Abstract
As ocean temperatures continue to rise due to climate change, many questions remain on how coastal species will cope with a changing environment. The effects of increased temperatures on bivalves has been well examined through single-species studies, showing reductions in tissue mass, shell growth, oxygen uptake, feeding rates, and survival. However, the consequences of these effects on predator–prey interactions remain poorly understood. We examined how increased temperatures (30, 32, 34 °C) and the presence of water-borne predation cues from blue crabs (Callinectes sapidus) affected the morphology and growth rate of southern ribbed mussels (Geukensia granosissima), as well as their handling times when attacked by predatory crabs. Although southern ribbed mussels were able to survive under chronic heat stress, exposure to higher temperatures resulted in more elongated shell shapes. Growth rates in mussel wet weight were higher for mussels reared in the presence of a predator than in the predator-free control, but only in the low-temperature treatment. Likewise, handling times were greater for crabs eating mussels grown in the presence of a predator, but the effect was lost at the mid- and high-temperature treatments. These findings suggest that predation-induced defenses were suppressed when prey were under chronic thermal stress, which could make mussels more vulnerable to predation. The presence of predation cues in natural environments should be taken in consideration when estimating or predicting the effects of climate change on organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increases in ocean temperatures as a consequence of climate change (Meehl et al. 2007) raise questions on how coastal species will cope with a changing environment. Estuarine and coastal marine environments can have highly variable ambient conditions, such as temperature and salinity. Because organisms living in these habitats have a wide tolerance to ambient abiotic conditions, many are already living at the edges of their environmental thresholds and can be sensitive to extreme changes (Connell 1972; Davenport and Davenport 2005; Somero 2002). Some intertidal organisms are already living near or at their physiological tolerance limit (Somero 2010). Sessile organisms, such as bivalves, are especially vulnerable to extreme environmental changes (Nicholson 2002), due to their inability to move from unfavorable conditions.
Coastal bivalves play important ecological roles, as they prevent erosion by providing structure and can increase concentration of nutrients in sediments (Bertness 1984). The southern ribbed mussel Geukensia granosissima is common in intertidal habitats across Florida, USA and is often associated with oyster-reef intertidal habitats. Ribbed mussels have a wide thermal tolerance and can occupy tropical intertidal habitats where both acute and chronic average temperatures can exceed 30 °C (Jost and Helmuth 2007; Read and Cumming 1967). Exposure to these and higher levels of chronic heat stress, however, can have detrimental effects. The effects of climate change on single species of coastal bivalves has been an area of special interest in recent years, with evidence that increased temperatures can drive reductions in shell growth and size-specific tissue mass (Fitzgerald-Dehoog et al. 2012; Miller et al. 2014; Zhao et al. 2017), reduced oxygen consumption (Ganser et al. 2015), metabolic energy deficiency (Tateda et al. 2015), increased cellular protein denaturation (Buckley et al. 2001), and decreased survival (Pincebourde et al. 2008; White et al. 2015). However, the consequences of these single-species effects on predator–prey interactions remain poorly understood and predictions regarding how predator–prey species interactions will be affected by climate change are primarily theoretical (Kordas et al. 2011).
Ribbed mussels in oyster-reef intertidal habitats serve as a food source for many predators including the blue crab Callinectes sapidus, common in oyster-reef habitats (Peterson et al. 2003) and an important predator on intertidal mussels (Macreadie et al. 2011; Sherwood and Petraitis 1998). Crab predators can also induce anti-predator defenses on bivalves through water-borne chemical cues (Caro and Castilla 2004; Freeman 2007), making this a model predator–prey system to study the interactive effects of increased temperature and predator presence on mussel defenses and their consequences for predation susceptibility.
Predator–prey relationships are complex and can be affected by biological and physical processes (Blundon and Kennedy 1982; Connell 1961; Gestoso et al. 2015; Hughes and Seed 1981). Biological processes, such as inducible defenses in the form of increased shell size and thickness in bivalves, can be especially important for protection against predators (Caro and Castilla 2004; Freeman 2007; Harper and Skelton 1993). Abiotic stressors, such as increased temperatures, can negatively affect growth and alter the morphology of bivalves (Fitzgerald-Dehoog et al. 2012; Rodland et al. 2009; Talmage and Gobler 2011; Tateda et al. 2015). Thus, bivalves may become more susceptible to predation as increased temperatures decrease the protection afforded by their shells. However, few studies have tested the effects of elevated temperatures on inducible defenses in bivalves and how these in turn affect predator–prey interactions. There is, therefore, a need to examine how bivalve prey respond to elevated temperatures while in the presence of predation cues to better understand the effects of these environmental changes through a broader ecological perspective.
