Abstract
The most important nesting site for the green turtle, Chelonia mydas, in the Atlantic basin is Costa Rica’s Tortuguero National Park (TNP). An important portion of this population nests on Playa Norte, an adjacent beach located to the north of TNP. Using 10 years (2006–2015) of mark-recapture data, we estimated demographic parameters for green turtles nesting at Playa Norte. During the 10-year study period, 1573 nesting encounters were recorded, representing 1101 distinct individuals. Open-population models estimated an abundance of 6004 (95% CI 5037–6970), representing the contingent of adult females that nested on Playa Norte at least once during the study period. This estimate includes females that died did not use Playa Norte as nesting site or became post-reproductive after the first nesting encounter as well as nesting and non-nesting adult females in a given year. This open-population abundance estimate and related demographic rates are also assumed to be robust against inter-nesting occurrences between Playa Norte and TNP, and between Playa Norte and other unmonitored and minor beaches. The mean annual survival rate was 0.85 (95% CI 0.76–0.91) and the realized annual population growth from 2006 to 2015 was 1.0179 (95% CI 0.7984–1.2978), indicating a positive trend characterized by an average 1.79% annual increase. The results presented here concur with results from studies of nesting females at TNP and highlight the importance of Playa Norte as a nesting ground for Atlantic green turtles. In addition, our study demonstrated that reliable demographic information can be derived from nesting sites at the edge of a main nesting area if proper survey designs and analytical methods are used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, green turtles (Chelonia mydas, Linnaeus 1758) are classified as endangered by the International Union for the Conservation of Nature (Seminoff 2004). The species is circumtropically distributed (Bowen et al. 1992) and nests on beaches in over 80 countries (Hirth 1997; Seminoff 2004). Nesting beaches are vulnerable to poaching of eggs and females, and these pressures have caused significant population declines (Heppell et al. 2003; Tomillo et al. 2008). Legal and illegal harvesting at sea, marine traffic, and pollution impose additional pressures on sea turtle populations (Seminoff 2004). Although sea turtles are iteroparous, the vast migration distances between feeding and breeding grounds require high energy expenditures that prevent individuals from reproducing annually (Spotila 2004; Rivalan et al. 2005), thus limiting population recovery rates. Several initiatives have been implemented to assist the recovery of sea turtles, including conservation programs aimed at the protection of nesting females, eggs, and hatchlings (Troëng and Rankin 2005; Chaloupka et al. 2008).
Quantifying population parameters such as abundance and population growth rates are important to evaluate status and track recovery. Population estimates through mark-recapture analysis have been applied to juvenile and nesting and foraging adult sea turtles (Sasso et al. 2006; Troëng and Chaloupka 2007; Colman et al. 2015). In addition to estimation of abundance and population growth, mark-recapture studies allow estimation of survival and recruitment rates as well as capture or encounter probabilities (Pradel 1996; Thompson et al. 1998; Lettink and Armstrong 2003). However, long-term data collection is necessary to detect changes in population trajectories of long-lived wide-ranging species (King et al. 1990; Gibbons et al. 2000) such as sea turtles, which have large foraging areas and restricted breeding grounds (Chaloupka 2001; Chaloupka et al. 2008). Sea turtle populations are mostly studied in their rookeries, with most data available from tagging and monitoring of females that come ashore to nest (Chaloupka and Limpus 2001; James et al. 2005). Natal homing (also known as “philopatry”) in sea turtles is well documented (Lohmann and Lohmann 1996), and the green turtle exhibits high nest site fidelity (Carr et al. 1978; Meylan et al. 1990). Nest site fidelity is an important life history trait that has allowed the study of population parameters in green turtles because data collected in rookeries can be safely assumed to be representative of a discrete population.
Tortuguero National Park (TNP) in Costa Rica encompasses a coastline spanning 30 km and hosts one of the most important green turtle rookeries in the world (Lahanas et al. 1998; Bjorndal et al. 1999; Troëng and Rankin 2005), at least an order of magnitude larger than any other Atlantic population (Bjorndal et al. 2005a). Large colonies such as TNP are extremely rare in the Atlantic (Piniak and Eckert 2011), and globally, it is only surpassed in size by Raine Island, Australia (Chaloupka et al. 2008). The previous annual counts of green turtle’s nests at TNP have ranged from 17,402 to 37,290 (Bjorndal et al. 2005a; Troëng and Rankin 2005). TNP green turtles have, however, experienced a 15–30 fold decrease over the last 300 years (Jackson et al. 2001). The sever depletion of TNP turtles has been attributed to human exploitation prior to the 20th century (McClenachan et al. 2006).
Although the majority of the population nests inside TNP, an important portion also uses the adjacent beach to the north, Playa Norte, which can be considered a spillover location at the edge of TNP. Leatherbacks (Dermochelys coriacea, Vandelli 1761), hawksbills (Eretmochelys imbricata, Linnaeus 1766), and loggerheads (Caretta caretta, Linneaus 1758) also nest on this beach in varying numbers; however, green turtles are by far the most abundant (COTERC, unpublished data). The green turtle nesting population at TNP and Playa Norte forms a distinct reproductive stock (Encalada et al. 1996; Bjorndal et al. 2005b; Shamblin et al. 2012) that forages mostly in the Miskitos Coast (Nicaragua), although they can be found throughout the Caribbean Sea (Carr et al. 1978; Bass et al. 1998; Troëng et al. 2005). In this study, the ensemble of TNP and Playa Norte nesting grounds is henceforth referred to as the “Tortuguero” population. It is known that Tortuguero green turtles also nest in neighboring areas on smaller and unmonitored beaches. This aggregation into a single discrete population is supported by the strong homing behavior (Peare and Parker 1996; Tiwari et al. 2005) and the genetic uniqueness (Bjorndal et al. 2005b) of Tortuguero’s green turtles. Spatial nesting of Tortuguero green turtles has been estimated to be within 10 km of an individual’s previous nest site (Tiwari et al. 2005).
