Abstract
Climate change has driven shifts in species distributions along latitudinal and elevational gradients, and such shifts are likely to continue as warming accelerates. However, little is known about the response of strongly interacting species, including whether multiple, interacting species are likely to shift concordantly or whether climate change will promote community disassembly. In rocky shore ecosystems, mussels are dominant foundation species that provide habitat and increase diversity of associated species. The New Zealand mussel guild is uniquely diverse as four species can be found within 1 m2 of shoreline. We integrated comparative ecophysiology and population ecology to evaluate whether air temperature sets elevational range limits and to quantify mussels’ warming tolerances. Air temperature appears to set upper intertidal limits across mid-intertidal species, based on findings that (1) lethal thermal limits coincided with temperatures experienced at upper tide-height limits, (2) species with higher thermal tolerances occurred higher on shore, and (3) lethal tolerances were highest at our warmest site. Based on predicted body temperatures in year 2100, mid-elevation habitat-forming mussels are likely to experience an increase in the frequency of thermal events causing 50% mortality at their upper elevation limit. Such events are predicted to occur 3.0–4.4 times more frequently in 2100 than present at a warmer site and to increase from 0 to 0.4/0.1 days per year for Perna/Aulacomya, but not Mytilus, at a cooler site. These results indicate that the mussel species’ ranges are all likely to contract at warmer sites in the future, decreasing habitat for associated organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent climate warming is associated with shifts in species distributions along latitude (Parmesan and Yohe 2003; Sorte et al. 2010; Chen et al. 2011; Wernberg et al. 2011), depth (Perry et al. 2005), and elevation (Chen et al. 2011) gradients. Physiological tolerances are highly variable both within and among species (Stillman 2003; Stillman and Somero 2000; Sorte et al. 2011), and populations living at or near their thermal limits could be most prone to distributional shifts (Helmuth et al. 2006; Somero 2012; Sunday et al. 2012) and local extinction (Calosi et al. 2008; Berg et al. 2010). Range shifts could have severe consequences for adaptive potential, community structure and ecosystem functioning, particularly when they occur for strongly interacting “leverage species”—such as foundation species—which have extensive influences on local biodiversity (Borrvall et al. 2000; Schiel et al. 2004; Harley et al. 2006). Furthermore, recent observations have shown that species often shift at disparate rates, leading to potential disassembly (“mismatch”) of trophic webs and overall communities (Chivers et al. 2017). Given projections that global surface temperatures will increase by 2.6–4.8 °C during the twenty-first century (Rogelj et al. 2012), understanding the species-specific thermal ecophysiology of multiple foundation species is essential for predicting their vulnerability to warming and preserving vital ecosystem services. The goal of our study was to link air temperatures, thermal tolerance limits, and distribution patterns across a guild of foundation species and use this mechanistic understanding to project distributional shifts under climate-warming scenarios.
Temperature often limits the latitudinal and elevational ranges of marine and terrestrial species (Cahill et al. 2014) because both non-lethal and lethal responses to warming influence demographic parameters (Somero 2002; Williams et al. 2007). However, population sizes—and, thus, range boundaries—can also be set by other abiotic factors (e.g., precipitation, nutrients and salinity), species interactions, and community composition (Keith et al. 2008; Sexton et al. 2009). To project range shifts under climate warming, we first need to determine the degree to which temperature sets distributional limits and estimate “warming tolerance”, or the difference between a species’ physiological tolerance limit and habitat temperature (Deutsch et al. 2008). Recent work has demonstrated that warming tolerances can differ greatly between species (e.g., Deutsch et al. 2008; Vinagre et al. 2016) and across available habitats (e.g., Miller et al. 2009; Faulkner et al. 2014; Mislan et al. 2014). However, few studies have considered warming tolerances among interacting species to anticipate shifts in community composition in response to rising global temperatures (Gilman et al. 2010).
In rocky intertidal ecosystems—where organisms living between the low- and high-tide water lines experience daily exposure to both marine and terrestrial conditions–maximum temperatures experienced during aerial emersion often set species’ upper intertidal range limits (Kennedy 1976; Menge 1976; Harley and Helmuth 2003; Stickle et al. 2016; 2017). At the same time, biological interactions (e.g., predation and competition) have been shown to drive lower limits (Connell 1961; Menge and Sutherland 1976; but see e.g., Seabra et al. 2016; Lourenço et al. 2016). These effects of abiotic and biotic factors on species’ distributions create well-defined zonation patterns, with mussel species dominating the mid zone of intertidal systems (Menge 1976; Menge and Branch 2001). Mussels act as foundation species, increasing the biodiversity of associated species by providing a refuge from abiotic and biotic stressors (Suchanek 1992; Gutiérrez et al. 2003; Borthagaray and Carranza 2007, Jurgens and Gaylord 2018). Given their role in promoting community diversity, it is particularly important to understand how climate change might affect the intertidal mussel guild.
We investigated the underlying mechanisms and consequences of climate change responses—specifically, increase in air temperature—for a mussel guild in the rocky shore community of New Zealand. Four mussel species co-occur in the intertidal zone of New Zealand’s South Island: the green-lipped mussel Perna canaliculus, blue mussel Mytilus galloprovincialis, ribbed mussel Aulacomya maoriana, and little black mussel Xenostrobus pulex (hereafter, referred to by genus) (Fig. 1). Most temperate rocky shorelines are inhabited by an average of two mussel species per site (Blanchette et al. 2008; Broitman et al. 2001; Branch and Steffani 2004; Sorte et al. 2017). The high mussel diversity in New Zealand is unique and makes this system ideal for conducting ecophysiological comparisons between species. Furthermore, the mussel guild in our study region of the South Island is composed of only native species, based on recent molecular evidence that the dominant local Mytilus is the native Southern Hemisphere lineage of Mytilus galloprovincialis (Westfall and Gardner 2010; Gardner et al. 2016).
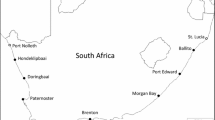
Field sites and mussel species on the South Island of New Zealand. Primary sites (in bold face) were Nile River (NR) and Box Thumb (BT) with additional distribution surveys conducted at Nine Mile Beach (NM) and Blue Duck (BD). Note that mussels are not shown on the same scale and vary in maximum size from the largest species on left to smallest on right (see Online Resource 2 for representative mussel sizes).
Although there are relatively few published papers on New Zealand mussels, previous studies in this system suggest that thermal physiology is important in biogeographic structuring and, by extension, that this guild may experience alterations under climate change (Kennedy 1976; Menge et al. 2007; Petes et al. 2007). Across the intertidal gradient, Mytilus and Perna tend to dominate mid- and low intertidal zones, respectively (Kennedy 1976; Menge et al. 2007), with Aulacomya typically residing within the mussel bed matrix and Xenostrobus occurring high on the shore. These distribution patterns may reflect physiological differences: Kennedy (1976) found that Mytilus tolerated higher water temperatures than Perna and Aulacomya, and Petes et al. (2007) reported higher survival of Mytilus than Perna following an intertidal heat wave. However, there have been no comparisons of lethal air temperature tolerances where all species are subject to the same experimental conditions, allowing for parallel comparisons and future projections.