This study was designed to investigate the morphological responses of southern ribbed mussels to two simultaneous stressors: elevated temperatures and the presence of water-borne predation cues from blue crabs. More specifically, we tested whether (1) mussel growth and morphology were affected by elevated temperatures and the presence of water-borne predation cues, and if (2) any effects of these treatments on growth and mussel morphology led to differences in handling times by predatory crabs.
Materials and methods
Laboratory setup
Southern ribbed mussels were held in one of six cross-factored temperature × predator treatments: 30, 32, or 34 °C [seawater temperatures of present day and those expected for the years 2050 and 2100, respectively (Meehl et al. 2007; Sokolov et al. 2009)], and the presence (P+) or absence (P−) of water-borne chemical cues from a predatory blue crab for a 4-week period. This medium-term exposure period has been an appropriate time frame to observe the effects of temperature on mussel morphology (Gestoso et al. 2016; Kroeker et al. 2014), with some studies observing effects in as little as 2 weeks (Keppel et al. 2015). Temperature levels were maintained using an Apex AquaController system (Neptune Systems), equipped with 300-W Finnex titanium heaters. The experimental system was exceptionally efficient, maintaining tank temperatures within 0.2–0.6 °C of target temperatures (Table 1).
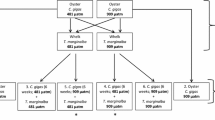
The experimental setup consisted of a flow-through, two-tank glass aquaria system (Fig. 1). Water originated from a temperature-controlled sump, flowed through a primary (predator) tank, and gravity fed to a secondary (mussel) tank. Water from all secondary (mussel) tanks overflowed into a temperature-controlled water bath to maintain constant and homogeneous temperatures across all tanks. Each primary (predator) tank held a single blue crab, and each secondary (mussel) tank held fifteen southern ribbed mussels. Predator-free control groups were held in the same two-tank experimental setup, but without a predator. The design of the experimental system allowed for movement of water-borne cues from the predators to the mussels, while preventing direct interactions. Each of the six temperature × predator presence treatments had 3 tank replicates, for a total of 18 experimental tanks. Each experimental tank held fifteen mussels, of which eight were monitored for shell growth (total mussels: 18 experimental tanks × 15 mussels per tank = 270 mussels; mussels monitored for growth: 18 experimental tanks × 8 mussels per tank = 144 mussels).
Schematic of experimental temperature-controlled system. Water was pumped from (1) a temperature-controlled 190 L sump to (2) the 9.5 L crab/control tanks. Water then gravity fed to (3) the 9.5 L mussel tanks and overflowed onto (4) a temperature-controlled water bath that maintained temperatures constant and homogeneous across all experimental tanks. There were a total of 15 mussels per experimental tank, of which 8 (shaded dark) were monitored for shell growth
Flow was maintained at a constant rate of 3.28 mL/min, resulting in complete water turnover every 48 h. To avoid external chemical cues, artificial sea water (mix of DI water and Instant Ocean sea salt) was used, mixed to a target salinity of 32 ppt. Experimental tanks were exposed to a controlled 12:12 photoperiod and aerated with ambient air. Southern ribbed mussels were fed 5.7 × 107 cells/day per mussel (163 mg of organic matter per day per mussel) of refrigerated phytoplankton (Shellfish Diet 1800), representing a minimum of 14% of the tissue dry weight of the mussels in the study. This amount of organic matter was greater than the recommended 3% by the food manufacturer (Reed Mariculture Inc. 2015; Helm et al. 2004), the amount of organic matter available in the mussels’ natural environment (Environmental Protection Commission of Hillsborough County 2018), and food concentrations used in similar studies (Kroeker et al. 2014). Homogenate conspecific signals have been shown to induce anti-predator traits in prey sometimes even more than the presence of a predator itself (Robson et al. 2010; Yamada et al. 1998); therefore, crabs were fed southern ribbed mussel conspecifics once a day to mimic natural conditions and increase the probability of eliciting induced defenses on mussel morphology. By feeding crabs with mussel conspecifics we aimed to mimic natural environmental conditions where crabs commonly consume mussels in the immediate proximity of other mussels. Using this experimental design, we compared growth in mussels exposed to a combination of crab and mussel conspecific water-borne cues vs. growth in mussels exposed to neither.
Specimen collection and response parameters
Southern ribbed mussels (mean ± SD shell length = 19.47 ± 5.75 mm, n = 270), were collected from intertidal habitats in Tampa Bay, Florida, USA (27.84°N, 82.61°W) in October 2014. Mussels were transported in aerated containers to the laboratory and allowed to acclimate at a baseline temperature of mean ± SD = 25.25 ± 0.03 °C for 10 days. After the acclimation period, temperatures were increased by 1 °C every 12 h from the baseline temperature until target temperatures were reached. Live blue crabs (mean ± SD carapace width = 150 ± 11 mm, n = 9) were obtained from local fishermen in Tampa Bay and were transported to the lab less than 24 h after capture.