The Canadian Organization for Tropical Education and Rainforest Conservation (COTERC; http://www.coterc.org/) has monitored nesting events and collected mark-capture data for green turtles nesting on Playa Norte since 2006 with the aim of helping the scientific and conservation community to compile information on the biological and population parameters of green turtles. The availability of this data is a great opportunity to evaluate the demographic information that can be derived from a spillover site and compare it with information derived from the main nesting area (TNP). This would have important implications for animal populations that use remote or inaccessible areas to scientists or that have large populations or use large areas, and therefore, mark-recapture studies necessarily need to target specific, accessible portions of the population, and/or habitat. The main goals of this study are: (1) to estimate green turtle demographic parameters based on 2006–2015 mark-recaptured data of females nesting in Playa Norte; and (2) to evaluate whether open-population mark-recapture models are robust to data collected outside the main sampling area by comparing demographic parameters from Playa Norte with those produced by TNP studies.
Materials and methods
Study area
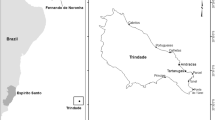
Playa Norte belongs to the “Barra del Colorado” Wildlife Refuge. This protected area borders TNP to the north and both beaches are separated by the Laguna Tortuguero river mouth (Fig. 1). Data collection on Playa Norte took place along a 5 km transect (Start: 10°35′38.4216″N–83°31′31.2234″W; End: 10°38′2.8536″N–83°32′29.8674″W), divided into 25 sections of 200 m each. The beach is closed to the public from March 1st to October 31st and the only permitted access is with a research permit. Playa Norte had been subject to high poaching pressure in the past, but since the start of patrols for the data collection used in this study, poaching of nesting adults and eggs has been significantly reduced. However, losses of nests to domestic dogs remain a problem. The beach itself is highly dynamic suffering increasingly heavy coastal erosion resulting in a number of nests being lost each year (COTERC, unpublished data).
Study area. Points A and B represent the start and end point of the nest monitoring transect, respectively. The study area, Playa Norte, is part of the Barra del Colorado Wildlife Refuge and it is located north of Tortuguero National Park (TNP). These two nesting beaches are separated by the Laguna Tortuguero river mouth and are located on the central Caribbean coast of Costa Rica
Surveys
Beach patrols were conducted every night (20:00–04:00) from 1 March to 31 October every year of the study (2006–2015). Nesting occurs in other months, but drops off dramatically outside this period. Each turtle encountered was checked for identification tags or new or replacement tags were implanted. Individual tags (Inconel #681; National Band and Tag Company, Newport, Kentucky, USA) were implanted proximal to the first scale in the front flippers as per Balazs (1999). Both flippers were tagged to reduce the risk of complete tag loss. The tags bore a return address which enabled the collection of some mortality data from encounters outside of Playa Norte. Any evidence of the previous tagging in the form of old tag holes or notches was recorded at the time of encounter and turtles with double-tag loss (i.e., turtles with no tags but showing evidence of old tag holes or notches) were re-tagged. In the single-tag loss cases, tags were replaced on the flipper with the notch or hole keeping track of the original tag record. An individual encounter history (IEH) was created for each turtle based on the tag information (see below). The monitoring protocol had little variation throughout the duration of the study and the survey effort remained nearly constant over time.
Analyses
Most modern mark-recapture analytical techniques and software require mark-recapture data in the form of IEHs. An IEH is a contiguous series of binary variables (0 or 1), each of which indicates encounter attributes of a unique individual; for example, whether or not it was encountered on a particular occasion, its state when it was encountered (live or dead), where it was encountered, and so forth. For each individual encountered, including for losses on capture (Schwarz et al. 1993) that represent individuals not released after an encounter either because they are dead or have been removed from the system, there is an IEH representing the sequence of encounter events. Each one of these events has a corresponding probability, and therefore, an explicit probability can be calculated for each IEH. Since sampling occasions were scaled by year, multiple encounters of a distinct individual in a given year were still considered a single encounter.
Modern Jolly–Seber estimators compute both gross (e.g., all green turtles accessing the system during the study) and net (gross minus permanent emigration and/or inter-nesting mortality) abundance (e.g., Schwarz and Arnason 1996). POPAN is a generalization of the Jolly–Seber model using a multinomial distribution from a super-population (i.e., the total number of animals that are ever available for capture or encounter in the population of interest over the course of the study), as described by Schwarz and Arnason (1996). We used 2006–2015 green turtle IEHs to implement an open-population POPAN modelling approach (Schwarz and Arnason 1996) to estimate the abundance of the contingent of adult females that uses Playa Norte as a nesting ground. POPAN models also allowed the estimation of survival and capture/encounter rates. POPAN models are defined by their explicit assumptions about the main probability components of the likelihood algorithm: capture probability (p; or encounter herein, since green turtles are not retained), survival probability (s), and recruitment (b; probability of new females recruiting into the adult population that use Playa Norte as nesting ground in a given year). Hence, encounter, survival, and recruitment rates can change during the study. The full POPAN model for a case with one group (adult females) and temporal (annual) stratification is herein represented as {p(t), s(t), b(t)}, indicating that encounter, survival, and recruitment probabilities vary across sampling occasions (t). Goodness-of-fit testing (see below) is based on the full model. However, abundance is not estimable at the first and last sampling occasions nor can recruitment be estimated for the first and last sampling interval because of confounding and non-identifiability of parameters, unless a constraint on pt is imposed (Cooch and White 2013). Two types of constraints were imposed: (i) a time-invariant p or (ii) fixing the value of p at 1.00 for the first (a) and last (z) sampling occasions (Vélez-Espino et al. 2016). Hence, the most general models with the capacity to estimate abundance without confounding and non-identifiability problems would be {p(*), s(t), b(t)} or {p(t; a & z = 1), s(t), b(t)}.
