Abstract
Climate change alters multiple physical drivers that act concurrently on ecological communities. Evidence suggests widespread non-additive effects between multiple drivers; most of this evidence, however, is based on species-level responses, which is problematic because community responses to environmental change also depend on species interactions. To address this knowledge gap, this study experimentally manipulated two physical drivers and examined the responses of a predator–prey interaction. The two drivers tested are fundamental in intertidal systems: air and water temperatures. The two species were the intertidal dogwhelk, Nucella ostrina, and its barnacle prey, Balanus glandula. The objective was to test alternative hypotheses that air and water warming have additive vs. non-additive effects on the whelk-barnacle interaction. A 14-day mesocosm experiment was conducted in which animals were subjected to one of four temperature treatments: ambient (no temperature manipulation; water 12 °C, air 13 °C), warm water (15 °C), warm air (27 °C), or combined (water 15 °C, air 27 °C). There were two key findings. First, air and water warming non-additively affected interaction strength: warm water mitigated a 35% decrease in mean whelk feeding rate caused by warm air. Second, air warming had contrasting effects on individual growth rates of predator and prey. While whelk growth decreased by ~ 60% in warm air, barnacle growth increased by 47%. These findings suggest that combined air and water warming will benefit barnacle populations more than their whelk predators. This study highlights the value of integrating species performances and interactions to understand how multiple physical drivers may affect community structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change affects biodiversity and ecosystem services by altering multiple physical drivers that act concurrently on ecological communities (Halpern et al. 2007; Crain et al. 2008; Gunderson et al. 2016). Modern ecology aims to understand how ecological communities respond to multiple drivers (Koussoroplis et al. 2017) in order to improve the accuracy of biological projections (Sala et al. 2000; Harley et al. 2006). Evidence suggests that interactive effects between multiple physical drivers are widespread (Paine et al. 1998; Folt et al. 1999; Harley et al. 2006; Crain et al. 2008; Darling and Côté 2008; Gunderson et al. 2016). Most of the evidence, however, is based on species-level responses (but see Cheng et al. 2017), which is problematic because community responses to environmental change also depend on interactions between species (Gilman et al. 2010; Kordas et al. 2011; Russell et al. 2012; Harley 2013). To address this important knowledge gap, this study experimentally tested the responses of a model predator–prey interaction to the combined effect of two physical drivers (air and water temperature).
Temperature is a fundamental driver of biological processes (Gillooly et al. 2001; Brown et al. 2004; Kordas et al. 2011). In the intertidal zone, air and water temperatures are different physical drivers that affect the physiology of intertidal organisms in distinct ways (Helmuth et al. 2006b; Koussoroplis et al. 2017). For example, intertidal organisms can undergo evaporative cooling when exposed to air, preventing thermal stress (Porter and Gates 1969; Bell 1995; Helmuth 1998). Desiccation stress associated with warmer temperatures occurs only in air. Furthermore, most intertidal animals respire aerobically in one medium but anaerobically in the other, leading to different effects of temperature changes on organism oxygen and energy balances in air and water (McMahon 1988; Somero 2002; Sokolova and Pörtner 2003).
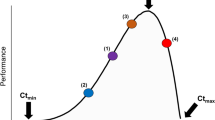
As both air and water temperatures continue to increase with climate change (IPCC 2014), intertidal organisms may face increased combined effects of these two drivers. A useful conceptual framework for considering such effects is thermal performance curves (TPCs), which describe organism performance (e.g., growth) as a function of body temperature (Huey and Stevenson 1979). Most ectotherms have unimodal TPCs (Dell et al. 2011), which means the direction of the effect of temperature change will depend on where the organism is currently on its TPC (Fig. 1). The unimodal shape of TPCs informs predictions of how intertidal organisms will potentially respond to climate warming (assuming no adaptation or acclimation), which is likely to occur faster in the air than in the water due to water’s high heat capacity (IPCC 2014). Since many intertidal species already live at the upper edges of their thermal tolerances during emersion (Tomanek and Helmuth 2002; Gilman et al. 2015), further air warming may bring them to the falling portions of their air TPCs and decrease performance (Fig. 1f). For example, studies have found that air warming decreased survival, growth, and feeding rates of intertidal whelks and sea stars, potentially due to the energetic costs of preparing for and recovering from thermal stress (Menge et al. 2002; Pincebourde et al. 2008; Yamane and Gilman 2009; Pincebourde et al. 2012; Vaughn et al. 2014). In contrast, water warming may bring intertidal organisms to the rising portions of their water TPCs and increase performance (Fig. 1a). Previous studies have found that warm water increased feeding rates of whelks and sea stars, although growth may decrease depending on the energetic costs of faster metabolic rates under warmer body temperatures (Sanford 1999, 2002a, b; Yamane and Gilman 2009; Pincebourde et al. 2012; Miller 2013; Fakhoury and Gosnell 2014). Thus, intertidal organisms may respond differently to air and water warming under future climates (e.g., Yamane and Gilman 2009; King et al. 2017).