Morphometric traits of the southern ribbed mussels (i.e., shell length, shell width, and wet weight) were measured at the beginning and end of the 4-week experimental period. Mussels were removed from their holding tanks, blot dried, and measured for shell length and shell width using digital calipers to the nearest 0.01 mm. Mussels were then immediately placed on a digital scale to measure wet weight to the nearest 0.001 g. Mortality was low, with a total of 4 mussels that died from different experimental treatments during the experimental period (overall survival = 98.5%). Mussels that did not survive the 4-week period were not included in the analysis. We considered a mussel dead when it did not close its valves after being mechanically stimulated. Growth was represented as the change in shell length, shell width, and wet weight from start to end of the 4-week period, normalized by the initial values and expressed as a percentage.
Handling time experiment
To determine how any effects of increased temperature and predation cues on southern ribbed mussel morphology affected predation susceptibility, we followed the 4-week growth experiment with a predation experiment, measuring handling times of mussels by blue crabs across experimental treatments. Blue crabs (mean ± SD carapace width = 146 ± 16 mm, n = 33) were acclimated to target temperatures for a period of 4 days prior to the feeding experiment. Crabs were fed southern ribbed mussel conspecifics ad libitum for 2 days and starved for 1 day prior to the feeding trial to normalize hunger among test crabs. Each crab was placed in a test tank and allowed to feed uninterrupted for 1 h on mussels reared in the experimental treatments. We lost 28 mussels to an escaped predator during the handling time experiments; therefore, a total of 242 mussels were presented to crabs for the handling time experiments (66, 88, and 88 for low-, mid-, and high-temperature treatments, respectively; note that most of the lost mussels were from the low-temperature treatment, hence the difference in sample size). To prevent altered feeding behaviors due to human presence, trials were videotaped, and handling times were measured via video analysis. Handling time was measured from the crab’s first crushing behavior to when it abandoned the empty shell. Not all crabs consumed all mussels made available to them during the handling time experiments; therefore, only handling times for mussels that were consumed were included in the analysis.
Statistical analyses
All statistical analyses were performed using the Fathom Toolbox for Matlab (Jones 2014). Data did not meet the assumption of normality; therefore, non-parametric permutation-based tests were used for all statistical analyses. All permutation-based tests were performed using 5000 permutations of the data and a significance level of α = 0.05. Outliers at two standard deviations were excluded from the analyses. Initially, 144 mussels were monitored for morphometric growth. After accounting for the 4 mussel deaths and one outlier removed, a total sample size of 139 mussels remained for all growth data analyses. We used three-way analyses of variance (npANOVA) with tank as a nested factor to test whether individuals in different tanks exhibited different responses independently of the temperature and predator treatments in which they were reared. Since there was no significant effect of rearing tank on growth of any morphometric, nor interactive effects of tank with either temperature or predator presence (all P values > 0.25), individuals within a tank were considered replicates for all further analyses.
The effects of temperature and presence of water-borne predation cues on percent growth were tested using two-way npANOVAs followed by pair-wise tests. The effects of temperature and presence of predation cues on crab handling times were tested through analyses of covariance (npANCOVAs) using crab size as a covariate, since handling times were dependent on crab size [r2 = 0.035, F (2,202) = 7.241, P = 0.006].
Results
Growth in shell length of southern ribbed mussels did not differ across temperature treatments (Table 2a, Fig. 2a). Percent growth in shell width varied across temperatures (Table 2a), with no differences between the low- and mid-temperature treatments, but significantly lower growth at the high-temperature treatment (Table 2b, Fig. 2b). These differences were largely driven by differences in the dispersion of the data [dispersion across temperature treatments npANOVA F (2,136) = 19.225, P < 0.001; low × high dispersion pair-wise test t90 = 5.244, P < 0.001; mid × high dispersion pair-wise test t89 = 5.987, P < 0.001]. The significant differences in data dispersion reflected the variability in the morphological response to temperature stress, with decreased magnitude and variance of percent growth in shell width as temperature increased (Fig. 2b). Percent growth in mussel wet weight was higher in the low-temperature treatment compared to the mid- and high-temperature treatments (Table 2a, b, Fig. 2c). Dispersions for percent growth in mussel wet weight were homogeneous across temperature treatments (all npANOVA P values > 0.05).
Boxplots of percent growth in a shell length, b shell width, and c mussel wet weight by temperature treatment. Dashed lines represent the mean and dots represent outliers at the 95th percentile. Lower case letters represent pairs with significant differences in mean percent growth and asterisks depict level of significance (***< 0.001)
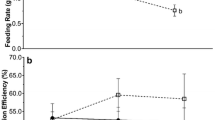
Water-borne predation cues did not affect percent growth in shell length or shell width, and had a marginally nonsignificant effect on mussel wet weight (Table 2a). Pair-wise comparisons revealed that at the low-temperature treatment, mussel wet weight was significantly higher in the presence than in the absence of predation cues (Fig. 3a) (pair-wise test t45 = 2.465, P = 0.017). This effect was not observed at the mid- (Fig. 3b) and high-temperature treatments (Fig. 3c) (pair-wise test P values > 0.05).