Mark-recapture analyses of nesting females followed the model selection approach recommended by Lebreton et al. (1992): (1) start from a global model compatible with species biology and assess with goodness-of-fit tests; (2) select the most parsimonious model (e.g., using Akaike’s Information Criterion AIC) to limit the number of formal tests; (3) compare the most parsimonious model with neighboring models using likelihood ratio tests (LRT); and (4) obtain maximum likelihood estimation (MLE) of model parameters with estimates of precision. We applied this approach to POPAN models using the program MARK (White and Burnham 1999; http://www.phidot.org/software/mark). MARK was also used for goodness-of-fit testing based on the program RELEASE (Burnham et al. 1987), AIC model selection, and derivation of MLEs. Goodness-of-fit testing was based on the more general, and fully time-dependent, open-population model (e.g., a POPAN’s full model). The information provided by RELEASE allowed the identification of the potential existence of encounter or survival heterogeneity as well as overdispersion, hence informing the model construction and selection processes.
Overdispersion, defined as a ratio between the residual deviance and the degrees of freedom (ĉ, a.k.a. variance inflation factor) significantly larger than 1.0, magnifies model parameter error rates, and produces skewed confidence intervals (McCullagh and Nelder 1983). Overdispersion was identified from the RELEASE results and addressed through ĉ adjustment within MARK. This adjustment changes the standard errors for each of the parameters in each of the candidate models as well as the AIC weights because these are originally calculated using the default ĉ value of 1.0 (Cooch and White 2013).
Population growth rate during 2006–2015 was estimated with Pradel models (Pradel 1996), which are a special (conditional) case of the more general Jolly–Seber model and, therefore, use the same IEHs prepared for POPAN, which can also be fitted using MARK. A similar model selection process was applied to Pradel models characterized by three structural parameters: p, s, and λ, where p and s are similar to POPAN’s and λ is the population growth rate parameter. As in POPAN, several parameters are inestimable under the fully time-dependent model {p(t), s(t), λ(t)} due to statistical non-identifiability. The same constraints used in POPAN models to deal with parameter confounding and non-identifiability (i.e., time-invariant p, or p[a] = 1.00 = P[z]) were included in the Pradel models to produce robust estimates of λ.
Results
Biological data
Over the 10-year survey period, 1573 nesting encounters from 1101 individual females were recorded. The occurrence of tag loss in both flippers took place during the study with 65 individuals (5.9% of total IEHs); these individuals were re-tagged. The number of nesting green turtles per year ranged from 48 in 2011 to 284 in 2013. Although nesting encounters occurred from March through October, the vast majority took place during the summer months (July–September) with the highest number of turtle encounters occurring in August (Fig. 2), except in 2014 and 2015 when more turtles were encountered in September (77 and 60, respectively). However, there was no significant difference in the number of nesting turtles encountered between August and September across the study period (t = 1.37; df = 336.178; p = 0.17).
Abundance
Twelve POPAN models were explored and evaluated via AIC (Table 1). RELEASE showed evidence for capture heterogeneity and overdispersion, and it was necessary to adjust ĉ from 1.0 to 3.2. Model {p(t), s(*), b(t)} ranked highest, followed by models {p(t), s(t), b(t)} and {p(*), s(*), b(t)}. However, the two models with the highest ranks include time-variant p parameters that are known to produce unidentifiable parameters at the first and last sampling occasions and thus were removed from the model selection exercise. Therefore, model {p(*), s(*), b(t)} was selected as the best biological model. LRT showed that the fit of this model to the data was significantly better (p < 0.001) than that of the closest neighbour model {p(*), s(t), b(t)}.
Net abundance of adult females nesting in Playa Norte was estimated as 5706 (95% CI 4096–7316) during the study period. This estimate does not include those adult females that died or permanently emigrated (e.g., were no longer reproductive or nested on TNP after the last encounter) during the study. Gross abundance, which includes deaths and permanent emigrants, was estimated as 6004 (95% CI 5037–6970). Annual survival during the study period averaged 0.85 (95% CI 0.76–0.91), whereas the probability of encountering a distinct adult female in a given year was 0.05 (95% CI 0.034–0.071). Since these encounter probabilities are conditional on survival, these can be properly defined as detection probabilities. Recruitment probabilities (i.e., the probability of new females recruiting into the adult portion of the nesting colony) during the study period averaged 0.069 (range 0.00–0.38), with the largest annual recruitment occurring during the 2011–2012 annual interval. Additional detail on population parameters derived from the best biological model is shown in Table 2.