General thermal performance curves (TPCs) of an intertidal ectotherm in air and water. Air and water TPCs likely have different shapes and locations. Performance (e.g., growth rate) can increase (a, d), decrease (c, f), or remain the same (b, e) with warming. Warming is not necessarily stressful; all nine combinations of a–f are possible even without considering potential non-additivity. Overall organism performance depends on both air and water temperatures, which may have non-additive effects
The combined effect of air and water warming will depend on the timing and intensity of each driver, which will determine whether the drivers are additive or non-additive (Gunderson et al. 2016; Koussoroplis et al. 2017). When air and water warming are additive, their combined influence will be the algebraic sum of the effect of each driver alone. When air and warming are non-additive, their combined effect will be different than the sum of the effect of each driver alone (covariance of co-limiting factors; see theory in Koussoroplis et al. 2017). Several categories of non-additive effects are known (Koussoroplis et al. 2017). Growing evidence indicates that air and water warming can have non-additive effects for intertidal predators and prey at the species level (Pincebourde et al. 2012; Schneider et al. 2012; Seabra et al. 2016), raising the question: how do intertidal predator–prey interactions respond to combined warming?
This study examined the combined effects of air and water warming on the species interaction between the dogwhelk, Nucella ostrina, and its barnacle prey, Balanus glandula. In the Northeast Pacific, B. glandula is an abundant ecosystem engineer (Barnes 2000). N. ostrina eat barnacles, altering barnacle abundance and distribution (Connell 1970; Dayton 1971). Both species respond to air or water warming (Yamane and Gilman 2009; Nishizaki and Carrington 2014; Vaughn et al. 2014), making them a prime system to examine potential non-additivity between the two drivers. The objective of this study was to test the hypothesis that air and water warming have a non-additive effect on the strength of the interaction between N. ostrina and B. glandula. A mesocosm experiment increased air and water temperatures factorially and measured the individual growth rates of N. ostrina and B. glandula, and their interaction strength (whelk feeding rate). This paper presents findings that air and water warming have a non-additive effect on N. ostrina feeding rate upon B. glandula. Contrasting responses of predator and prey growth to air warming are also presented. Along with other recent studies, this paper underscores the importance of considering species interactions in understanding how multiple physical drivers affect ecological communities.
Methods
Field temperature recordings
To understand the thermal context that whelks and barnacles may experience in nature, temperature loggers were used to measure field air and water temperatures at intertidal sites on San Juan Island, Washington, USA (Supplement Table S1). The four sites (Reuben Tarte, Colin’s Cove, Friday Harbor Labs, and Cattle Point) were protected from full oceanic conditions; see Dethier and Williams (2009) for general site descriptions. At each site, two to three iButton temperature loggers (DS1921G-F5, Maxim Integrated; ± 0.5 °C), wrapped in parafilm and embedded in marine epoxy (Z-Spar Splash Zone A-788), recorded approximate rock temperatures in the barnacle zone on vertical walls and boulder faces (+ 0.94 to + 1.91 m) hourly from late May to mid-August 2017. Temperature recordings were categorized as emersion or immersion based on predicted tidal elevations [http://tbone.biol.sc.edu/tide/ (accessed August 2017)].
Collection and preparation of organisms
Nucella ostrina were collected from Eagle Cove (48°27′N 123°01′W) on San Juan Island in early September 2016. San Juan Island experiences warm summer temperatures that coincide with daytime low-tides, making it a “hot spot” for climate warming (Helmuth et al. 2006a). Only whelks showing recent shell growth were used. To minimize the confounding effects of body size, whelks were selected within a narrow size range (mean shell length ± SD; 2.27 ± 0.07 cm, n = 48).
B. glandula on the valves of mussels (Mytilus trossulus) were collected from a pier at Jackson Beach (48°31′N 123°00′W) on San Juan Island in early September 2016. Mussels were killed and the valves separated from each other. Mussel valves with barnacles were glued to plastic disks (similar to Vaughn et al. 2014). Barnacles with operculum length > 1 mm were used (mean barnacle operculum length ± SD; 3.24 ± 0.74 mm, n = 1327).
To give replicate whelks and barnacles similar thermal histories, animals were acclimated in outdoor tanks at Friday Harbor Laboratories (FHL) for 1–2 weeks before the experiment. Barnacles were continuously submerged in running seawater (~ 12 °C). Whelks experienced the ambient tidal cycle (air ~ 13 °C, water ~ 12 °C) and were fed B. glandula from the same population as those used in the study ad libitum.
Temperature treatments
To quantify how air and water temperatures affect the whelk-barnacle interaction, animals were subjected to one of four treatments manipulating air and water temperatures factorially: ambient (no temperature manipulation; water 12 °C, air 13 °C), water warming (15 °C), air warming (27 °C), or combined (water 15 °C, air 27 °C). These temperatures were chosen based on previous studies which found that warm water increased whelk (12 °C, Sanford 2002a; 13.5 °C, Yamane and Gilman 2009) and barnacle (14 °C, Nishizaki and Carrington 2014) performance and warm air decreased whelk performance (28 °C, Yamane and Gilman 2009; Vaughn et al. 2014). The water warming treatment was expected to be on the rising portions of the TPCs for both species whereas the air warming treatment was expected to be on the falling portion for whelks.