Boxplots a–c show comparisons of percent mussel wet weight growth between predator absent (P−) and predator present (P+) treatments at low-, mid- and high-temperature treatment levels, respectively. Dashed lines represent the mean and dots represent outliers at the 95th percentile. Lower case letters represent pairs with significant differences in mean percent growth and asterisks depict level of significance (*< 0.05). Shading represents temperature treatment level: white (low), light grey (mid), and dark grey (high)
Temperature did not influence mussel handling times by crab predators [npANCOVA F (1,202) = 1.096, P = 0.363]. However, after removing the effect of crab size, residuals for mussel handling time in the low temperature treatment reflected the pattern observed in mussel wet weight growth (Fig. 4a). Crabs spent more time handling and consuming mussels reared in the presence of predation cues than those in the predator-free control group [npANCOVA F (1,57) = 4.108, P = 0.048], but only at the low-temperature treatment. This effect was not observed between predator treatments at the mid- [Fig. 4b, npANCOVA F (1,79) = 0.170, P = 0.675] and high-temperature treatments [Fig. 4c, npANCOVA F (1,62) = 0.589, P = 0.456].
Boxplots a–c show comparisons of npANCOVA residuals for handling time (after correcting for the effect of crab size) between predator absent (P−) and predator present (P+) treatments at low-, mid- and high-temperature treatment levels, respectively. Dashed lines represent the mean and dots represent outliers at the 95th percentile. Lower case letters represent pairs with significant differences in mean handling time and asterisks depict level of significance (*< 0.05). Shading represents temperature treatment level: white (low), light grey (mid), and dark grey (high)
Discussion
Responses to climate change across taxa are diverse and vary by species (Kroeker et al. 2013). Through this study we demonstrated how elevated temperatures affected southern ribbed mussel morphology and its capacity to develop inducible defenses in the presence of predation cues. When exposed to increased temperatures, southern ribbed mussels: (1) exhibited altered shell shapes, (2) lacked apparent inducible defenses, and (3) experienced similar handling times by predatory crabs regardless of their predator treatment, demonstrating the consequences of loss of inducible defenses.
Reductions in mussel growth
Growth in shell width and mussel wet weight decreased as temperature increased. Differences in dispersion of growth data suggested heterogeneity among individual mussels in their response to stressors. Southern ribbed mussels survived at temperatures above 30 °C, but with significant reductions in growth, supporting recent findings (Fitzgerald-Dehoog et al. 2012; Miller et al. 2014; Zhao et al. 2017). This effect could be due to physiological limitations. Many marine species have limited growth rates above 31 °C (Goodwin et al. 2001; Schöne and Giere 2005; Schöne et al. 2002) due to limitations in oxygen uptake and metabolic activity (Rodland et al. 2009; Schöne and Giere 2005). Moreover, many bivalves, including ribbed mussels, respire more as temperature increases (Noisette et al. 2015; Wilbur and Hilbish 1989). Thus, the negative effects of increased temperature on mussel growth observed in this study may be due to physiological energy demands.
Heat stress can increase the energy demands on the production of proteins that prevent and repair heat-induced cellular damage. In intertidal mussels, thermal stress is linked to denaturation and structural damage of proteins, evidenced by the up-regulation of oxidative-stress (Fields et al. 2012), cytoskeletal (Fields et al. 2012; Tomanek and Zuzow 2010), and heat-shock chaperone proteins (Fields et al. 2012; Hofmann and Somero 1995; Roberts et al. 1997; Tomanek and Zuzow 2010). The energy costs associated with producing and maintaining the activities of these proteins likely reduces the energy available for biological demands, such as reproduction and growth (Han et al. 2013; Hofmann and Somero 1995). The demands on energy allocation due to thermal stress may cause diversion of energy to growth in specific morphological traits that may be especially beneficial to the organism’s survival. We hypothesize, then, that these energetic physiological demands due to thermal stress affected mussels’ abilities to respond to water-borne predation cues.