Population growth
Twelve Pradel models were explored and evaluated via AIC (Table 3). Models {p(t), s(*), λ(t)} and full model {p(t), s(t), λ(t)} ranked highest but produced unreasonable output due to the non-identifiability of some parameters and thus were removed from the model selection exercise. LRT showed that the fit of the best model {p(*), s(*), λ(t)} to the data was significantly better (p < 0.001) than that of the closest neighbour models. The best model produced variable estimates of realized population growth between the annual intervals in the study period (Fig. 3). These changes in realized population growth between annual intervals represent not only the balance between deaths and new recruits into the adult female contingent but also the balance between “emigration” and “immigration” to and from neighbour nesting beaches (i.e., TNP and other minor nesting beaches part of the same population). Most likely, new recruits are TNP females nest clutching sporadically on Playa Norte. Thus, permanent emigration is probably quite common due to the spillover nature of Playa Norte’s contingent. The geometric mean of realized annual population growth from 2006 to 2015 was 1.0179 (95% CI 0.7984–1.2978), indicating a positive trend characterized by an average 1.79% annual increase.
Discussion
Population parameters
The number of nesting turtles found over the study period (48–284 per year) shows the typical fluctuations of nesting behavior in green turtles (Limpus 1996; Bjorndal et al. 1999, 2005a). Within season demographic parameters could not be estimated, because our mark-recapture data set was too sparse (from the 1101 unique individuals in our study, 68% were encountered only once, 23% in two different years, 8% in three different years, and 1% during 4 years). The fact that the majority of females at Playa Norte are seen only once reflects the spillover nature of this site with most nesting activities surely occurring within TNP and Playa Norte getting a small percentage of the total number of beach landings. Numerous green turtle females bearing tags applied by the Sea Turtle Conservancy, while nesting on TNP were observed during our study. Nonetheless, the availability of data from a long-term study of a smaller segment of a population (such as Playa Norte to Tortuguero) has the potential to contribute valuable information and produce robust assessments of sea turtle biological and population parameters to better understand sea turtle population dynamics. However, these studies face several challenges, including tag loss, variable female reproductive output, and incomplete beach coverage (Rivalan et al. 2005; Hatase and Tsukamoto 2008; Tucker 2010). Variation in remigration intervals alongside inconsistent clutch frequencies (Bjorndal et al. 1999) contributes to natural population fluctuations (Troëng and Rankin 2005). Due in part to this natural annual variation in numbers of nesting females, long-term monitoring projects are especially important to assess sea turtle population sizes and trends (Schroeder and Murphy 1999; Troëng and Rankin 2005).
Tagging in both flippers significantly reduced the complete loss of data for an individual turtle in the present study. Given the small percentage of re-tagged turtles in both flippers at Playa Norte (5.9%) and that the probability of encountering an individual in a given year was 0.05, the probability of duplicating an IEH as a result of re-tagging was extremely low (0.3%), but nonetheless with the potential to overestimate abundance. Bjorndal et al. (1996) calculated the percentage of Inconel tag losses over 3 years at TNP as 14%. Campbell and Lagueux (2005) reported single flipper tag loss in one season at TNP to be 3.1% in 2000 and 3.3% in 2001. If the proportion of re-tagged turtles in both flippers were much higher, assigning “tags at risk” in a random process could have been incorporated to account for their effect on population parameter estimation (Vélez-Espino et al. 2016). Estimation problems due to tag loss such as parameter biases and reduction in precision (Arnason and Mills 1981; Pollock et al. 1990) could be exacerbated in long-lived species in the presence of high rates of tag loss (Nelson et al. 1980; Campbell and Lagueux 2005). To address estimation uncertainty due to lost tags, tag losses should be minimized as much as possible with good-quality tags and tag-application techniques.
Mark-recapture studies of sea turtles have used similar methodologies and analytical tools (e.g., Pradel models, RELEASE, MARK, etc.) to evaluate abundance, survival, and population growth of juvenile and adult sea turtles (Sasso et al. 2006; Troëng and Chaloupka 2007). The mean annual survival probability found in our study, 0.85, is identical to that reported by Troëng and Chaloupka (2007) but with wider 95% confidence intervals (0.76–0.91 vs. 0.83–0.87), probably due to a noticeably smaller sample size. These authors used similar analytical methods, basing their estimates on an open robust model design. Moreover, our estimate of survival was slightly higher than that reported by Campbell and Lagueux (2005) (0.82; 95% CI 0.73–0.89). According to Lagueux et al. (2014), Tortuguero’s green turtle population has a lower survival probability than other populations due to the strong fishing pressure in its foraging grounds. The Nicaraguan coast sustains the second largest legal turtle fishery in the world (Humber et al. 2014) in which an average of 8169 green turtles per year were fished between 1991 and 2011 (Lagueux et al. 2014), including primarily large juveniles and adults (Troëng and Chaloupka 2007). Failure of fishers to return tags or delays in tag returns generally result in the underreporting of turtle mortality that would overestimate survival probabilities (Campbell and Lagueux 2005). Both these situations may be resolved with improved reporting of captures by Nicaraguan and other Caribbean fishers. However, this would require greater effort on the part of un-invested third parties (Troëng and Chaloupka 2007).
The gross abundance of Playa Norte’s nesting females in our study, 6004 (95% CI 5037–6970), needs to be interpreted as Tortuguero’s portion of adult females that utilized Playa Norte as a nesting ground during the study period, subject to inter-nesting occurrences between Playa Norte and TNP, and between Playa Norte and other unmonitored and minor beaches. This abundance number is substantially smaller than the abundance of the TNP females estimated by Troëng and Rankin (2005) between 1999 and 2003 (17,402–37,290) and the 26,535 reported by 2001 in Seminoff (2004). This is an expected difference since TNP’s nesting area is approximately six times larger than Playa Norte. Ideally, coordination with other monitoring organizations in possession of mark-recapture data would be important to yield better and comprehensive estimates of population size and trends for the Tortuguero green turtles.