Since physiological processes depend on body temperatures, which can differ from environmental temperatures (Helmuth et al. 2006b), biomimetic sensors were used to estimate body temperatures in each treatment. N. ostrina biomimetics were thermocouples (Type T, OMEGA Engineering, Inc.) inserted into empty N. ostrina shells flooded with clear epoxy (Gilman et al. 2015). B. glandula biomimetics were made of thermocouples embedded in ~ 1 cm diameter balls of white epoxy (modified from Gilman et al. 2015). Biomimetics placed in the centers of eight separate mesocosms estimated body temperatures for each species and treatment. A central datalogger (21× Micrologger, Campbell Scientific, Inc.) recorded biomimetic temperatures every 3 min throughout the experiment.
Mesocosm set-up
The 14-day experiment was conducted outdoors at FHL in September 2016. An opaque canopy shaded the set-up such that animals experienced indirect but naturally varying light. Clear plastic tanks (946 mL) with plastic mesh lids served as mesocosms (Supplement Figure S2). Four mesocosms were randomly assigned to each treatment. Plastic mesh divided each mesocosm into two compartments: one with barnacles and whelks and one with only barnacles. The barnacles in the two compartments were used to determine interaction strength (amount of predation) and barnacle performance (growth in the absence of predation) respectively. Barnacle density in the latter compartment was manipulated by removing neighboring individuals so that barnacles could grow without crowding. Free water movement between the compartments allowed barnacles to experience whelk presence, including potential chemical cues, without being eaten. Each mesocosm started with 10–20 barnacles without whelks and 40–50 barnacles with 3 whelks (enough for whelks to feed on for at least a week), all randomly assigned.
All mesocosms experienced the same tidal regime. Mesocosms were subject to one low tide per day, approximating the ambient tidal regime at + 1.0 m in the intertidal zone on San Juan Island during the study period (total emersion duration mean ± SD; 6.1 ± 1.8 h). Tidal regimes were simulated using separate aquarium pumps (#VS-HG16-2, VicTsing) that continuously circulated seawater between mesocosms and clear storage tanks. At low tide the aquarium pumps turned off and water drained back into the storage tanks. To maintain water quality, a trickle of new seawater continuously fed each storage tank and all water was changed every 2–3 days. Every mesocosm had its own storage tank.
During immersion, separate aquarium heaters (#GH200, Aquatop) increased water temperature in each storage tank of mesocosms assigned to the warm water treatment. Temperature controllers (True Temp #JB1235, JBJ Lighting) for each aquarium heater continuously sensed and adjusted water temperatures in storage tanks by turning aquarium heaters on or off. During emersion, separate ceramic heat lamps (#LF-15, Zoo Med) raised air temperatures in each mesocosm assigned to the warm air treatment (including nighttime low tides). Separate temperature controllers continuously sensed and adjusted air temperatures in each mesocosm by turning heat lamps on or off. Heat lamps turned on 1 h into each low tide (Pincebourde et al. 2012) to allow organisms to adjust to aerial exposure. Timers (#FD60-U5, Titan Controls) automatically switched temperature controllers for heat lamps and aquarium pumps on or off. All mesocosms had lamps hanging directly above them; lamps in mesocosms without warm emersion remained off to control for potential effects of lamp presence on the animals.
Predator and prey growth rates
To examine how whelk growth responded to temperature treatments, absolute changes in shell height, total mass, and shell lip were measured for each whelk. Change in total mass (body and shell together) was measured as the blotted wet mass of whelks immediately before and after the experiment (following Palmer 1982). The lip (apertural growing edge) of each whelk shell was painted with nail polish at the start of the experiment. Photos of each whelk were taken at the start and end of the experiment and analyzed to determine change in shell lip (degrees of new shell extending beyond the nail polish line) and change in shell height (distance from apex to edge of siphonal canal).
To examine how barnacle growth responded to temperature treatments, absolute change in operculum length was measured from photos of each barnacle taken at the start and end of the experiment. All photo analyses in this experiment were conducted using ImageJ v1.48 (Schneider et al. 2012).
Predation rate
To examine how the interaction strength between whelks and barnacles responded to temperature treatments, whelk feeding rates were measured. Whelks were given a fresh set of barnacles each week. Number of barnacles eaten in each mesocosm was counted after days 7 and 14. Barnacles were considered eaten if their opercular plates split when gently tapped and their tests were empty. Counts from days 7 and 14 were summed to calculate the total number of barnacles eaten in each mesocosm.
Per capita feeding rates were calculated by dividing the total number of barnacles eaten by the number of whelks in each mesocosm. For the mesocosms where whelks died during the experiment (three deaths total in two mesocosms), the numbers of whelks used in the denominators were calculated by summing the products of the number of whelks present and the fraction of days in which that number of whelks was present.
Statistical analysis
To test the effects of air and water warming on the whelk-barnacle interaction, linear models were developed for each response variable. All models had air warming, water warming, and their interaction as categorical fixed effects. For whelk and barnacle growth, separate linear mixed effects models with Gaussian response distribution and identity link function were used, with mesocosm as a random effect. Gaussian response distribution was chosen to accommodate negative values in response variables. Statistical significance of fixed effects in these models was determined using type III F tests with Satterthwaite approximated degrees of freedom.