Alterations in shell shape
Southern ribbed mussels grew in length, but had limited growth in width, with increasing temperatures, creating a more elongated shell shape. Blue crabs exhibit preference for ribbed mussel with shells shorter than 25 mm to maximize energy ingestion while minimizing energy expenditure and handling time (Elner and Hughes 1978; Hughes and Seed 1981). Therefore, it is possible that mussels in our study expended energy in growth in shell length rather than shell width to move out of their predators’ preferred feeding size range. However, mollusks have been observed to adopt rounder, flatter shells, the opposite of the alterations in shell shape due to increased temperatures observed in this study, to better protect themselves from shell-crushing predators (Bronmark et al. 2011). These differences in shell morphology can alter predation rates and feeding preference by predators, affecting predator–prey interactions and resulting in diet shifts (Lopez et al. 2010). Therefore, the observed elongation of shell shapes could be detrimental for mussel survival and could have further consequences for intertidal predator–prey relationships.
Suppression of inducible defenses
Southern ribbed mussels reared in the presence of predation cues grew significantly more in terms of wet weight than those reared in the absence of predation cues, but only in the low-temperature treatment. Loss of this predator effect at the experimental mid- and high-temperature treatments demonstrated the capacity of increased temperatures to affect the production of inducible defenses in mussels. This effect was further evidenced by the differences in predator handling times only observed at the low-temperature treatment.
The significant increase in wet weight observed did not match increases in shell length or shell width, and therefore, must be due to growth in a different morphometric. We were unable to calculate shell thickness with the available data; however, a probable explanation for increased mussel wet weight is an increase in shell thickness, a common inducible anti-predatory response used by mollusks for defense against predation, often at the expense of growth in shell length and width (Caro and Castilla 2004; Freeman 2007; Freeman and Byers 2006; Smith and Jennings 2000). Predator-induced shell thickening is essential for southern ribbed mussels, as it defends against crab predation by increasing handling times (Freeman 2007) and makes them less energetically feasible prey. In laboratory studies, blue crabs have been observed to abandon mussel prey with high handling times to minimize energy usage (Hughes and Seed 1995), and exhibit preference for feeding on thin-shelled mussels (Caro and Castilla 2004). Southern ribbed mussels in our study may have adopted this shell thickening approach to respond to the presence of predation cues at the low-temperature treatment. At elevated temperatures, however, the demands on energy allocation (Ganser et al. 2015; Hofmann and Somero 1995) may have attributed to the apparent disruption of the predator effect.
The effects of temperature on behavioral inducible defenses have been recorded for scallops in the form of altered escape performance (Schalkhausser et al. 2014) and snails as alterations in righting behavior (Schram et al. 2014). However, to our knowledge, this is the first documentation of increased temperatures interfering with morphological inducible defenses, and thus predator handling times, in southern ribbed mussels.
Study limitations
The results of this study provide evidence on the effects of increased temperatures and predation cues on southern ribbed mussel growth. However, it is possible that the length of the experiment was insufficient to reflect the full scope of the synergistic effects of elevated temperatures and predation cues. 4 weeks may have been insufficient for mussels to grow out of blue crabs’ preferred prey size (< 25 mm) (Hughes and Seed 1981). Although a longer experimental period may have been beneficial, it is interesting that we were able to observe differences in shell shape and disruption of inducible defenses so clearly, which attests to the strength of the temperature effect on southern ribbed mussel morphology. Our efforts are a step forward from classic studies that have examined the effects of increased temperatures on single species, by including and measuring the effect of another crucial stressor in natural systems: the presence of predation cues.
Conclusions
We have demonstrated that chronic heat stress can have detrimental morphological effects on intertidal mussels. We documented the ability of southern ribbed mussels to survive chronic thermal stress up to 34 °C, although with significant reductions of both growth in shell width and wet weight compared to lower temperatures. Mussels reared in elevated temperatures also manifested more elongated shell shapes, which could affect their ability to protect themselves against predation. Mussels under constant heat stress may be energetically limited by cellular-repairing processes (Fields et al. 2012; Hofmann and Somero 1995; Roberts et al. 1997; Tomanek and Zuzow 2010), leading to limitations in growth and the loss of predator-induced defenses. Here we provided evidence for the disruption of the predator effect on inducible defenses by thermal stress in temperatures over 30 °C for southern ribbed mussels, followed by decreases in predator handling times. The observed shell elongation and lack of anti-predatory responses in elevated temperatures could make southern ribbed mussels more vulnerable to predation by blue crabs and other predators. The effects of temperature alone on single species can induce shifts in species abundances, distributions, and interspecies interactions, leading to important consequences for coastal ecosystem dynamics (Miller et al. 2014; Schiel et al. 2004). Therefore, the presence of predation cues in natural environments should be considered when addressing the effects of elevated temperatures on marine organisms and when making inferences on the effects of climate change on coastal ecosystems.