Population trends
Our study identified a positive abundance trend in green turtle adult females at Playa Norte (1.79% annual growth rate) with high inter-annual fluctuations. This is consistent with other studies of Tortuguero’s population (Bjorndal et al. 1999, 2005a; Troëng and Rankin 2005; Troëng and Chaloupka 2007) and reflects the fact that Playa Norte’s positive growth rate is a direct result of the growing Tortuguero population and the resulting increased spillover from the main TNP nesting area. The IUCN marine turtle survival group report found that, alongside other Atlantic populations, TNP nesting events had more than doubled from the previous 20–30 years (Broderick et al. 2006). Chaloupka et al. (2008) reported high inter-annual fluctuations with an average nesting population increase of 4.9% in the TNP rookery (1971–2003). It is important to note, however, that whereas studies based on nest counts (e.g., Troëng and Rankin 2005; Chaloupka et al. 2008) only reflect realized nesting occurrences in a given year, abundance and population growth estimates from mark-recapture studies of nesting females (like the present study) attempt to reflect the population dynamics of adult females using a probabilistic framework and accounting for imperfect information (Pfaller et al. 2013). Population estimates from mark-recapture studies are meant to represent nesting and non-nesting adult females in a given year as well as females that die or become post-reproductive (i.e., reproductive senescence; Broderick et al. 2003) after the first nesting encounter. Despite these differences, positive trends in abundance of reproductive females identified in our study are concomitant with nesting increases reported by other studies (e.g., Troëng and Rankin 2005; Chaloupka et al. 2008).
Several factors have contributed to recovery of sea turtles, including increasing conservation efforts (Chaloupka et al. 2008) such as beach patrols (aiming to protect females, eggs, and hatchlings), hatcheries, head start programs, and environmental education (Ehrenfeld 1995; García et al. 2003). Although beach conservation efforts seem to be successful at helping population growth, factors affecting mortality at other life stages need to be considered (Frazer 1992; Spotila et al. 2000; García et al. 2003). Turtle excluder devices (TEDs) have been implemented in shrimp trawl fisheries to protect turtles foraging at sea (Crowder et al. 1994). Recovery has been more obvious for sea turtle populations exhibiting short-distance migration patterns (Eckert and Abreu-Grobois 2001; Troëng and Rankin 2005; Broderick et al. 2006), because energy otherwise expended on migration can be reallocated to reproduction (Bjorndal 1995; Troëng et al. 2005). The Tortuguero population has one of the shortest remigration intervals of green turtles, with the majority of females nesting every 2 or 3 years (Carr and Carr 1970; Troëng and Chaloupka 2007). While TNP and Playa Norte individuals disperse to feeding grounds across the Caribbean, an estimated 80–82% of Tortuguero green turtles migrate the relatively short 830 km distance to feeding grounds along the Miskitos Coast of Nicaragua (Bjorndal 1995; Troëng et al. 2005). Variable remigration intervals are considered to be a driver of inter-annual variation in nesting events and are closely associated with environmental influences (Hays 2000). For instance, a positive correlation between sea surface temperatures and remigration intervals has been identified for the Tortuguero population (Solow et al. 2002).
Other factors such as density-dependent compensatory mechanisms (Rose et al. 2001; Vélez-Espino and Koops 2012) could also play an important role influencing positively population growth rates in Tortuguero green turtles. Density-dependent reproductive compensation from a heavily depleted population following years of human exploitation has been invoked as a potential factor contributing to positive trends in population growth (Troëng and Rankin 2005; Troëng and Chaloupka 2007; Chaloupka et al. 2008).
The importance of Playa Norte
This study showed that thousands of green turtles use Playa Norte as a nesting ground and hence highlights the importance of this beach in the broader conservation strategy for Atlantic green turtles. Playa Norte’s number of females and increasing abundance trend are positive indicators of both Tortuguero’s and the global status of the species (see also Troëng and Rankin 2005). A positive abundance trend in Tortuguero nesting females is particularly important given green turtles are globally listed as endangered mostly due to noticeable declining trends caused by overexploitation and fishery bycatch (Seminoff 2004). Currently, Playa Norte experiences a lower level of government enforcement and protection from poaching due to its remoteness. Despite the patrol teams being active during the 10 years of this study, several cases of nesting turtles killed and nests excavated due to poaching were observed. Nevertheless, conservation efforts that focus on rookery protection, such as the COTERC patrols on Playa Norte, no doubt contribute to improved population performance and their value should not be underestimated (see García et al. 2003; Dutton et al. 2005). The implementation of patrols every day of the study minimized the undesirable influence of poaching on population estimates via additional non-recorded mortalities and potential loss of tagged individuals.