For whelk feeding rate, a generalized linear model with a Gamma response distribution and inverse link function was used. Here gamma response distribution was chosen because it is useful for positive continuous values and inverse link function was chosen because it is useful for bounded data (where the upper limit of feeding rate was the number of barnacles available; Faraway 2006). Statistical significance of fixed effects in this model was determined using t tests. Switching the order of terms in the model did not change interpretation of results. Post hoc pairwise comparisons (using the same linear models) were only conducted if an interaction between fixed effects was detected. Residuals were visually inspected to check that response distributions and link functions for all models were reasonable. All tests used α = 0.05. Statistical tests were conducted using R v3.3.1 (R Core Team 2016) and the R packages lme4 (Bates et al. 2015) and lmerTest (Kuznetsova et al. 2016).
Results
Field and mesocosm temperatures
A wide range of emersion and immersion temperature combinations were observed in the field (Fig. 2). Daily median emersion temperatures were generally warmer than immersion temperatures due to summer daytime low tides. Field emersion temperatures ranged 10–35 °C, with 90 records ≥ 25 °C. Field immersion temperatures ranged 10–17 °C, with 27 records ≥ 15 °C. In the mesocosm experiment, emersion and immersion temperature combinations for all treatments were within the range of the field observations (Fig. 2). Biomimetic temperatures were influenced by cool air during nighttime low tides, but were ~ 3 °C warmer in water and ~ 11 °C warmer in air when comparing warmed vs. ambient treatments (Table 1; Fig. 2; Supplemental Figure S1).
Median emersed temperature during low tide compared to median immersed temperature during high tide in the field (crosses) and in the mesocosm experiment (points). In the field, iButton dataloggers measured summer temperatures in the barnacle zone at 4 intertidal sites (n = 742 records total). In the mesocosm experiment, biomimetic dataloggers estimated whelk and barnacle body temperatures (aggregated across species) in each treatment: ambient (circles), warm water (squares), warm air (diamonds), and combined (triangles). Crosses and grey points indicate median temperatures for a given day in the field and in the mesocosm experiment respectively. Black points indicate median temperatures for each treatment in the mesocosm experiment overall
Predator and prey growth rates
Warm air reduced whelk growth rates. Compared to whelks that did not experience any warm air, whelks that did experience warm air added 55% less shell lip (Fig. 3), grew on average 54% less in shell height, and added 77% less body mass (Supplement Figure S3). No effects of warm water nor of the interaction between warm air and warm water were detected on whelk growth rates (Table 2; Supplement Table S2).
N. ostrina (top) and B. glandula (bottom) growth after the 14 day experiment. P values listed are for each fixed effect, where “interaction” indicates the statistical interaction between warm air and warm water, based on linear mixed effects models. The statistical tests used Satterthwaite approximated denominator df = 12.20 and 141 for change in whelk shell lip and barnacle operculum length, respectively. Circles indicate individual animals, triangles indicate mesocosm means, and squares indicate treatment means. Bars indicate standard errors. Note different y-axes
In contrast to whelks, warm air increased barnacle growth rates, as did warm water (Fig. 3). Compared to barnacles that did not experience any warm air, barnacles that did experience warm air increased mean operculum length by 47% (Table 2; Supplement Table S3). Similarly, compared to barnacles that did not experience any warm water, barnacles that did experience warm water increased mean operculum length by 36%. No interaction effect was detected between warm air and warm water on barnacle growth.
Predation rate
Warm air reduced mean whelk per capita feeding rate by 35% compared to whelks that did not experience any warm air (Table 3). However, there was no effect of warm water. There was a significant interaction between warm air and warm water; warm water mitigated the negative effects of warm air on whelk feeding rates (Fig. 4). Post hoc pairwise comparisons indicated that whelk feeding rates in combined warming were similar to those in the ambient and warm water treatments (Supplement Table S4).
N. ostrina per capita feeding rate on B. glandula over the 14-day experiment. n = 4 mesocosms per treatment (triangles). P values listed are for each fixed effect, where “interaction” indicates the statistical interaction between warm air and warm water. Different letters indicate significantly different means (squares) based on pairwise comparisons. Bars indicate standard errors
Discussion
Evidence suggests that multiple physical drivers can have non-additive effects on individual species (Folt et al. 1999; Crain et al. 2008; Darling and Côté 2008; Gunderson et al. 2016); however, to better understand the community-level consequences of such effects, more information on the responses of species interactions is needed. Two findings from this study helped address this knowledge gap. First, air and water warming non-additively affected the strength of the whelk-barnacle interaction (Fig. 4). Second, air warming had contrasting effects on whelk and barnacle growth (Fig. 3). Together, these findings suggest that combined air and water warming under climate change will benefit barnacle populations more than whelks, highlighting the value of using a multi-species perspective to examine how multiple drivers affect ecological communities.