We combined data on species distributions, thermal exposures, and thermal tolerance limits (e.g., Miller et al. 2009; Mislan et al. 2014) to predict how climate warming is likely to affect New Zealand’s mussel guild and intertidal community (Fig. 2). Our specific questions were: (1) Are species’ intertidal distribution patterns related to thermal tolerance limits? (2) What are the warming tolerances of New Zealand mussel species? (3) How are distribution patterns likely to shift under future climate warming? (4) Are range shift projections similar or different across multiple species in the foundational guild?
Conceptual diagram of a performance as a function of temperature and b intertidal distributions under present and future temperatures. As temperature exceeds tolerance limits, increases in mortality at upper elevation limits would likely lead to downward range contractions. Such shifts could result in changes in community structure and diversity, especially when they occur for foundation species such as the mussels depicted here
Materials and methods
Approach and study locations
We evaluated potential impacts of climate warming on the New Zealand mussel guild using an integrative, ecophysiological approach. Our predictions relied on three data sets: (1) mussel abundance and upper tide-height distribution limits determined by field surveys, (2) lethal aerial temperature limits based on lab assays, and (3) mussel body temperatures predicted by a biophysical model.
Study locations were rocky shorelines with moderate–high wave exposure (Menge et al. 1999). Mussel distribution limit, abundance (cover), thermal environment, and tolerance data were collected at two primary sites on New Zealand’s South Island: Nile River (NR; southwest of Westport; 41.90°S, 171.44°E) on the west coast and Box Thumb (BT; located at Godley Head/Taylor’s Mistake on the Banks Peninsula; 43.58°S, 172.78°E) on the east coast (Fig. 1). We also quantified mussel cover (described below) at two additional sites: Nine Mile Beach (NM; 42.34°S, 171.26°E) on the west coast and Blue Duck (BD; 42.28°S, 173.75°E) on the east coast. Climatic conditions vary between sites, with cloud cover and precipitation higher on the west coast than east coast (Walter et al. 1975), while wave forces are comparable between coasts (Menge et al. 1999). Thermal conditions can also be strongly influenced by the timing of the low tides (Helmuth et al. 2002), and Box Thumb experiences more midday low tides than Nile River: during the summer months of 2016 (Jan, Feb and Dec), 27% and 21% of extreme low tides occurred between the hours of 10:00 and 16:00 for our ‘warmer’ (Box Thumb) and ‘cooler’ (Nile River) site, respectively (tidal data for Lyttleton and Westport, New Zealand; available at http://tbone.biol.sc.edu/tide/). Further details of site characteristics are available in previous studies (e.g., Vincent et al. 1991; Menge et al. 1999; Rilov and Schiel 2006; Petes et al. 2007; Callander 2012; Bracken et al. 2012; Schiel et al. 2016).
Mussel distributions
We quantified the relative abundance of each mussel species across tide heights in austral summer 2015. Percent cover was visually estimated in ¼ × ¼ m quadrats (which is more effective than point-contact approaches at enumerating rare species; Dethier et al. 1993) at 0.5-m vertical intervals (using a stadia rod and sight level) in tide height. We surveyed the shoreline from 1.0 m above lowest astronomical tide (based on predictions by http://tbone.biol.sc.edu/tide/) to the upper edge of the mussel zone (as in Sorte et al. 2017). The location of quadrats (N = 3) assessed at each tide height was established by first laying a 30-m transect tape parallel (horizontal) to the water line in an area of contiguous mussel bed. Three vertical transects were then laid perpendicular to the horizontal transect at locations determined using random numbers generated in MS Excel. We also provide percent cover data collected similarly in 2003 as context for the temporal stability of distributions patterns (Online Resource 1).
To assess each species’ elevational range limit, we measured the maximum tide height for each mussel species at the NR and BT sites in austral summer 2017. Five vertical transects were established at 5-m intervals (between 5 and 25 m) along a 30-m horizontal transect. Along each vertical transect, we identified the highest individual of each mussel species that fell within 5 cm of the transect tape, and we used a stadia rod and sight level to survey this maximum tide height in meters in reference to the water level at the time of surveying (tidal data for Christchurch and Westport, New Zealand; available at http://tbone.biol.sc.edu/tide/).
Lethal aerial temperature limits
We estimated thermal limits for Mytilus, Aulacomya, and Perna as the temperature lethal to 50% of individuals (LT50) following a 6-h emersed thermal exposure and 18-h immersed recovery period at 17 °C (chosen to represent ambient ocean temperature in the month of March based on 2015 and 2016 satellite data available at http://www.niwa.co.nz). In March 2017, following summer spawning (Buchanan 2001; Petes et al. 2007), mussels were collected haphazardly from the NR and BT sites at the upper vertical limit of each species’ tide-height distributions (as these individuals should demonstrate maximum thermal tolerance due to selection and environmental filtering) and were transported in a cooler (~ 1 h) to the laboratory. To characterize the current maximum thermal tolerance limits of the population, we collected individuals from the upper limits and assessed tolerance immediately upon return from the field (to avoid any tolerance changes during laboratory acclimation), using survival in ambient temperature controls as our indicator of any handling stress. Thermal tolerance assays were conducted in air in 50-ml centrifuge tubes with holes in the caps to allow for airflow (oxygen consumption rates reported by Marsden and Weatherhead 1998 indicate that anoxia would be unlikely even in closed chambers). A seawater-soaked, 4 cm2 chamois was added to each tube to prevent desiccation. Three individuals (N = 3; 1 individual per tube) of average size for each mussel species (Online Resource 2) were randomly assigned to four aerial temperature treatments that we expected to span the tolerance thresholds of these species based on previous studies (e.g., Kennedy 1976). All species were assayed at air temperatures of 17 °C (ambient control), 30 °C, 33.5 °C, and 37 °C except that Mytilus was not assayed at 30 °C for the NR population, but was also tested at 39 °C (BT only) and 41 °C (NR and BT) based on initial observations of low mortality at 37 °C. For all treatments, temperature in the chambers was increased (using heated water baths) from ambient at a rate of 1 °C every 4–5 min (similar to rapid warming events of, e.g., 11 °C in 30 min, at our NR field site in 2015; C. Sorte unpubl. data) until the treatment temperature was reached. Experimental temperatures were maintained using an electric cooler (control) and heated water baths (treatments) and were recorded every 10 min. After a total thermal exposure of 6 h (including the temperature ramp), mussels entered an 18-h recovery period in aerated, ambient temperature (17 °C) seawater. This assay was designed to simulate heat stress during a ~ 6-h low-tide exposure followed by an 18-h recovery prior to the next daytime low tide (Heath 1985). Survival was assessed after the recovery period via inspection for movement or responsiveness to probing, and mussel size was determined using calipers.