Final remarks
The Tortuguero nesting population is increasing, as evidenced by our research and that of Bjorndal et al. (1999) and Troëng and Rankin (2005). However, while we report positive news regarding adult female abundance increases, we suggest exercising caution when applying this optimism to the population as a whole. Nesting populations are not representative of other life stages and omit information on male turtles (Crowder et al., 1994). It cannot be assumed that all populations of turtle have a 50:50 sex ratio. For instance, temperature-dependent sex determination often skews sea turtle population sex ratios (Standora and Spotila 1985). Positive population growth rates in nesting females are not necessarily evidence of overall population increases. Positive rates may merely echo favourable environmental conditions that increase reproduction whilst disguising overall population declines (Chaloupka 2001). Confounding the problem is the challenge of assessing long-lived, slow-maturing species and the interval between implementing a conservation management strategy and the visibility of its impact on the population (Bjorndal et al. 1999). In sea turtles, problems or improvements at the juvenile level may not be detected until decades later when nesting females are encountered. Some have suggested that changes will not be apparent until 20–25 years after conservation strategies have been implemented (Eckert 1995; Troëng and Rankin 2005; Bjorndal et al. 2005a). Bjorndal et al. (2005a) found a significant disparity between the increasing Tortuguero nesting population and the declining foraging populations elsewhere in the Caribbean. In Nicaragua, the main feeding ground for the Tortuguero population, this discrepancy is most pronounced, with capture rates at the foraging ground showing a steady decrease (Lagueux et al. 2014). Even if green turtles in the Caribbean are truly recovering, the population still remains far from the historical abundance estimates which likely reached millions during pre-Columbian times (Jackson 1997).
We estimated demographic parameters based on 10 years of consistent monitoring efforts and compilation of mark-recapture data from green turtle females nesting at Playa Norte. Our analyses provided information on abundance and growth rate as well as survival, recruitment, and encounter probabilities. Information derived from this study is meant to enhance the state of knowledge about green turtle population dynamics, therefore, assisting regional management and organizations with planning effective green turtle conservation strategies. Our study demonstrated that reliable demographic information can be derived from nesting sites at the edge of a main nesting area if proper survey designs and analytical methods are used. Nevertheless, as Playa Norte is an important spillover site for the Tortuguero population, inter-agency exchange of information on tagging and other biological data as well as initiatives to conduct coordinated surveys and mark-recapture studies in TNP and Playa Norte would no doubt improve population estimates and produce a better understanding of Tortuguero’s population dynamics.
References
Arnason AN, Mills KH (1981) Bias and loss of precision due to tag loss in Jolly–Seber estimates for mark-recapture experiments. Can J Fish Aquat Sci 38:1077–1095
Balazs GH (1999) Factors to consider in the tagging of sea turtles. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication 4, Gland, pp 101–109
Bass AL, Lagueux CJ, Bowen BW (1998) Origin of green turtles, Chelonia mydas, at ‘‘sleeping rocks’’ off the northeast coast of Nicaragua. Copeia 1998:1064–1069
Bjorndal KA (1995) The consequences of herbivory for the life history pattern of the Caribbean green turtle, Chelonia mydas. In: Bjorndal KA (ed) Biology and conservation of sea turtles, revised edition. Smithsonian Institution, Washington, pp 111–116
Bjorndal KA, Bolten AB, Lagueux CJ, Chaves A (1996) Probability of tag loss in green turtles nesting at Tortuguero, Costa Rica. J Herpetol 30:566–571
Bjorndal KA, Wetherall JA, Bolten AB, Mortimer JA (1999) Twenty-six years of green turtle nesting at Tortuguero, Costa Rica: an encouraging trend. Conserv Biol 13:126–134
Bjorndal KA, Bolten AB, Chaloupka M (2005a) Evaluating trends in abundance of immature green turtles, Chelonia mydas, in the greater Caribbean. Ecol Appl 15:304–314
Bjorndal KA, Bolten AB, Troëng S (2005b) Population structure and genetic diversity in green turtles nesting at Tortuguero, Costa Rica, based on mitochondrial DNA control region sequences. Mare Biol 147:1449–1457
Bowen BW, Meylan AB, Ross JP, Limpus CJ, Balazs GH, Avise JC (1992) Global population structure and natural history of the green turtle (C. mydas) in terms of matriarchal phylogeny. Evolution 46:865–881
Broderick AC, Glen F, Godley BJ, Hays GC (2003) Variation in reproductive output of marine turtles. J Exp Mar Biol Ecol 288:95–109
Broderick AC, Frauenstein R, Glen F, Hays GC, Jackson AL, Pelembe T, Ruxton GD, Godley BJ (2006) Are green turtles globally endangered? Glob Ecol Biogeogr 15:21–26
Burnham KP, Anderson DR, White GC, Brownie C, Pollock KH (1987) Design and analysis methods for fish survival experiments based on release-capture. Am Fish Soc, Monog, p 5
Campbell CL, Lagueux CJ (2005) Survival probability estimates for large juvenile and adult green turtles (Chelonia mydas) exposed to an artisanal marine turtle fishery in the western Caribbean. Herpetologica 61:91–103
Carr A, Carr MH (1970) Modulated reproductive periodicity in Chelonia. Ecology 51:335–337