Differences among species and studies in the consequences of intertidal warming
Differences between the responses of N. ostrina and B. glandula to warming in this study (Fig. 3) emphasize that stress is species-specific (Maestre et al. 2009). The air warming treatment increased whelk and barnacle body temperatures similarly (Table 1) but was apparently only stressful to whelks. Other studies have also found that 25–28 °C body temperature in air decreased N. ostrina growth and feeding (Yamane and Gilman 2009; Vaughn et al. 2014). Lower whelk growth rates under aerial warming in this study were likely caused by decreased energy intake (due to decreased feeding), increased energetic costs of heat stress response (Somero 2002), or both. Aerial stress affected whelk feeding rates even though whelks initiate feeding during immersion (Bertness and Schneider 1976) likely because thermal stress recovery took longer than the duration of aerial exposure (average 6 h). Heat shock protein expression in marine organisms may not peak until 15 h after stress exposure (Gunderson et al. 2016) and take days to return to baseline levels (Tomanek and Sanford 2003).
Warmer water may have increased barnacle growth by increasing their cirri beating rates and food capture efficiencies (Cole 1929; Southward 1955, 1957; Nishizaki and Carrington 2014). It is also possible that warmer temperatures in the water storage tanks promoted plankton growth, leading to more food for barnacles. While it was known that warmer water (14–15 °C) can increase barnacle growth rate (Fig. 1a; Nishizaki and Carrington 2015), this may be the first study to find a similar effect of warmer air (Fig. 1d). Increases in barnacle growth under air warming in this experiment were variable and slight overall; further tests are needed to determine if this pattern is robust. Although barnacles feed only when underwater, higher body temperatures in air may have increased barnacle growth by increasing digestion efficiency (shown for other ectotherms, e.g., Harlow et al. 1976). More efficient digestion during emersion could have freed up stomach space for new food and allowed barnacles to allocate more energy to growth during immersion. Higher barnacle body temperatures in air may have also increased underwater feeding rates (shown for sea stars exposed to acute aerial warming; Pincebourde et al. 2008). In the laboratory, warm emersion temperatures up to 30 °C have been observed to stimulate B. glandula feeding rate upon submersion (Sarah Gilman pers comm).
This study did not find that water warming increased Nucella feeding rates (Fig. 4), in contrast to other studies (Sanford 2002a; Yamane and Gilman 2009; Miller 2013; Fakhoury and Gosnell 2014). Differences between studies may be due to the different species and temperatures tested. Three of the studies (Sanford 2002a; Miller 2013; Fakhoury and Gosnell 2014) tested other Nucella species that have different distributions than N. ostrina and potentially different thermal performance across water temperatures. Yamane and Gilman (2009) tested N. ostrina feeding on B. glandula from San Juan Island in late summer to early autumn, similar to this study. However, Yamane and Gilman (2009) compared underwater body temperatures of 11 vs. 13.5 °C while this study compared 12 vs. 15 °C. If 15 °C is near the peak of the N. ostrina water TPC, then it is possible that Yamane and Gilman (2009) examined a part of the TPC with steep increases in performance (Fig. 1a) while this study examined a part with little or no change in performance (Fig. 1b). Even if there was a subtle change in feeding rate near 15 °C, this study probably lacked the power to detect it (n = 4). Furthermore, body temperatures in this study (Fig. 2; Supplement Figure S1) were more variable than in Yamane and Gilman (2009), and magnitude of temperature variation can affect physiological performance and feeding rates (Pincebourde et al. 2012; Dowd et al. 2015).
Non-additivity in response to air and water warming
Two physical drivers that are temporally separated can have non-additive effects if the responses of organisms to each driver overlap (Gunderson et al. 2016). Results from this study contribute to a growing body of evidence that air and water warming can have non-additive effects on intertidal organisms (Schneider 2008; Pincebourde et al. 2012; Seabra et al. 2016). In this study, whelk feeding rate responded non-additively to sequential air and water warming: air warming strongly decreased whelk feeding rate when the water was at ambient temperatures but had no effect when the water was warmed (Fig. 4). This suggests that warmer water may have increased whelk tolerance to aerial heat stress. Generally, non-lethal warm temperatures can prime ectotherms for thermal stress by upregulating heat shock protein production (Schneider 2008 and references therein; Gunderson et al. 2016). Warmer water may have increased the standing stock of heat shock proteins in whelks, allowing them to feed at normal rates (i.e., rates similar to those in ambient conditions). It is also possible that whelks in the warm air treatment experienced more thermal stress than those in the combined treatment due to slightly lower air temperatures in the latter (< 1 °C; Table 1).
In contrast to whelk feeding rate, whelk growth responded additively to combined warming: decreased whelk growth caused by warmer air was not “rescued” by warmer water (Fig. 3). The energetic costs of dealing with aerial thermal stress (Somero 2002) apparently overwhelmed energy intake from normal feeding rates. In general, warmer body temperatures increase metabolic rates, which can increase energy demand (Gillooly et al. 2001; Brown et al. 2004; but see Marshall and McQuaid 2011). Studies on sea stars and other whelks have found that decreased feeding in cooler water may be balanced by decreased metabolic costs (Sanford 2002a, b; Pincebourde et al. 2008) and that lower metabolic rates may contribute to rescuing sea stars from aerial thermal stress (Pincebourde et al. 2012). In this study, however, there was no evidence for the increased metabolic costs of warmer water because whelks in the warm water treatment grew at similar rates as those in the ambient treatment. Fine scale TPC measurements will help clarify how changes in body temperatures can alter the relationships between N. ostrina metabolic, growth, and feeding rates.