For Mytilus, Aulacomya, and Perna, we calculated LT50 for each species × site combination (i.e., 12–15 total replicates) using individual generalized linear models with binomial error distributions, with mussel survival modeled as a function of aerial temperature. Xenostrobus LT50 values were not calculated because low survival in the controls suggested either handling stress, difficulty in confirming initial–or assessing final–survival due to mussel size, or a negative response to submerged recovery for this high zone mussel. Models were run using R statistical software v. 3.2.2 (R Core Team 2015). Raw data for all four species are provided in Online Resource 2.
Temperatures
We estimated temperatures from the recent past (1997–2009) and projected future (2100) using a biophysical model of mussel body temperature developed by Wethey et al. (2011). This biophysical model predicts temperatures within the mussel bed as a layer (on top of impermeable rock) in a meteorological land surface model (Chen and Dudhia 2001; Ek et al. 2003) which includes tidal (Egbert and Erofeeva 2002) and wave data (US Army Corps of Engineers 2002; Tolman 2009). This model has been extensively validated, performing as well or better than similar models in predicting temperatures measured by mussel biomimetic sensors over 4 years and across sites spanning 1700 km along the U.S. West Coast (Wethey et al. 2011). Across all six of the sites included in the study by Wethey et al. (2011), the model under-predicted temperatures measured by biomimics, by an average of 1.8 °C. This model has since been applied to predict climate change vulnerability of the California mussel Mytilus californianus at its upper elevational range limit across the U.S. west coast (Mislan and Wethey 2011; Mislan et al. 2014). It is possible to use the biophysical model for this study of New Zealand mussels because the physical properties of mussel beds on the U.S. West Coast and New Zealand coast are similar. The longwave emissivity would be the same because the mussel beds are similar in color, and both NZ and U.S. mussel beds are on rocky intertidal benches so the soil type (rock) and tidal inundation are the same (Wethey et al. 2011). CFSR (Climate Forecast System Reanalysis), which we used as input data in this study, was one of the types of input weather data verified in Mislan and Wethey (2011) for the U.S. west coast. The three U.S. west coast field sites evaluated by Mislan and Wethey (2011) had different errors, ranging from 1.9 to 3.4 °C. The errors for the New Zealand mussel beds would be expected to fall within this range, given similarity in morphological characteristics (color, size structure, and form of aggregation) of mussel beds between the two locations (Fig. 1; Smith et al. 2006a; Mislan and Wethey 2011).
We used this model to calculate the temperature in the middle of a 5-cm-deep mussel bed across tide heights (0.5–2.5 m at 0.1-m intervals) during 30-min time intervals at both sites for the years 1997–2009 (‘historical’). The model uses weather data as input to the equations, which simulate the physics of how atmospheric conditions influence the temperature of mussels in a mussel bed. Input data for the model included downwelling short-wave and long-wave radiation, atmospheric pressure at the earth’s surface, air temperature and relative humidity at 2 m above the surface, wind speed at 10-m elevation, and precipitation rate from the NOAA Climate Forecast System Reanalysis (Saha et al. 2006, 2010). Sea surface temperature was from the Naval Research Laboratory Modular Ocean Data Assimilation (MODAS) (Barron and Kara 2006). Tides were predicted using the Oregon State University Tidal Inversion Software (OTIS) in conjunction with the Oregon State University Tidal Prediction Software (Egbert et al. 1994; Egbert and Erofeeva 2002). Wave height and wave period data from global versions of NOAA Wavewatch III models (Tolman 1998, 2007) were used to calculate wave run-up using equations from the U.S. Army Corps of Engineers Coastal Engineering Manual (Smith 2003).
New Zealand mean air temperature at 2 m above sea level is projected to increase by 3 °C by the year 2100 under the RCP 8.5 climate scenario. Projections for New Zealand were calculated using results from five earth system models in the Coupled Model Intercomparison Project Phase 5 (CMIP5) archive (Online Resource 3; Collins et al. 2011; Jones et al. 2011; Dunne et al. 2012, 2013; Taylor et al. 2012; Dufresne et al. 2013; Giorgetta et al. 2013; Ilyina et al. 2013). We projected future mussel temperatures by adding 3 °C to the air temperatures from years 1997 to 2009 in the input data for the mussel model. This approach is conservative in that it does not include predicted increases in temperature variance (e.g., Meehl and Tebaldi 2004).
Statistical analyses
We addressed our specific questions by examining the relationships between tide-height distributions, lethal temperature limits, and mussel body temperatures across species and study sites. We used a two-way ANOVA to assess differences in vertical abundance distributions across mussel species and sites, and a mixed-effects model (with site as a random effect) to test the relationship between upper tide-height limit and LT50 (using the lme4 package; Bates et al. 2015), both in R (R Core Team 2015). To account for variation in tidal amplitude, which we expect leads to differences in absolute upper limits between sites, hourly tidal predictions (available at http://tbone.biol.sc.edu/tide/) from 2016 were used to convert upper tide-height limits into emersion time (predicted percent of time above the waterline). Normality and homoscedasticity were visually assessed for all models using quantile–quantile plots and box plots of residuals. Emersion time was log-transformed to meet model assumptions. We analyzed differences between mussel vertical distributions using a Tukey post hoc test.
To assess climate change vulnerability of each mussel species, we integrated the lethal tolerance (LT50) and temperature data (1997–2009 and 2100 projections). We predicted the average frequency (days per year) that LT50s are exceeded (both based on a 6-h period) from 12 years of extrapolated temperature data across tide heights, species, and sites.
Results
Within the New Zealand mussel guild, species inhabited different zones from the water line to upper edge of the intertidal habitat, which were characteristic and largely consistent across four field sites. At most sites, the dominant species (in terms of space occupancy) switched from Perna to Mytilus to Xenostrobus, with Aulacomya present at low abundance up to the mid-intertidal zone (Fig. 3; although Perna were never dominant at Blue Duck). These patterns of differential species dominance across tidal elevations were also apparent in surveys conducted in early summer 2003 (Online Resource 1).