Carr A, Carr MH, Meylan AB (1978) The ecology and migrations of sea turtles, 7. The west Caribbean green turtle colony. Bull Am Mus Nat Hist 162:1–46
Chaloupka M (2001) Historical trends, seasonality and spatial synchrony in green sea turtle egg production. Biol Conserv 101:263–279
Chaloupka M, Limpus CJ (2001) Trends in the abundance of sea turtles resident in Southern Great Barrier Reef waters. Biol Conserv 102:235–249
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17:297–304
Colman LP, Patrício ARC, McGowan A, Santos AJB, Marcovaldi MA, Bellini C, Godley BJ (2015) Long-term growth and survival dynamics of green turtles (Chelonia mydas) at an isolated tropical archipelago in Brazil. Mar Biol 162:111–122
Cooch EG, White GC (2013) Program Mark: a gentle introduction. Edition12. http://www.phidot.org/software/mark/docs/book/
Crowder LB, Crouse DT, Heppel SS, Martin TH (1994) Predicting the impact of turtle excluder devices on loggerhead sea turtle populations. Ecol Appl 4:437–445
Dutton DL, Dutton PH, Chaloupka M, Boulon R (2005) Increase of a Caribbean leatherback Dermochelys coriacea nesting population linked to long-term nest protection. Biol Conserv 126:186–194
Eckert KL (1995) Draft general guidelines and criteria for management of threatened and endangered marine turtles in the Wider Caribbean Region. UNEP (OCA)/CAR WG.19/INF.7. In: Prepared by WIDECAST and adopted by the third meeting of the interim scientific and technical advisory committee to the SPAW protocol, Kingston, 11–13 October 1995. United Nations Environment Programme, Kingston
Eckert KL, Abreu-Grobois FA (2001) Proceedings of the regional meeting ‘Marine turtle conservation in the Wider Caribbean Region: a dialogue for effective regional management’, Santo Domingo, Dominican Republic, 16–18 November 1999. WIDECAST, IUCNMTSG, WWF and UNEP-CEP, Kingston
Ehrenfeld D (1995) Options and limitations in the conservation of sea turtles. In: Bjorndal KA (ed) Biology and conservation of sea turtles, revised edn. Smithsonian Institution Press, Washington, DC, pp 457–463
Encalada SE, Lahanas PN, Bjorndal KA, Bolten AB, Miyamoto MM, Bowen BW (1996) Phylogeography and population structure of the Atlantic and Mediterranean green turtle Chelonia mydas: a mitochondrial DNA control region sequence assessment. Mol Ecol 5:473–483
Frazer NB (1992) Sea-turtle conservation and halfway technology. Conserv Biol 2:179–184
García A, Ceballos G, Adaya R (2003) Intensive beach management as an improved sea turtle conservation strategy in Mexico. Biol Conserv 111:253–261
Gibbons JW, Scott DE, Ryan TJ, Bulhmann KA, Tuberville TD, Metts BS, Greene JL, Mills T, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, déja vu amphibians. Bioscience 50:653–666
Hatase H, Tsukamoto K (2008) Smaller longer, larger shorter: energy budget calculation explain intrapopulation variation in remigrant intervals for loggerhead sea turtles (Caretta caretta). Can J Zool 86:595–600
Hays GC (2000) The implications of variable remigration intervals for the assessment of population size in marine turtles. J Theor Biol 206:221–227
Heppell SS, Snover ML, Crowder LB (2003) Sea turtle population ecology. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles, vol II. CRC Press, Boca Raton, pp 275–306
Hirth HF (1997) Synopsis of the biological data on the green turtle Chelonia mydas (Linnaeus 1758). U.S. Fish and Wildlife Service, Biological Report 97:i-v, 1–120
Humber F, Godley BJ, Broderick AC (2014) So excellent a fishe: a global overview of legal marine turtle fisheries. Divers Distrib 2:579–590
Jackson JBC (1997) Reefs since Columbus. Coral Reefs 16(Suppl):S23–S32
Jackson JB, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CM, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
James MC, Ottensmeyer CA, Myers RA (2005) Identification of high-use habitat and threats to leatherback sea turtles in northern waters: new directions for conservation. Ecol Lett 8:195–201
King FW, Espinal M, Cerrato C (1990) Distribution and status of the crocodilians of Honduras. Proceedings Working Meeting Crocodile Specialist Group, Crocodiles. IUCN, Gland, pp 313–354
Lagueux CJ, Campbell CL, Strindberg S (2014) Artisanal green turtle, Chelonia mydas, fishery of Caribbean Nicaragua: I. Catch rates and trends, 1991–2011. PLoS One 9(4):e94667. https://doi.org/10.1371/journal.pone.0094667
Lahanas PN, Bjorndal KA, Bolten AB, Encalada SE, Miyamoto MM, Valverde RA, Bowen BW (1998) Genetic composition of a green turtle (Chelonia mydas) feeding ground population: evidence for multiple origins. Mar Biol 130:345–352
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lettink M, Armstrong DP (2003) An introduction to using mark-recapture analysis for monitoring threatened species. Dep Conserv Tech Ser 28A:5–32
Limpus CJ (1996) Myths, reality, and limitations of green turtle census data. In: Keinath JA, Barnard DA, Musick JA, Bell BA (eds) Proceedings of the fifteenth annual workshop on sea turtle biology and conservation, NOAA Technical Memorandum NMFS-SEFSC-387, pp 170–173
Lohmann KJ, Lohmann CMF (1996) Orientation and open-sea navigation in sea turtles. J Exp Biol 199:73–81
McClenachan L, Jackson JBC, Newman MJH (2006) Conservation implications of historic sea turtle nesting beach loss. Front Ecol Evol 4:290–296
McCullagh P, Nelder JA (1983) Generalized linear models. Chapman and Hall, New York
Meylan AB, Bowen BW, Avise JC (1990) A genetic test of the natal homing versus social facilitation models for green turtle migration. Science 248:724–728
Nelson LJ, Anderson DR, Burnham KP (1980) The effect of band loss on estimates of annual survival. J Field Ornithol 51:30–38