The limitations of this study temper its conclusions and point out valuable questions for future study. First, this study used mesocosms that enforced body temperatures, focusing on the physiological rather than behavioral responses of whelks and barnacles to warming. Nucella, however, are known to behaviorally adjust their environment (Berlow and Navarrete 1997; Hayford et al. 2015); thus, the non-additive effects of air and water warming on Nucella predation observed in this study establishes a null expectation against which future studies can test the effects of combined warming in the field, where whelks can thermoregulate. Second, in this study body temperatures of animals in treatments with air warming were more variable than in other treatments because aerial warming sometimes occurred during nighttime low tides (Table 1; Supplement Figure S1), and the results should be interpreted in this context. Future studies examining temperature variation between and within aerial and aquatic environments will contribute to the growing demand for understanding how changes in temperature mean and variation affect ecological systems (Pincebourde et al. 2012; Dillon et al. 2016; Koussoroplis et al. 2017). Lastly, while this study focused on organismal responses to changes in air and water body temperatures, other factors may have impacted performance. For example, humidity and desiccation affect performance of Balanus (Barnes et al. 1963; Foster 1971) and Nucella (Stickle et al. 2017), and can alter the aerial thermal tolerances of intertidal invertebrates (as shown for limpets, Miller et al. 2009). Mesocosms in this experiment had open tops and humidity was not measured. Complementary tests are needed to tease apart the direct and interactive effects of changes in temperature, desiccation, and respiration on organismal responses to intertidal warming.
Community-level responses to multiple drivers
This study demonstrates that using a multi-species perspective to examine the consequences of multiple drivers yields useful information worth the logistical challenges. Using only whelk growth and feeding rates, it could be inferred that combined air and water warming might decrease whelk populations, potentially benefitting barnacle populations (Yamane and Gilman 2009); however, combined warming could also decrease barnacle growth. Using only barnacle growth rates, it would be unclear whether combined warming would also strengthen whelk predation upon barnacles, mitigating the direct benefits of warming on barnacle populations. Synthesizing the whelk and barnacle perspectives in this study suggests that combined warming may allow barnacles to more quickly reach size escape from whelk predation and, all else being equal, would lead to increased barnacle populations under projected climate warming. Simultaneously examining within and between species responses allowed this study to provide a more complete picture of the consequences of intertidal warming for this interacting pair. Future studies may also benefit by integrating species performances and interactions (Harley et al. 2006; Gilman et al. 2010; Kordas et al. 2011) to examine how multiple drivers affect ecological communities under global change.
References
Barnes M (2000) The use of intertidal barnacle shells. In: Oceanography and marine biology an annual review. pp 157–187
Barnes H, Finlayson DM, Piatigorsky J (1963) The effect of desiccation and anaerobic conditions on the behaviour, survival and general metabolism of three common cirripedes. J Anim Ecol 32:233. https://doi.org/10.2307/2537
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Bell EC (1995) Environmental and morphological influences on thallus temperature and desiccation of the intertidal alga Mastocarpus papillatus Kützing. J Exp Mar Biol Ecol 191:29–55. https://doi.org/10.1016/0022-0981(95)00037-R
Berlow EL, Navarrete SA (1997) Spatial and temporal variation in rocky intertidal community organization: lessons from repeating field experiments. J Exp Mar Biol Ecol 214:195–229. https://doi.org/10.1016/S0022-0981(97)00023-3
Bertness MD, Schneider DE (1976) temperature relations of puget sound thaids in reference to their intertidal distribution. Veliger 19:47–58
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Cheng BS, Komoroske LM, Grosholz ED (2017) Trophic sensitivity of invasive predator and native prey interactions: integrating environmental context and climate change. Funct Ecol 31:642–652. https://doi.org/10.1111/1365-2435.12759
Cole WH (1929) The relation between temperature and the pedal rhythm of Balanus. J Gen Physiol 12:599
Connell JH (1970) A predator-prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecol Monogr 40:49–78. https://doi.org/10.2307/1942441
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315. https://doi.org/10.1111/j.1461-0248.2008.01253.x
Darling ES, Côté IM (2008) Quantifying the evidence for ecological synergies. Ecol Lett 11:1278–1286. https://doi.org/10.1111/j.1461-0248.2008.01243.x
Dayton PK (1971) Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41:351–389. https://doi.org/10.2307/1948498
Dell AI, Pawar S, Savage VM (2011) Systematic variation in the temperature dependence of physiological and ecological traits. Proc Natl Acad Sci 108:10591–10596. https://doi.org/10.1073/pnas.1015178108