Abundance of mussel species across tidal elevations (m above lowest astronomical tide) at four rocky intertidal sites in New Zealand, including our primary sites a Nile River and b Box Thumb as well as abundance-only sites c Nine Mile Beach and d Blue Duck. Values are percent cover (± 1 SE) based on estimates within N = 3 quadrats (0.0625 m2) surveyed in March 2015. Note that the dependent variable (abundance) is on the x axis to illustrate the vertical orientation of the independent variable (tide height)
Maximum tide height differed across species (F3,32 = 40.04, P < 0.001) and sites (F1,32 = 23.57, P < 0.001), with a significant interaction between species and site (F3,32 = 3.19, P = 0.037) driven by site differences in Xenostrobus distribution (Fig. 4, Online Resource 2). Maximum tide height did not differ between the two low intertidal species (Tukey P = 0.99), which extended at NR/BT up to 1.3/1.5 m (Perna) and 1.5/1.3 m (Aulacomya). However, Mytilus extended to 1.9 m at both sites, higher than these low intertidal species (Tukey P < 0.002), and Xenostrobus extended significantly higher than Mytilus (Tukey P < 0.001), to 3.9 m at NR and 2.4 m at BT (Online Resource 2). Mussels extended higher into the intertidal at NR than BT, the cooler and warmer site, respectively (Table 1, Figs. 3, 4).
Maximum tide height increases with LT50 (P < 0.001, log(y) ~ 0.09x + 1.01) across species (Perna = triangle, Aulacomya = square, Mytilus = circle) and sites (NR = grey, BT = black). Upper tide height limit values were surveyed on N = 5 transects and are presented as the mean percent of time emersed (± SE). LT50 values are the temperature lethal to 50% of individuals (Online Resource 4). Dashed lines indicate the 95% confidence interval of the model fit. Site was included as a random effect in the model, and emersion time was log-transformed for analysis and back-transformed for this figure
Distribution patterns were related to thermal tolerances. Higher maximum tide heights were associated with species–site pairs with higher LT50 values (F1,27 = 19.49, P < 0.001, marginal R2 = 0.37; Fig. 4). Calculated LT50 values for NR and BT populations were, respectively, 31.8 and 33.3 °C for Perna, 31.8 and 33.1 °C for Aulacomya, and 35.2 and 37.9 °C for Mytilus (Online Resource 4).
The predicted frequency with which LT50s were exceeded was remarkably similar between species but differed greatly between sites. At Box Thumb, the LT50 was exceeded by an average of 0.69, 1.46, and 1.38 days annually during the years 1997–2009 at the maximum tide height of Aulacomya, Perna, and Mytilus, respectively (Table 1). At the end of the century, our model predicted that LT50 temperatures would be exceeded 3–4.4 times more often at these species’ current maximum tide heights (Table 1). At Nile River, our model predicted 0 days with thermal stress exceeding the LT50 of any of these three species at their current maximum tide height, with increases to 1–2 days by the year 2100 only at tide heights beyond these species’ present distributions.
Discussion
We provide evidence that temperature is an important driver of distribution limits for species within the New Zealand mussel guild, suggesting that shifts in distributions are likely to occur under climate warming. Specifically, we found that (1) across species and sites, lethal thermal limits were related to air temperatures experienced at upper tide-height limits, (2) species with higher thermal tolerances occurred higher on the shore, and (3) lethal tolerance limits were higher at the warmer site. These findings are consistent with the few previous studies of these species’ thermal biology (Kennedy 1976; Petes et al. 2007; Callander 2012; Dunphy et al. 2015). Prior experimental studies, however, report tolerance limits from assays where mussels were submerged during thermal exposure (Kennedy 1976; Dunphy et al. 2015), and physiological responses differ between organisms exposed in water versus air (e.g., Jones et al. 2009; Yamane and Gilman 2009; Seabra et al. 2016). At our field sites, maximum temperatures are encountered during low tide when mussels are exposed to air. Although temperature stress is often thought to limit upper extents of rocky intertidal species (Menge and Branch 2001), this is not always the case. For example, Mislan et al. (2014) found that lethal temperature and upper elevation limits of the North American species Mytilus californianus coincided at only 2 of 15 sites along the U.S. West Coast, suggesting that upper limits are set by factors other than temperature at the remaining sites. Water temperature can limit species distributions in the intertidal, as shown by Seabra et al. (2016) for an Eastern Atlantic limpet. The primary importance of air temperature for setting the upper limit of New Zealand’s intertidal mussels is supported by our findings of a significant relationship between tolerance and aerial temperatures. Demonstrating an association between temperature and vertical range limits strengthens our assertions of projected distributional shifts under climate warming (Helmuth et al. 2006).
Based on measured lethal thermal limits and projected future temperatures under climate change, Perna, Aulacomya and Mytilus are all likely to experience vertical range contractions by the year 2100 at the Box Thumb site. To maintain an equivalent thermal environment over the next century, these three species would need to shift 0.2–0.4 m lower in the intertidal zone at this and similar sites. However, any shifts at Nile River or other colder sites are predicted to be of lower magnitude because body temperatures in the mussel bed at this site were predicted to elicit less than 50% mortality through the year 2100. It is important to note that the biophysical model simulates mussel bed layers and not individual mussels. Therefore, differences in the physical size and shape of Perna, Aulacomya and Mytilus as individuals are not accounted for by the model. The future projections of mussel temperatures made using the biophysical model are most relevant for mussels in a bed (which describes the current landscape at these sites; Fig. 1; C. Sorte pers. obs.) rather than individuals of specific species. We also note that the model error (estimated at 1.9–3.4 °C for similar U.S. west coast mussels; Mislan and Wethey 2011) is of the same magnitude as predicted warming over the coming century (3 °C). This suggests that our results are most appropriately interpreted in a comparative sense, with future range contractions expected to be relatively similar across three species in the New Zealand mussel guild.
Changes to mussel abundances and distributions could lead to subsequent shifts in overall community structure and diversity. This is because mussels, as foundation species (Bruno and Bertness 2001; Yakovis et al. 2008; Angelini et al. 2015), provide structurally heterogeneous habitat for a diverse assemblage of associated organisms, many of which could not persist on bare rock due to stressors such as waves and desiccation (Suchanek 1992; Borthagaray and Carranza 2007). Research has shown that declines in mussel (Mytilus californianus) bed thickness in southern California, USA (Smith et al. 2006a) was associated with decreased diversity (Smith et al. 2006b). In New Zealand, where mussel beds are formed by up to four co-occurring species, the impact of mussel declines on diversity at a particular elevation might vary by species because of differing body sizes and, consequently, bed depth. For example, Perna-dominated mussel beds at NR and BT are 1.7 times as deep as those dominated by Mytilus (~ 35 vs. ~ 21 mm; C. Sorte and M. Bracken, unpubl. data). Although we were not able to evaluate the likelihood of shifts in Xenostrobus, the high zone mussel, it provides less habitat area (~ 6-mm bed depth) than the other three species.
