Cham, pp 19–49. https://doi.org/10.1007/978-3-319-27721-9_2
Partan SR, Marler P (2005) Issues in the classification of multimodal communication signals. Am Nat 166(2):231–245. https://doi.org/10.1086/431246
Patek SN, Caldwell RL (2006) The stomatopod rumble: low frequency sound production in Hemisquilla californiensis. Mar Freshw Behav Physiol 39(2):99–111. https://doi.org/10.1080/10236240600563289
Patek SN, Shipp LE, Staaterman ER (2009) The acoustics and acoustic behavior of the California spiny lobster (Panulirus interruptus). J Acoust Soc Am 125(5):3434–3443. https://doi.org/10.1121/1.3097760
Payne R, Webb D (1971) Orientation by means of long range acoustic signalling in baleen whales. Ann NY Acad Sci 188:110–141. https://doi.org/10.1111/j.1749-6632.1971.tb13093.x
Popper A, Salmon M, Horch K (2001) Acoustic detection and communication by decapod crustaceans. J Comp Physiol A 187:83–89. https://doi.org/10.1007/s003590100184
Radford CA, Tay K, Goeritz ML (2016) Hearing in the paddle crab, Ovalipes catharus. Proc Mtgs Acoust 27:010013. https://doi.org/10.1121/2.0000259
Rondeau A, Sainte-Marie B (2001) Variable mate-guarding time and sperm allocation by male snow crabs (Chionoecetes opilio) in response to sexual competition, and their impact on the mating success of females. Biol Bull 201(2):204–217. https://doi.org/10.2307/1543335
Ryan MJ (1990) Sexual selection, sensory systems and sensory exploitation. In: Douglas F, Janis A (eds) Oxford Surveys in Evolutionary Biology, vol 7, pp 157–195
Salmon M (1967) Coastal distribution, display and sound production by Florida fiddler crabs (genus Uca). Animal Behav 15(4):449–459. https://doi.org/10.1016/0003-3472(67)90043-7
Salmon M, Atsaides SP (1968) Visual and acoustical signalling during courtship by fiddler crabs (genus Uca). Am Zool 8:623–639. https://doi.org/10.1093/icb/8.3.623
Salmon M, Horch KW (1972) Acoustic signalling and detection by semiterrestrial crabs of the family Ocypodidae. In: Winn HE, Olla BL (eds) Behavior of marine animals. Springer, Boston, pp 60–96. https://doi.org/10.1007/978-1-4684-0907-9_2
Selverston A, Szücs A, Huerta R, Pinto RD, Reyes MB (2009) Neural mechanisms underlying the generation of the lobster gastric mill motor pattern. Front Neural Circuit 3:12. https://doi.org/10.3389/neuro.04.012.2009
Sneddon LU, Huntingford FA, Taylor AC, Clare AS (2003) Female sex pheromone-mediated effects on behavior and consequences of male competition in the shore crab (Carcinus maenas). J Chem Ecol 29(1):55–70. https://doi.org/10.1023/A:1021972412694
Sørensen PW, Scott AP (1994) The evolution of hormonal sex pheromones in teleost fish: poor correlation between the pattern of steroid release by goldfish and olfactory sensitivity suggests that these cues evolved as a result of chemical spying rather than signal specialization. Acta Physiol Scand 152(2):191–205. https://doi.org/10.1111/j.1748-1716.1994.tb09799.x
Staaterman E, Claverie T, Patek S (2010) Disentangling defense: the function of spiny lobster sounds. Behaviour 147(2):235–248. https://doi.org/10.1163/000579509X12523919243428
Staaterman ER, Clark CW, Gallagher AJ, deVries MS, Claverie T, Patek SN (2011) Rumbling in the benthos: acoustic ecology of the California mantis shrimp Hemisquilla californiensis. Aquat Biol 13(2):97–105. https://doi.org/10.3354/ab00361
Stephenson W (1969) The morphology of stridulatory structures in the genus Ovalipes Rathbun. Trans R Soc New Zealand, Bio Sci 11(4):43–71
van Oosterom L, Montgomery JC, Jeffs AG, Radford CA (2016) Evidence for contact calls in fish: conspecific vocalisations and ambient soundscape influence group cohesion in a nocturnal species. Sci Rep 6:19098. https://doi.org/10.1038/srep19098
Wear RG, Haddon M (1987) Natural diet of the crab Ovalipes catharus (Crustacea, Portunidae) around central and northern New Zealand. Mar Ecol Prog Ser 35(1/2):39–49
Wood DE, Derby CD (1995) Coordination and neuromuscular control of rhythmic behaviors in the blue crab, Callinectes sapidus. J Comp Physiol A 177(3):307–319. https://doi.org/10.1007/BF00192420
Wyatt TD (2010) Pheromones and Behavior. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 22–38. https://doi.org/10.1007/978-0-387-77101-4_2
Acknowledgements
We would like to thank the staff at the Leigh Marine Laboratory, in particular Errol Murray and Peter Browne for their help building the experimental setup and assistance collecting animals. We would also like to thank Katya Ruggiero for statistical advice and Colin McLay for his expertise. We also thank our two anonymous reviewers and editor Martin Thiel for their valuable input and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained. All procedures involving animals were performed in accordance with the Ethical Standards of the University of Auckland and were approved by the Animal Ethics Committee (AEC), approval number 001721.
Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Additional information
Responsible Editor: M. Thiel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by I. Hinojosa and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Flood, A.S., Goeritz, M.L. & Radford, C.A. Sound production and associated behaviours in the New Zealand paddle crab Ovalipes catharus. Mar Biol 166, 162 (2019). https://doi.org/10.1007/s00227-019-3598-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3598-x