Peare T, Parker PG (1996) Local genetic structure within rookeries of Chelonia mydas (the green turtle). Heredity 77:619–628
Pfaller JB, Bjorndal KA, Chaloupka M, Williams KL, Frick MG, Bolten AB (2013) Accounting for imperfect detection is critical for inferring marine turtle nesting population trends. PLoS One 8(4):e62326. https://doi.org/10.1371/journal.pone.0062326
Piniak WED, Eckert KL (2011) Sea turtle nesting habitat in the Wider Caribbean Region. Endanger Species Res 15:129–141
Pollock KH, Nichols JD, Brownie C, Hines JE (1990) Statistical inference for capture–recapture experiments. Wildlife Monog 107
Pradel R (1996) Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 52:703–709
Rivalan P, Prévot-Julliard A, Pradel R, Jacquemin B, Girondot M (2005) Trade-off between current reproductive effort and delay to next reproduction in the leatherback sea turtle. Oecologia 145:564–574
Rose KA, Cowan JH, Winemiller KO, Myers RA, Hilborn R (2001) Compensatory density dependence in fish populations: importance, controversy, understanding and prognosis. Fish Fish 2:293–327
Sasso CR, Braun-McNeill J, Avens L, Epperly SP (2006) Effects of transients on estimating survival and population growth in juvenile loggerhead turtles. Mar Ecol Prog Ser 324:287–292
Schroeder B, Murphy S (1999) Population surveys (ground and aerial) on nesting beaches. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication, Washington, D.C., pp 45–55
Schwarz CJ, Arnason AN (1996) A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52:860–873
Schwarz CJ, Bailey RE, Irvine JR, Dalziel FC (1993) Estimating salmon spawning escapement using capture-recapture methods. Can J Fish Aquat Sci 50:1181–1197
Seminoff JA (2004) Marine Turtle Specialist Group Chelonia mydas Assessment. Marine Turtle Specialist Group. IUCN Red List status assessment
Shamblin BM, Bjorndal KA, Bolten AB, Hillis-Starr ZM, Lundgren I, Naro-Maciel E, Nairn CJ (2012) Mitogenomic sequences better resolve stock structure of southern Greater Caribbean green turtle rookeries. Mol Ecol 21:2330–2340
Solow AR, Bjorndal KA, Bolten AB (2002) Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on remigration intervals. Ecol Lett 5:742–746
Spotila JR (2004) Sea turtles: a complete guide to the biology, behaviour and conservation. John Hopkins University Press, Maryland
Spotila JR, Reina RD, Steyermark AC, Plotkin PT, Paladino FV (2000) Pacific leatherback turtles face extinction. Nature 405:529–530
Standora EA, Spotila JR (1985) Temperature dependent sex determination in sea turtles. Copeia 3:711–722
Thompson WL, White GC, Gowan C (1998) Monitoring vertebrate populations. Academic, San Diego
Tiwari M, Bjorndal KA, Bolten AB, Bolker BM (2005) Intraspecific application of the mid-domain effect model: spatial and temporal nest distributions of green turtles, Chelonia mydas, at Tortuguero, Costa Rica. Ecol Lett 8:918–924
Tomillo PS, Saba VS, Piedra R, Paladino FV, Spotila JR (2008) Effects of illegal harvest of eggs on the population decline of leatherback turtles in Las Baulas Marine National Park, Costa Rica. Conserv Biol 22:1216–1224
Troëng S, Chaloupka M (2007) Variation in adult annual survival probability and remigration intervals of sea turtles. Mar Biol 151:1721–1730
Troëng S, Rankin E (2005) Long-term conservation of the green turtle Chelonia mydas nesting population at Tortuguero, Costa Rica. Biol Conserv 121:111–116
Troëng S, Evans D, Harrison E, Lagueux C (2005) Migration of green turtles Chelonia mydas nesting at Tortuguero, Costa Rica. Mar Biol 148:435–447
Tucker AD (2010) Nest site fidelity and clutch frequency of loggerhead turtles are better elucidated by satellite telemetry than by nocturnal tagging effort: implications for stock estimation. J Exp Mar Biol Ecol 383:48–55
Vélez-Espino LA, Koops MA (2012) Capacity for increase, compensatory reserves, and catastrophes as determinants of minimum viable population in freshwater fishes. Ecol Model 247:319–326. https://doi.org/10.1016/j.ecolmodel.2012.09.022
Vélez-Espino LA, Irvine JR, Winther I, Dunlop R, Mullins G, Singer K, Trouton N (2016) Robust and defensible mark–recapture methodologies for salmonid escapement: modernizing the use of data and resources. N Am J Fish Manag 36:183–206. https://doi.org/10.1080/02755947.2015.1114540
White GC, Burnham KP (1999) Program MARK: survival rate estimation from both live and dead encounters. Bird Study 46(Suppl):S123–S139
Acknowledgements
We would like to thank to all the volunteers, interns, research coordinators and managers of Caño Palma Biological Station whose effort and dedication made this project possible. Our heartfelt gratitude goes to the Sea Turtle Conservancy for their unwavering support from the start of the project and Costa Rica’s MINAE (Ministerio de Ambiente y Energía) which facilitated the permits. We also thank Joseph Pfaller for his suggestions that noticeably improved the manuscript. This project was funded by COTERC and the Donner Foundation.
Funding
This project was funded by The Canadian Organization for Tropical Education and Rainforest Conservation (COTERC) and the Donner Canadian Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: P. Casale.
Reviewed by M. Girondot and an undisclosed expert.
Rights and permissions
About this article
Cite this article
Velez-Espino, A., Pheasey, H., Araújo, A. et al. Laying on the edge: demography of green sea turtles (Chelonia mydas) nesting on Playa Norte, Tortuguero, Costa Rica. Mar Biol 165, 53 (2018). https://doi.org/10.1007/s00227-018-3305-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3305-3