Changes in the New Zealand mussel guild could also shift competitive interactions (Angelini et al. 2011), biotic resistance to invasions (Kimbro et al. 2013), or prey availability (Menge 1972; Abrams and Matsuda 1996). Mussels are an important prey species for subtidal predators including fish and crabs (Rilov and Schiel 2006), and there may be cascading effects on these mussel predators and subsequently adjacent communities if population sizes decline. The changes in mussel species distributions forecasted here could also have implications for ecosystem functioning (such as carbon inputs; Bracken et al. 2012; Bracken 2017) at local scales.
Our projections for the New Zealand mussel guild are based on the results of short-term thermal tolerance assays and predicted increases in mean temperature. Indeed, future changes in mussel distributions will also depend on many factors including altered intra- and interspecific interactions (e.g., Sanford 1999; Kordas et al. 2011; Gilman et al. 2010), the presence of endoliths in mussel shells (as they have shown to buffer mussels from extreme heating events; Zardi et al. 2016), and transgenerational plasticity (e.g., Ghalambor et al. 2007). Multiple exposures to stressful temperatures can also increase thermal tolerance (Sorte et al. 2011) including via inducible and reversible mechanisms such as up-regulation of stress proteins (Feder and Hofmann 1999). To the degree that our findings indicate higher thermal tolerances for mussels at the warmer BT site than the cooler NR site, this suggests that these New Zealand mussels may have some capacity to acclimatize or adapt to increased temperature. However, it is unknown if adaptation will keep pace with the current rate of climate warming. Tolerances reported here should be representative of the current maximum thermal limits for these populations. We assayed mussels inhabiting the upper edge of their tide-height distributions (where selection and environmental filtering should maximize proportional abundance of the most tolerant individuals) and at the end of the austral summer (when individuals have survived selection for temperature tolerance and also achieved maximal heat-hardening via phenotypic plasticity). Although local adaptation has been shown for some broadcast spawning species like mussels, it can be limited by dispersal within meta-populations. For example, thermal tolerance comparisons between populations of Mytilus californianus inhabiting the U.S. west coast have shown that tolerances differ between Washington and southern California populations (Logan et al. 2012) but not those spanning ~ 1000 km of coastline from central California to Oregon (Logan et al. 2012; Mislan et al. 2014). Similarly, thermal tolerances did not differ between Perna populations spanning the length of the South Island (Dunphy et al. 2013).
While warming impacts could be ameliorated by acclimatization or local adaptation, they could also be exacerbated due to increases in temperature variability and extremes as well as sub-lethal effects of thermal stress. Our warming projections were based on mean predicted changes in global temperature, but extreme heating events (i.e., heat waves) are also predicted to increase in frequency, severity, and duration (Meehl and Tebaldi 2004). Thus, it is likely that mussels will experience days with temperatures above their LT50 more often than we predicted (Table 1). Furthermore, short-term (18-h) recovery periods can miss delayed mortality (Dowd and Somero 2013), and sub-lethal responses to climate warming can also depress demographic rates (Somero 2002; Williams et al. 2007) with mussels that survive temperature stress exhibiting lower growth rates (Petes et al. 2007) or fecundity (Petes et al. 2007, 2008). Over time, these sub-lethal impacts can accrue, leading to shifts in species’ abundances and distributions. Under more stressful conditions, individuals tend to be smaller in size due to metabolic trade-offs (e.g., Petes et al. 2007, 2008), which would decrease size-dependent habitat provisioning with potential cascading impacts on local biodiversity.
Our results indicate that aerial temperature sets current upper elevation limits and that vulnerability to climate warming—at least at our warmer site—is likely to increase greatly over the following century. To maintain their current thermal niche, the upper elevation limit of these mussels would need to shift downward, decreasing habitat availability for associated organisms. Facilitation becomes more important as environmental stress increases (He et al. 2013); thus, mussel declines may occur when these foundation species are needed the most. At the same time, our results suggest that interactions between multiple species in the New Zealand guild may be maintained, allowing continued functioning albeit at a lower elevation on the shoreline. However, this conclusion is based on the expectation that lower elevations provide refuge from thermal stress, an expectation that may become increasingly unfounded as ocean temperatures warm and metabolic rates increase during tidal immersion. Future research would ideally evaluate the adaptation ability of these species under warming aerial conditions, as well as the potential modifying effects of ocean warming, sea level rise, and topographic buffering of thermal regimes (Helmuth and Hofmann 2001; Jimenez et al. 2015; Jurgens and Gaylord 2018). Our study, and others that integrate ecophysiological and population metrics, have the potential to forecast shifts in species’ distributions across community members and the likelihood of cascading extinctions (Borrvall et al. 2000; Steneck et al. 2002; Ellison et al. 2005; Hughes et al. 2009; Wernberg et al. 2016).
References
Abrams PA, Matsuda H (1996) Positive indirect effects between prey species that share predators. Ecology 77:610–616. https://doi.org/10.2307/2265634
Angelini C, Altieri AH, Silliman BR, Bertness MD (2011) Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. Bioscience 61:782–789. https://doi.org/10.1525/bio.2011.61.10.8
Angelini C, Van der Heide T, Griffin JN, Morton JP, Derksen-Hooijberg M, Lamers LP, Smolders AJ, Silliman BR (2015) Foundation species’ overlap enhances biodiversity and multifunctionality from the patch to landscape scale in southeastern United States salt marshes. Proc R Soc B 282:20150421. https://doi.org/10.1098/rspb.2015.0421
Barron CN, Kara AB (2006) Satellite-based daily SSTs over the global ocean. Geophys Res Lett 33:L15603. https://doi.org/10.1029/2006GL026356
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Berg MP, Kiers E, Driessen G, Van Der Heijden M, Kooi BW, Kuenen F, Liefting M, Verhoef HA, Ellers J (2010) Adapt or disperse: understanding species persistence in a changing world. Glob Change Biol 16:587–598. https://doi.org/10.1111/j.1365-2486.2009.02014.x