References
Bertness MD (1984) Ribbed mussels and Spartina alterniflora production in a New England salt-marsh. Ecology 65:1794–1807. https://doi.org/10.2307/1937776
Blundon JA, Kennedy VS (1982) Refuges for infaunal bivalves from blue crab, Callinectes sapidus (Rathbun), predation in Chesapeake Bay. J Exp Mar Biol Ecol 65:67–81. https://doi.org/10.1016/0022-0981(82)90176-9
Bronmark C, Lakowitz T, Hollander J (2011) Predator-induced morphological plasticity across local populations of a freshwater snail. PLoS One 6:6. https://doi.org/10.1371/journal.pone.0021773
Buckley BA, Owen M-E, Hofmann GE (2001) Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 204:3571–3579
Caro AU, Castilla JC (2004) Predator-inducible defences and local intrapopulation variability of the intertidal mussel Semimytilus algosus in central Chile. Mar Ecol Prog Ser 276:115–123. https://doi.org/10.3354/meps276115
Connell JH (1961) Effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecol Monogr 31:61–104
Connell JH (1972) Community interactions on marine rocky intertidal shores. Annu Rev Ecol Syst 3:169–192. https://doi.org/10.1146/annurev.es.03.110172.001125
Davenport J, Davenport JL (2005) Effects of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Mar Ecol Prog Ser 292:41–50. https://doi.org/10.3354/meps292041
Elner RW, Hughes RN (1978) Energy maximization in the diet of the shore crab, Carcinus maenas. J Anim Ecol 47:103–116. https://doi.org/10.2307/3925
Environmental Protection Commission of Hillsborough County (2018) EPC Water Quality Data, Tampa. http://www.epchc.org/divisions/water-management/water-monitoring-maps-and-data. Accessed 18 May 2018
Fields PA, Cox KM, Karch KR (2012) Latitudinal variation in protein expression after heat stress in the salt marsh mussel Geukensia demissa. Integr Comp Biol 52:636–647. https://doi.org/10.1093/icb/ics086
Fitzgerald-Dehoog L, Browning J, Allen BJ (2012) Food and heat stress in the California Mussel: evidence for an energetic trade-off between survival and growth. Biol Bull 223:205–216
Freeman AS (2007) Specificity of induced defenses in Mytilus edulis and asymmetrical predator deterrence. Mar Ecol Prog Ser 334:145–153
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833. https://doi.org/10.1126/science.1125485
Ganser AM, Newton TJ, Haro RJ (2015) Effects of elevated water temperature on physiological responses in adult freshwater mussels. Freshw Biol 60:1705–1716. https://doi.org/10.1111/fwb.12603
Gestoso I, Arenas F, Olabarria C (2015) Feeding behaviour of an intertidal snail: does past environmental stress affect predator choices and prey vulnerability? J Sea Res 97:66–74. https://doi.org/10.1016/j.seares.2014.12.006
Gestoso I, Arenas F, Olabarria C (2016) Ecological interactions modulate responses of two intertidal mussel species to changes in temperature and pH. J Exp Mar Biol Ecol 474:116–125. https://doi.org/10.1016/j.jembe.2015.10.006
Goodwin DH, Flessa KW, Schone BR, Dettman DL (2001) Cross-calibration of daily growth increments, stable isotope variation, and temperature in the Gulf of California bivalve mollusk Chione cortezi: implications for paleoenvironmental analysis. Palaios 16:387–398. https://doi.org/10.2307/3515578
Han GD, Zhang S, Marshall DJ, Ke CH, Dong YW (2013) Metabolic energy sensors (AMPK and SIRT1), protein carbonylation and cardiac failure as biomarkers of thermal stress in an intertidal limpet: linking energetic allocation with environmental temperature during aerial emersion. J Exp Biol 216:3273–3282. https://doi.org/10.1242/jeb.084269
Harper E, Skelton P (1993) A defensive value of the thickened periostracum in the Mytiloidea. Veliger 36:36–42
Helm MM, Bourne N, Lovatelli A (2004). Hatchery culture of bivalves — a practical manual. FAO Fisheries Technical Paper. FAO, Rome
Hofmann GE, Somero GN (1995) Evidence for protein damage at environmental temperatures—seasonal changes in levels of ubiquitin conjugates and HSP70 in the intertidal mussel Mytilus trossulus. J Exp Biol 198:1509–1518
Hughes RN, Seed R (1981) Size selection of mussels by the blue crab Callinectes sapidus: energy maximizer or time minimizer? Mar Ecol Prog Ser 6:83–89. https://doi.org/10.3354/meps006083
Hughes RN, Seed R (1995) Behavioural mechanisms of prey selection in crabs. J Exp Mar Biol Ecol 193:225–238. https://doi.org/10.1016/0022-0981(95)00119-0
Jones D (2014) Fathom Toolbox for Matlab: software for multivariate ecological and oceanographic data analysis. College of Marine Science, University of South Florida, St Petersburg