Dethier MN, Williams SL (2009) Seasonal stresses shift optimal intertidal algal habitats. Mar Biol 156:555–567. https://doi.org/10.1007/s00227-008-1107-8
Dillon ME, Woods HA, Wang G, Fey SB, Vasseur DA, Telemeco RS, Marshall K, Pincebourde S (2016) Life in the frequency domain: the biological impacts of changes in climate variability at multiple time scales. Integr Comp Biol 56:14–30. https://doi.org/10.1093/icb/icw024
Dowd WW, King FA, Denny MW (2015) Thermal variation, thermal extremes and the physiological performance of individuals. J Exp Biol 218:1956–1967. https://doi.org/10.1242/jeb.114926
Fakhoury WA, Gosnell JS (2014) Limits to local adaptation: some impacts of temperature on Nucella emarginata differ among populations, while others do not. Mar Biol 161:1943–1948. https://doi.org/10.1007/s00227-014-2459-x
Faraway JJ (2006) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Chapman & Hall/CRC, London
Folt CL, Chen CY, Moore MV, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44:864–877. https://doi.org/10.4319/lo.1999.44.3_part_2.0864
Foster BA (1971) Desiccation as a factor in the intertidal zonation of barnacles. Mar Biol 8:12–29
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251. https://doi.org/10.1126/science.1061967
Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331. https://doi.org/10.1016/j.tree.2010.03.002
Gilman S, Hayford H, Craig C, Carrington E (2015) Body temperatures of an intertidal barnacle and two whelk predators in relation to shore height, solar aspect, and microhabitat. Mar Ecol Prog Ser 536:77–88. https://doi.org/10.3354/meps11418
Gunderson AR, Armstrong EJ, Stillman JH (2016) Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annu Rev Mar Sci 8:357–378. https://doi.org/10.1146/annurev-marine-122414-033953
Halpern BS, Selkoe KA, Micheli F, Kappel CV (2007) Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv Biol 21:1301–1315. https://doi.org/10.1111/j.1523-1739.2007.00752.x
Harley CDG (2013) Linking ecomechanics and ecophysiology to interspecific interactions and community dynamics: linking ecomechanics to community ecology. Ann N Y Acad Sci. https://doi.org/10.1111/nyas.12228
Harley CDG, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241. https://doi.org/10.1111/j.1461-0248.2005.00871.x
Harlow HJ, Hillman SS, Hoffman M (1976) The effect of temperature on digestive efficiency in the herbivorous lizard, Dipsosaurus dorsalis. J Comp Physiol B 111:1–6. https://doi.org/10.1007/BF00691105
Hayford H, Gilman S, Carrington E (2015) Foraging behavior minimizes heat exposure in a complex thermal landscape. Mar Ecol Prog Ser 518:165–175. https://doi.org/10.3354/meps11053
Helmuth BST (1998) Intertidal mussel microclimates: predicting the body temperature of a sessile invertebrate. Ecol Monogr 68:51–74
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006a) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst 37:373–404
Helmuth B, Broitman BR, Blanchette CA, Gilman S, Halpin P, Harley CDG, O’Donnell MJ, Hofmann GE, Menge B, Strickland D (2006b) Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76:461–479. https://doi.org/10.1890/0012-9615(2006)076[0461:MPOTSI]2.0.CO;2
Huey RB, Stevenson RD (1979) Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool 19:357–366. https://doi.org/10.1093/icb/19.1.357
IPCC (2014) Climate change 2014: synthesis report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, p 151
King NG, Wilcockson DC, Webster R, Smale DA, Hoelters LS, Moore PJ (2017) Cumulative stress restricts niche filling potential of habitat-forming kelps in a future climate. Funct Ecol 00:1–12. https://doi.org/10.1111/1365-2435.12977
Kordas RL, Harley CDG, O’Connor MI (2011) Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol 400:218–226. https://doi.org/10.1016/j.jembe.2011.02.029
Koussoroplis A-M, Pincebourde S, Wacker A (2017) Understanding and predicting physiological performance of organisms in fluctuating and multifactorial environments. Ecol Monogr 87:178–197. https://doi.org/10.1002/ecm.1247
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) lmerTest: tests in linear mixed effects models. R package version 2.0-32. https://CRAN.R-project.org/package=lmerTest
Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009) Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol 97:199–205. https://doi.org/10.1111/j.1365-2745.2008.01476.x
Marshall DJ, McQuaid CD (2011) Warming reduces metabolic rate in marine snails: adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proc R Soc B Biol Sci 278:281–288. https://doi.org/10.1098/rspb.2010.1414
McMahon RF (1988) Respiratory response to periodic emergence in intertidal molluscs. Am Zool 28:97–114
Menge BA, Olson AM, Dahlhoff EP (2002) Environmental stress, bottom-up effects, and community dynamics: integrating molecular-physiological and ecological approaches. Integr Comp Biol 42:892–908. https://doi.org/10.1093/icb/42.4.892
Miller LP (2013) The effect of water temperature on drilling and ingestion rates of the dogwhelk Nucella lapillus feeding on Mytilus edulis mussels in the laboratory. Mar Biol 160:1489–1496. https://doi.org/10.1007/s00227-013-2202-z
Miller LP, Harley CDG, Denny MW (2009) The role of temperature and desiccation stress in limiting the local-scale distribution of the owl limpet, Lottia gigantea. Funct Ecol 23:756–767. https://doi.org/10.1111/j.1365-2435.2009.01567.x