Blanchette CA, Miner CM, Raimondi PT, Lohse D, Heady KE, Broitman BR (2008) Biogeographical patterns of rocky intertidal communities along the Pacific coast of North America. J Biogeogr 35:1593–1607. https://doi.org/10.1111/j.1365-2699.2008.01913.x
Borrvall C, Ebenman B, Jonsson T, Jonsson T (2000) Biodiversity lessens the risk of cascading extinction in model food webs. Ecol Lett 3:131–136. https://doi.org/10.1046/j.1461-0248.2000.00130.x
Borthagaray AI, Carranza A (2007) Mussels as ecosystem engineers: their contribution to species richness in a rocky littoral community. Acta Oecol 31:243–250. https://doi.org/10.1016/j.actao.2006.10.008
Bracken MES (2017) Coexistence, complementarity, and resource partitioning in a guild of marine filter feeders. In: Abstract 28403 presented at ASLO 2017 aquatic sciences meeting, Honolulu, Hawaii, USA
Bracken MES, Menge BA, Foley MM, Sorte CJB, Lubchenco J, Schiel DR (2012) Mussel selectivity for high-quality food drives carbon inputs into open-coast intertidal ecosystems. Mar Ecol Prog Ser 459:53–62. https://doi.org/10.3354/meps09764
Branch GM, Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Biol Ecol 300:189–215. https://doi.org/10.1016/j.jembe.2003.12.007
Broitman BR, Navarrete SA, Smith F, Gaines SD (2001) Geographic variation of southeastern Pacific intertidal communities. Mar Ecol Prog Ser 224:21–34. https://doi.org/10.3354/meps224021
Bruno JF, Bertness MD (2001) Habitat modification and facilitation in benthic marine communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 201–216
Buchanan S (2001) Measuring reproductive condition in the Greenshell™ mussel Perna canaliculus. NZ J Mar Freshwat Res 35:859–870
Cahill AE, Aiello-Lammens ME, Fisher-Reid MC, Hua X, Karanewsky CJ, Ryu HY, Sbeglia GC, Spagnolo F, Waldron JB, Wiens JJ (2014) Causes of warm-edge range limits: systematic review, proximate factors and implications for climate change. J Biogeogr 41:429–442. https://doi.org/10.1111/jbi.12231
Callander DC (2012) Effects of environmental stress on gene expression in mussels. Dissertation, University of Canterbury, New Zealand
Calosi P, Bilton DT, Spicer JI (2008) Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol Lett 4:99–102. https://doi.org/10.1098/rsbl.2007.0408
Chen F, Dudhia J (2001) Coupling an advanced land surface-hydrology model with the Penn State-NCAR MM5 modeling system. Part I. Model implementation and sensitivity. Mon Weather Rev 129:569–585
Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026. https://doi.org/10.1126/science.1206432
Chivers WJ, Walne AW, Hays GC (2017) Mismatch between marine plankton range movements and the velocity of climate change. Nat Commun 8:14434. https://doi.org/10.1038/ncomms14434
Collins WJ, Bellouin N, Doutriaux-Boucher M, Gedney N et al (2011) Development and evaluation of an Earth-System model—HadGEM2. Geosci Model Dev 4:1051–1075
Connell JH (1961) The Influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723. https://doi.org/10.2307/1933500
Dethier MN, Graham ES, Cohen S, Tear LM (1993) Visual versus random-point percent cover estimations: ‘objective’ is not always better. Mar Ecol Progr Ser 96:93–100
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672. https://doi.org/10.1073/pnas.0709472105
Dowd WW, Somero GN (2013) Behavior and survival of Mytilus congeners following episodes of elevated body temperature in air and seawater. J Exp Biol 216:502–514
Dufresne J-L, Foujols M-A, Denvil S et al (2013) Climate change projections using the IPSL-CM5 earth system model: from CMIP3 to CMIP5. Clim Dyn 40:2123–2165
Dunne JP, John JG, Adcroft AJ et al (2012) GFDL’s ESM2 global coupled climate-carbon earth system models. Part I: physical formulation and baseline simulation characteristics. J Clim 25:6646–6665
Dunne JP, John JG, Shevliakova E et al (2013) GFDL’s ESM2 global coupled climate-carbon earth system models. Part II: carbon system formulation and baseline simulation characteristics. J Clim 26:2247–2267
Dunphy BJ, Ragg NL, Collings MG (2013) Latitudinal comparison of thermotolerance and HSP70 production in F2 larvae of the greenshell mussel (Perna canaliculus). J Exp Biol 216:1202–1209. https://doi.org/10.1242/jeb.076729
Dunphy BJ, Watts E, Ragg NL (2015) Identifying thermally-stressed adult green-lipped mussels (Perna canaliculus Gmelin, 1791) via metabolomic profiling. Am Malacol Bull 33:127–135. https://doi.org/10.4003/006.033.0110
Egbert GD, Erofeeva SY (2002) Efficient inverse modeling of barotropic ocean tides. J Atmos Ocean Technol 19:183–204
Egbert GD, Bennett A, Foreman M (1994) TOPEX/poseidon tides estimated using a global inverse model. J Geophys Res 99:24821–24852
Ek MB, Mitchell KE, Lin Y et al (2003) Implementation of NOAH land surface model advances in the National Centers for Environmental Prediction operational mesoscale Eta model. J Geophys Res 108:8851. https://doi.org/10.1029/2002JD003296
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486. https://doi.org/10.1890/1540-9295(2005)003%5b0479:LOFSCF%5d2.0.CO;2
Faulkner KT, Clusella-Trullas S, Peck LS, Chown SL (2014) Lack of coherence in the warming responses of marine crustaceans. Funct Ecol 28:895–903. https://doi.org/10.1111/1365-2435.12219
Feder M, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Gardner J, Zbawicka M, Westfall KM, Wenne R (2016) Invasive blue mussels threaten regional scale genetic diversity in mainland and remote offshore locations: the need for baseline data and enhanced protection in the Southern Ocean. Glob Change Biol 22:3182–3195. https://doi.org/10.1111/eva.12415
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331
Giorgetta MA, Jungclaus J, Reick CH et al (2013) Climate and carbon cycle changes from 1850 to 2100 in MPI-ESM simulations for the coupled model intercomparison project phase 5. J Adv Model Earth Syst 5:572–597
Gutiérrez JL, Jones CG, Straver DL, Iribarne OO (2003) Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101:79–90. https://doi.org/10.1034/j.1600-0706.2003.12322.x
Harley CD, Helmuth BS (2003) Local-and regional-scale effects of wave exposure, thermal stress, and absolute versus effective shore level on patterns of intertidal zonation. Limnol Oceanogr 48:1498–1508. https://doi.org/10.4319/lo.2003.48.4.1498
Harley CD, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 2:228–241. https://doi.org/10.1111/j.1461-0248.2005.00871.x
He Q, Bertness MD, Altieri AH (2013) Global shifts towards positive species interactions with increasing environmental stress. Ecol Lett 16:695–706