Jost J, Helmuth B (2007) Morphological and ecological determinants of body temperature of Geukensia demissa, the Atlantic ribbed mussel, and their effects on mussel mortality. Biol Bull 213:141–151
Keppel EA, Scrosati RA, Courtenay SC (2015) Interactive effects of ocean acidification and warming on subtidal mussels and sea stars from Atlantic Canada. Mar Biol Res 11:337–348. https://doi.org/10.1080/17451000.2014.932914
Kordas RL, Harley CDG, O’Connor MI (2011) Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol 400:218–226. https://doi.org/10.1016/j.jembe.2011.02.029
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896. https://doi.org/10.1111/gcb.12179
Kroeker KJ, Gaylord B, Hill TM, Hosfelt JD, Miller SH, Sanford E (2014) The role of temperature in determining species’ vulnerability to ocean acidification: a case study using Mytilus galloprovincialis. PLoS One 9:10. https://doi.org/10.1371/journal.pone.0100353
Lopez MS, Coutinho R, Ferreira CEL, Rilov G (2010) Predator-prey interactions in a bioinvasion scenario: differential predation by native predators on two exotic rocky intertidal bivalves. Mar Ecol Prog Ser 403:101–112. https://doi.org/10.3354/meps08409
Macreadie PI, Geraldi NR, Peterson CH (2011) How small-scale variation in oyster reef patchiness influences predation on bivalves. Mar Ecol Prog Ser 429:87–91. https://doi.org/10.3354/meps09115
Meehl G, Stocker T, Collins W, Friedlingstein P, Gaye A, Gregory J, Kitoh A, Knutti R, Murphy J, Noda A, Raper S, Watterson I, Weaver A, Zhao Z (2007) Contribution of working group 1 to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University, Cambridge
Miller LP, Matassa CM, Trussell GC (2014) Climate change enhances the negative effects of predation risk on an intermediate consumer. Glob Change Biol 20:3834–3844. https://doi.org/10.1111/gcb.12639
Nicholson S (2002) Ecophysiological aspects of cardiac activity in the subtropical mussel Perna viridis (L.) (Bivalvia: Mytilidae). J Exp Mar Biol Ecol 267:207–222. https://doi.org/10.1016/s0022-0981(01)00362-8
Noisette F, Richard J, Le Fur I, Peck LS, Davoult D, Martin S (2015) Metabolic responses to temperature stress under elevated pCO2 in Crepidula fornicata. J Mollus Stud 81:238–246. https://doi.org/10.1093/mollus/eyu084
Peterson CH, Grabowski JH, Powers SP (2003) Estimated enhancement of fish production resulting from restoring oyster reef habitat: quantitative valuation. Mar Ecol Prog Ser 264:249–264. https://doi.org/10.3354/meps264249
Pincebourde S, Sanford E, Helmuth B (2008) Body temperature during low tide alters the feeding performance of a top intertidal predator. Limnol Oceanogr 53:1562–1573. https://doi.org/10.4319/lo.2008.53.4.1562
Read KRH, Cumming KB (1967) Thermal tolerance of the bivalve molluscs Modiolus modiolus L., Mytilus edulis L. and Brachidontes demissus Dillwyn. Comp Biochem Physiol 22:149–155. https://doi.org/10.1016/0010-406X(67)90176-4
Reed Mariculture, Inc. (2015) Shellfish diet: smaller cell sizes & more DHA for first feeding larvae, Campbell
Roberts DA, Hofmann GE, Somero GN (1997) Heat-shock protein expression in Mytilus californianus: acclimatization (seasonal and tidal-height comparisons) and acclimation effects. Biol Bull 192:309–320. https://doi.org/10.2307/1542724
Robson AA, De Leaniz CG, Wilson RP, Halsey LG (2010) Behavioural adaptations of mussels to varying levels of food availability and predation risk. J Mollus Stud 76:348–353. https://doi.org/10.1093/mollus/eyq025
Rodland DL, Schone BR, Baier S, Zhang ZJ, Dreyer W, Page NA (2009) Changes in gape frequency, siphon activity and thermal response in the freshwater bivalves Anodonta cygnea and Margaritifera falcata. J Mollus Stud 75:51–57. https://doi.org/10.1093/mollus/eyn038
Schalkhausser B, Bock C, Portner HO, Lannig G (2014) Escape performance of temperate king scallop, Pecten maximus under ocean warming and acidification. Mar Biol 161:2819–2829. https://doi.org/10.1007/s00227-014-2548-x
Schiel DR, Steinbeck JR, Foster MS (2004) Ten years of induced ocean warming causes comprehensive changes in marine benthic communities. Ecology 85:1833–1839
Schöne B, Giere O (2005) Growth pattern and shell isotope ratios of the deep-sea hydrothermal vent bivalve mollusk Bathymodiolus brevior from the North Fiji Basin, Pacific Ocean. Deep Sea Res I 52:1896–1910
Schöne BR, Goodwin DH, Flessa KW, Dettman DL, Roopnarine PD (2002) Sclerochronology and growth of the bivalve mollusks Chione (Chionista) fluctifraga and C. (Chionista) cortezi in the northern Gulf of California, Mexico. Veliger 45:45–54