Nishizaki MT, Carrington E (2014) The effect of water temperature and flow on respiration in barnacles: patterns of mass transfer versus kinetic limitation. J Exp Biol 217:2101–2109. https://doi.org/10.1242/jeb.101030
Nishizaki MT, Carrington E (2015) The effect of water temperature and velocity on barnacle growth: quantifying the impact of multiple environmental stressors. J Therm Biol 54:37–46. https://doi.org/10.1016/j.jtherbio.2015.02.002
Paine RT, Tegner MJ, Johnson EA (1998) Compounded perturbations yield ecological surprises. Ecosystems 1:535–545. https://doi.org/10.1007/s100219900049
Palmer AR (1982) Growth in marine gastropods. A non-destructive technique for independently measuring shell and body weight. Malacologia 23:63–74
Pincebourde S, Sanford E, Helmuth B (2008) Body temperature during low tide alters the feeding performance of a top intertidal predator. Limnol Oceanogr 53:1562–1573
Pincebourde S, Sanford E, Casas J, Helmuth B (2012) Temporal coincidence of environmental stress events modulates predation rates. Ecol Lett 15:680–688. https://doi.org/10.1111/j.1461-0248.2012.01785.x
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244. https://doi.org/10.2307/1948545
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Russell BD, Harley CDG, Wernberg T, Mieszkowska N, Widdicombe S, Hall-Spencer JM, Connell SD (2012) Predicting ecosystem shifts requires new approaches that integrate the effects of climate change across entire systems. Biol Lett 8:164–166. https://doi.org/10.1098/rsbl.2011.0779
Sala OE, Chapin FS, Iii Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. https://doi.org/10.1126/science.287.5459.1770
Sanford E (1999) Regulation of keystone predation by small changes in ocean temperature. Science 283:2095–2096. https://doi.org/10.1126/science.283.5410.2095
Sanford E (2002a) The feeding, growth, and energetics of two rocky intertidal predators (Pisaster ochraceus and Nucella canaliculata) under water temperatures simulating episodic upwelling. J Exp Mar Biol Ecol 273:199–218. https://doi.org/10.1016/S0022-0981(02)00164-8
Sanford E (2002b) Water temperature, predation, and the neglected role of physiological rate effects in rocky intertidal communities. Integr Comp Biol 42:881–891
Schneider KR (2008) Heat stress in the intertidal: comparing survival and growth of an invasive and native mussel under a variety of thermal conditions. Biol Bull 215:253–264
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Seabra R, Wethey DS, Santos AM, Gomes F, Lima FP (2016) Equatorial range limits of an intertidal ectotherm are more linked to water than air temperature. Glob Change Biol 22:3320–3331. https://doi.org/10.1111/gcb.13321
Sokolova IM, Pörtner H-O (2003) Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J Exp Biol 206:195–207. https://doi.org/10.1242/jeb.00054
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol 42:780–789. https://doi.org/10.1093/icb/42.4.780
Southward AJ (1955) On the behavior of barnacles I. The relation of cirral and other activities to temperature. J Mar Biol Assoc UK 34:403–422
Southward AJ (1957) On the behaviour of barnacles III. Further observations on the influence of temperature and age on cirral activity. J Mar Biol Assoc UK 36:323–334
Stickle WB, Carrington E, Hayford H (2017) Seasonal changes in the thermal regime and gastropod tolerance to temperature and desiccation stress in the rocky intertidal zone. J Exp Mar Biol Ecol 488:83–91. https://doi.org/10.1016/j.jembe.2016.12.006
Tomanek L, Helmuth B (2002) Physiological ecology of rocky intertidal organisms: a synergy of concepts. Integr Comp Biol 42:771–775
Tomanek L, Sanford E (2003) Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (Genus: Tegula). Biol Bull 205:276–284
Vaughn D, Turnross OR, Carrington E (2014) Sex-specific temperature dependence of foraging and growth of intertidal snails. Mar Biol 161:75–87. https://doi.org/10.1007/s00227-013-2316-3
Yamane L, Gilman S (2009) Opposite responses by an intertidal predator to increasing aquatic and aerial temperatures. Mar Ecol Prog Ser 393:27–36. https://doi.org/10.3354/meps08276
Acknowledgements
Emily Carrington and the FHL maintenance crew lent essential equipment. Comments from Jennifer Ruesink, Hilary Hayford, Lauren Buckley, Megan Dethier, Eliza Heery, Alexander Lowe, Ryan Kelly, and two reviewers improved this and an earlier version of this manuscript. Animal collectors, button pressers, and troubleshooters included Fred Ellis, Matt Schlinger, Tom Campbell, Molly Roberts, Dara Yiu, Lyda Harris, Katie Dobkowski, and Sasha Seroy. This work was supported by FHL (Richard and Megumi Strathmann Fellowship and Patricia L. Dudley Fellowship); the University of Washington Department of Biology (Biology-Friday Harbor Labs Award); and the Pacific Northwest Shell Club (Trevor Roberts Award).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animals were collected with permission of Friday Harbor Laboratories.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: F. Bulleri.
Reviewed by S. Pincebourde and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
King, W., Sebens, K.P. Non-additive effects of air and water warming on an intertidal predator–prey interaction. Mar Biol 165, 64 (2018). https://doi.org/10.1007/s00227-018-3320-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3320-4