Heath RA (1985) A review of the physical oceanography of the seas around New Zealand—1982. N Z J Mar Freshw 19:79–124. https://doi.org/10.1080/00288330.1985.9516077
Helmuth BS, Hofmann GE (2001) Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol Bull 201:374–384
Helmuth B, Harley CDG, Halpin PM, O’Donnell M, Hofmann GE, Blanchette CA (2002) Climate change and latitudinal patterns of intertidal thermal stress. Science 298:1015–1017
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst 37:373–404
Hughes AR, Williams SL, Duarte CM, Heck KL, Waycott M (2009) Associations of concern: declining seagrasses and threatened dependent species. Front Ecol Environ 7:242–246. https://doi.org/10.1073/pnas.0905620106
Ilyina T, Six KD, Segschneider J, Maier-Reimer E, Li H, Núñez-Riboni I (2013) Global ocean biogeochemistry model HAMOCC: model architecture and performance as component of the MPI-Earth system model in different CMIP5 experimental realizations. J Adv Model Earth Syst 5:287–315
Jimenez AG, Jayawardene S, Alves S, Dallmer J, Dowd WW (2015) Micro-scale environmental variation amplifies physiological variation among individual mussels. Proc R Soc B 282:20152273. https://doi.org/10.1098/rspb.2015.2273
Jones SJ, Mieszkowska N, Wethey DS (2009) Linking thermal tolerances and biogeography: Mytilus edulis (L.) at its southern limit on the east coast of the United States. Biol Bull 217:73–85
Jones CD, Hughes JK, Bellouin N et al (2011) The HadGEM2-ES implementation of CMIP5 centennial simulations. Geosci Model Dev 4:543–570
Jurgens LJ, Gaylord B (2018) Physical effects of habitat-forming species override latitudinal trends in temperature. Ecol Lett 21:190–196. https://doi.org/10.1111/ele.12881
Keith DA, Akçakaya HR, Thuiller W, Midgley GF, Pearson RG, Phillips SJ, Regan HM, Araújo MB, Rebelo TG (2008) Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biol Lett 4:560–563. https://doi.org/10.1098/rsbl.2008.0049
Kennedy VS (1976) Desiccation, higher temperatures and upper intertidal limits of three species of sea mussels (Mollusca: Bivalvia) in New Zealand. Mar Biol 35:127–137. https://doi.org/10.1007/BF00390934
Kimbro DL, Cheng BS, Grosholz ED (2013) Biotic resistance in marine environments. Ecol Lett 16:821–833
Kordas RL, Harley CD, O’Connor MI (2011) Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol 400:218–226
Logan CA, Kost LE, Somero GN (2012) Latitudinal differences in Mytilus californianus thermal physiology. Mar Ecol Prog Ser 450:93–105. https://doi.org/10.3354/meps09491
Lourenço CR, Zardi GI, McQuaid CD, Serrao EA, Pearson GA, Jacinto R, Nicastro KR (2016) Upwelling areas as climate change refugia for the distribution and genetic diversity of a marine macroalga. J Biogeogr 43:1595–1607. https://doi.org/10.1111/jbi.12744
Marsden ID, Weatherhead MA (1998) Effects of aerial exposure on oxygen consumption by the New Zealand mussel Perna canaliculus (Gmelin, 1791) from an intertidal habitat. J Exp Mar Biol Ecol 230:15–29
Meehl GA, Tebaldi C (2004) More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305:994–997. https://doi.org/10.1126/science.1098704
Menge BA (1972) Foraging strategy of a starfish in relation to actual prey availability and environmental predictability. Ecol Monogr 42:25–50. https://doi.org/10.2307/1942229
Menge BA (1976) Organization of the New England rocky intertidal community: role of predation, competition, and environmental heterogeneity. Ecol Monogr 46:355–393. https://doi.org/10.2307/1942563
Menge BA, Branch GM (2001) Rocky intertidal communities. In: Bertness MD, Gaine SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 221–251
Menge BA, Sutherland JP (1976) Species diversity gradients: synthesis of the roles of predation, competition, and temporal heterogeneity. Am Nat 110:351–369
Menge BA, Daley BA, Lubchenco J, Sanford E, Dahlhoff E, Halpin PM, Hudson G, Burnaford JL (1999) Top-down and bottom-up regulation of New Zealand rocky intertidal communities. Ecol Monogr 69:297–330. https://doi.org/10.1890/0012-9615(1999)069%5b0297:TDABUR%5d2.0.CO;2
Menge BA, Daley BA, Sanford E, Dahlhoff EP, Lubchenco J (2007) Mussel zonation in New Zealand: an integrative eco-physiological approach. Mar Ecol Prog Ser 345:129–140. https://doi.org/10.3354/meps06951
Miller LP, Harley CD, Denny MW (2009) The role of temperature and desiccation stress in limiting the local-scale distribution of the owl limpet, Lottia gigantea. Funct Ecol 23:756–767. https://doi.org/10.1111/j.1365-2435.2009.01567.x
Mislan KAS, Wethey DS (2011) Gridded meteorological data as a resource for mechanistic macroecology in coastal environments. Ecol Appl 21:2678–2690
Mislan KAS, Helmuth B, Wethey DS (2014) Geographical variation in climatic sensitivity of intertidal mussel zonation. Glob Ecol Biogeogr 23:744–756. https://doi.org/10.1111/geb.12160
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. https://doi.org/10.1038/nature01286
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915. https://doi.org/10.1126/science.1111322
Petes LE, Menge BA, Murphy GD (2007) Environmental stress decreases survival, growth, and reproduction in New Zealand mussels. J Exp Mar Biol Ecol 351:83–91. https://doi.org/10.1016/j.jembe.2007.06.025
Petes LE, Menge BA, Harris AL (2008) Intertidal mussels exhibit energetic trade-offs between reproduction and stress resistance. Ecol Monogr 78:387–402. https://doi.org/10.1890/07-0605.1
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rilov G, Schiel DR (2006) Seascape-dependent subtidal–intertidal trophic linkages. Ecology 87:731–744. https://doi.org/10.1890/04-1853
Rogelj J, Meinshausen M, Knutti R (2012) Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nat Clim Change 2:248–253. https://doi.org/10.1038/nclimate1385
Saha S et al (2006) The NCEP climate forecast system. J Clim 19:2483–3517
Saha S et al (2010) The NCEP climate forecast system reanalysis. Bull Am Meterol Soc 91:1015–1057
Sanford E (1999) Regulation of keystone predation by small changes in ocean temperature. Science 283:2095–2097
Schiel DR, Steinbeck JR, Foster MS (2004) Ten years of induced ocean warming causes comprehensive changes in marine benthic communities. Ecology 85:1833–1839. https://doi.org/10.1890/03-3107
Schiel DR, Lilley SA, South PM, Coggins JH (2016) Decadal changes in sea surface temperature, wave forces and intertidal structure in New Zealand. Mar Ecol Prog Ser 548:77–95
Seabra R, Wethey DS, Santos AM, Gomes F, Lima FP (2016) Equatorial range limits of an intertidal ectotherm are more linked to water than air temperature. Glob Change Biol 22:3320–3331. https://doi.org/10.1111/gcb.13321
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436. https://doi.org/10.1146/annurev.ecolsys.110308.120317
Smith JM (2003) Surf zone hydrodynamics. U.S. Army Corps of Engineers coastal engineering manual. Part II, chapter 4. U.S. Army Engineer Research and Development Center, Vicksburg