Schram JB, Schoenrock KM, McClintock JB, Amsler CD, Angus RA (2014) Multiple stressor effects of near-future elevated seawater temperature and decreased pH on righting and escape behaviors of two common Antarctic gastropods. J Exp Mar Biol Ecol 457:90–96. https://doi.org/10.1016/j.jembe.2014.04.005
Sherwood RM, Petraitis PS (1998) Mortality differences of two intertidal mussels, Mytilus edulis L. and Geukensia demissa (Dillwyn), in a New Jersey salt marsh. J Exp Mar Biol Ecol 231:255–265. https://doi.org/10.1016/s0022-0981(98)00095-1
Smith LD, Jennings JA (2000) Induced defensive responses by the bivalve Mytilus edulis to predators with different attack modes. Mar Biol 136:461–469. https://doi.org/10.1007/s002270050705
Sokolov AP et al (2009) Probabilistic forecast for twenty-first-century climate based on uncertainties in emissions (without policy) and climate parameters. J Clim 22:5175–5204. https://doi.org/10.1175/2009jcli2863.1
Somero GN (2002) Thermal limits to life: underlying mechanisms and adaptive plasticity. Integr Comp Biol 42:1316
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920. https://doi.org/10.1242/jeb.037473
Talmage SC, Gobler CJ (2011) Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of Northwest Atlantic bivalves. PLoS One 6:12. https://doi.org/10.1371/journal.pone.0026941
Tateda Y, Sakaguchi I, Itani G (2015) Scope for growth of Mytilus galloprovincialis and Perna viridis as a thermal stress index in the coastal waters of Japan: field studies at the Uranouchi inlet and Yokohama. J Exp Mar Biol Ecol 470:55–63. https://doi.org/10.1016/j.jembe.2015.03.021
Tomanek L, Zuzow MJ (2010) The proteomic response of the mussel congeners Mytilus galloprovincialis and M. trossulus to acute heat stress: implications for thermal tolerance limits and metabolic costs of thermal stress. J Exp Biol 213:3559–3574. https://doi.org/10.1242/jeb.041228
White JD, Hamilton SK, Sarnelle O (2015) Heat-induced mass mortality of invasive zebra mussels (Dreissena polymorpha) at sublethal water temperatures. Can J Fish Aquat Sci 72:1221–1229. https://doi.org/10.1139/cjfas-2015-0064
Wilbur AE, Hilbish TJ (1989) Physiological energetics of the ribbed mussel Geukensia demissa (Dillwyn) in response to increased temperature. J Exp Mar Biol Ecol 131:161–170. https://doi.org/10.1016/0022-0981(89)90005-1
Yamada SB, Navarrete SA, Needham C (1998) Predation induced changes in behavior and growth rate in three populations of the intertidal snail, Littorina sitkana (Philippi). J Exp Mar Biol Ecol 220:213–226
Zhao LQ, Schone BR, Mertz-Kraus R, Yang F (2017) Insights from sodium into the impacts of elevated pCO2 and temperature on bivalve shell formation. J Exp Mar Biol Ecol 486:148–154. https://doi.org/10.1016/j.jembe.2016.10.009
Acknowledgements
We are grateful to Kevin Stichnot and Zach Ostroff for their assistance in building the temperature-controlled experimental system used for this study and to Dr. David Jones (College of Marine Science, University of South Florida) and Dr. David Kimbro (Department of Earth and Environmental Science, Northeastern University) for their support in data analysis and experimental design. Many thanks also to Destiny Reese and Ethan Taylor for their technical support throughout the experimental period. We acknowledge Pinellas County Parks & Conservation Resources for providing permission to collect organisms from Weedon Island Preserve, Florida, USA. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (Award No. 1746051) and Florida-Georgia Louis Stokes Alliance for Minority Participation (FGLSAMP) Bridge to the Doctorate award (HRD No. 1139850). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Further funding was provided by the Alfred P. Sloan Foundation Minority Ph.D. (MPHD) Program (Grant No. 2012-3-07) and the University of South Florida, Bridge to the Doctorate Endowed Graduate Fellowship (Fund No. 266005). This work was supported, in part, by the University of South Florida Research & Innovation Internal Awards Program (Grant No. 0084477). Animal illustrations courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: A. Checa.
Reviewed by E. Cacabelos and undisclosed experts.
Rights and permissions
About this article
Cite this article
Freytes-Ortiz, I.M., Stallings, C.D. Elevated temperatures suppress inducible defenses and alter shell shape of intertidal mussel. Mar Biol 165, 113 (2018). https://doi.org/10.1007/s00227-018-3371-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3371-6