Smith JR, Fong P, Ambrose RF (2006a) Long-term change in mussel (Mytilus californianus Conrad) populations along the wave-exposed coast of southern California. Mar Biol 149:537–545. https://doi.org/10.1007/s00227-005-0223-y
Smith JR, Fong P, Ambrose RF (2006b) Dramatic declines in mussel bed community diversity: response to climate change? Ecology 87:1153–1161. https://doi.org/10.1890/0012-9658(2006)87%5b1153:DDIMBC%5d2.0.CO;2
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol 42:780–789. https://doi.org/10.1093/icb/42.4.780
Somero GN (2012) The physiology of global change: linking patterns to mechanisms. Annu Rev Mar Sci 4:39–61. https://doi.org/10.1146/annurev-marine-120710-100935
Sorte CJB, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Glob Ecol Biogeogr 19:303–316. https://doi.org/10.1111/j.1466-8238.2009.00519.x
Sorte CJB, Jones SJ, Miller LP (2011) Geographic variation in temperature tolerance as an indicator of potential population responses to climate change. J Exp Mar Biol Ecol 400:209–217. https://doi.org/10.1016/j.jembe.2011.02.009
Sorte CJB, Davidson VE, Franklin MC, Benes KM, Doellman MM, Etter RJ, Hannigan RE, Lubchenco J, Menge BA (2017) Long-term declines in an intertidal foundation species parallel shifts in community composition. Glob Change Biol 23:341–352. https://doi.org/10.1111/gcb.13425
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459. https://doi.org/10.1017/S0376892902000322
Stickle WB, Lindeberg M, Rice SD, Munley K, Reed V (2016) Seasonal changes in the thermal regime and gastropod tolerance to temperature and desiccation stress in the rocky intertidal zone in southeast Alaska. J Exp Mar Biol Ecol 482:56–63
Stickle WB, Carrington E, Hayford H (2017) Seasonal changes in the thermal regime and gastropod tolerance to temperature and desiccation stress in the rocky intertidal zone. J Exp Mar Biol Ecol 488:83–91
Stillman JH (2003) Acclimation capacity underlies susceptibility to climate change. Science 301:65. https://doi.org/10.1126/science.1083073
Stillman JH, Somero GN (2000) A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool 73:200–208. https://doi.org/10.1086/316738
Suchanek TH (1992) Extreme biodiversity in the marine-environment-Mussel bed communities of Mytilus californianus. Northwest Environ J 8:150–152
Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Change 2:686–690. https://doi.org/10.1038/nclimate1539
Taylor KE, Stouffer RJ, Meehl GA (2012) An overview of the CMIP5 and the experiment design. Bull Am Meteorol Soc 93:485–498
Tolman HL (1998) A new global wave forecast system at NCEP. In: Edge BL, Helmsley JM (eds) Ocean wave measurements and analysis. American Society of Civil Engineers, Reston, pp 777–786
Tolman HL (2007) The 2007 release of WAVEWATCH III. NOAA/NWS/NCEP/OMB Tech Note 262
Tolman HL (2009) User manual and system documentation of WAVEWATCH III version 3.14. NOAA/NWS/NCEP/OMB Tech Rep 276
US Army Corps of Engineers (2002) Coastal engineering manual. Engineer manual 1110-2-1100. U.S. Army Corps of Engineers, Washington, DC
Vinagre C, Leal I, Mendonca V, Madeira D, Narciso L, Diniz MS, Flores AAV (2016) Vulnerability to climate warming and acclimation capacity of tropical and temperate coastal organisms. Ecol Indic 62:317–327. https://doi.org/10.1016/j.ecolind.2015.11.010
Vincent WF, Howard-Williams C, Tildesley P, Butler E (1991) Distribution and biological properties of oceanic water masses around the South Island, New Zealand. N Z J Mar Freshw 25:21–42. https://doi.org/10.1080/00288330.1991.9516451
Walter H, Harnickell E, Mueller-Dombois D (1975) Climate diagram maps. Supplement to vegetation monographs. Springer, New York
Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD (2011) Seaweed communities in retreat from ocean warming. Curr Biol 21:1828–1832. https://doi.org/10.1016/j.cub.2011.09.028
Wernberg T, Bennett S, Babcock RC, de Bettignies T, Cure K, Depczynski M, Dufois F, Fromont J, Fulton CJ, Hovey RK, Harvey ES (2016) Climate-driven regime shift of a temperate marine ecosystem. Science 353:169–172. https://doi.org/10.1126/science.aad8745
Westfall KM, Gardner J (2010) Genetic diversity of Southern hemisphere blue mussels (Bivalvia: Mytilidae) and the identification of non-indigenous taxa. Biol J Linn Soc 101:898–909. https://doi.org/10.1111/j.1095-8312.2010.01549.x
Wethey DS, Brin LD, Helmuth B, Mislan KAS (2011) Predicting intertidal organism temperatures with modified land surface models. Ecol Model 222:3568–3576. https://doi.org/10.1016/j.ecolmodel.2011.08.019
Williams JW, Jackson ST, Kutzbach JE (2007) Projected distributions of novel and disappearing climates by 2100 AD. Proc Natl Acad Sci USA 104:5738–5742. https://doi.org/10.1073/pnas.0606292104
Yakovis EL, Artemieva AV, Shunatova NN, Varfolomeeva MA (2008) Multiple foundation species shape benthic habitat islands. Oecologia 155:785–795. https://doi.org/10.1007/s00442-007-0945-2
Yamane L, Gilman SE (2009) Opposite responses by an intertidal predator to increasing aquatic and aerial temperatures. Mar Ecol Progr Ser 393:27–36
Zardi GI, Nicastro KR, McQuaid CD, Ng TPT, Lathlean J, Seuront L (2016) Enemies with benefits: parasitic endoliths protect mussels against heat stress. Sci Rep 6:31413
Acknowledgements
We particularly thank M. Bracken and A. Gannett for collaborating on the 2015 fieldwork and D. Schiel and members of MERG at University of Canterbury for facilitating this New Zealand-based research. We thank H. Frenzel for technological help and M. Bracken, M. Foley, M. Peich, D. Wethey, and Sorte Lab members for additional assistance and feedback. This is CSUN Marine Biology Contribution #280.
Funding
This work was supported by start-up funds from the University of California, Irvine to CS, an Erskine Fellowship from University of Canterbury to M. Bracken, and a UCI GAANN travel grant to LP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Data availability
Data and code for temperature modeling and projected frequency of lethal temperature events are available on GitHub at https://github.com/kallisons/NZMusselTemperatures and https://github.com/njsilbiger/NZMussels, respectively. Additional data (mussel abundances, maximum tide heights, and thermal tolerances) are available in Online Resource 2.
Additional information
Responsible Editor: M. G. Chapman.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sorte, C.J.B., Bernatchez, G., Mislan, K.A.S. et al. Thermal tolerance limits as indicators of current and future intertidal zonation patterns in a diverse mussel guild. Mar Biol 166, 6 (2019). https://doi.org/10.1007/s00227-018-3452-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3452-6