Abstract
Variation in levels of multiple paternity (MP) among species, populations and individuals has important ecological and evolutionary ramifications including maintenance of genetic diversity and offspring fitness benefits. Within species, differences in breeding experience and body size may affect the levels of MP via mate choice preferences. The present study tested these ideas in an Australian loggerhead turtle (Caretta caretta) population at Mon Repos Beach (24°48°S, 152°27°E, Queensland) to determine if variation in MP was related female breeding history or body size, or influenced embryo and hatchling outcomes in clutches from 29 females and 552 hatchlings. MP was moderately high (65.5%), but experienced females did not have higher levels of MP than first-time breeders (neophytes), nor was female size related to the number of sires. Instead, more subtle patterns emerged: multiply sired clutches of experienced females were sired by more males than those of multiply sired neophyte clutches and primary fathers sired a greater proportion of offspring when mated to larger females. These findings are consistent with cross-seasonal sperm storage in experienced breeders contributing to a small proportion of paternity and size-dependent variation in polyandrous mating behaviour. MP did not influence offspring size, levels of within-clutch morphological variation or hatching success. However, the number of sires of a clutch was positively correlated with proportion of developed embryos, suggesting a fitness advantage of MP. From a population perspective, male-biased sex ratios likely contribute to the MP levels observed, and levels could decrease with projected feminisation of populations due to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple paternity, where offspring from the same cohort of a particular female are sired by different males, is a common phenomenon that has arisen independently in numerous taxa (Birkhead and Moller 1998; Uller and Olsson 2008). Multiple paternity can provide fitness advantages that serve to increase survivorship of offspring via direct resource access benefits to the female or indirect genetic benefits to the offspring (Jennions and Petrie 2000). Levels of multiple paternity vary greatly among species, reflecting differences in mating systems, female and male mate choice preferences, sperm storage ability and sperm competition levels (Adams et al. 2005; Soulsbury 2010; Coleman and Jones 2011). Levels of multiple paternity can also vary substantially among populations of the same species (Coleman and Jones 2011) and among individuals within populations (Pearse et al. 2002). Numerous interacting factors may contribute to variation within and among populations including variation in demographic parameters (Emlen and Oring 1977; Sorin 2004), mate choice preferences (Birkhead and Moller 1993), and local selection pressures (Magnhagen 1991); however, the relative importance of different factors and their interactions is not well understood for many taxa (Uller and Olsson 2008; Wells et al. 2017). Empirical data are required to identify correlates of variation in multiple paternity within populations, and how this may generate population-level variation in multiple paternity.
Demographic parameters of population size, sex ratio and age structure are all potential influencing factors on levels of multiple paternity within and among populations. A positive relationship between population density and multiple paternity has been noted in mammal (Weston Glenn et al. 2009), bird (Westneat and Sherman 1997), and reptile populations (Jensen et al. 2006), but the relationship can be complex (Wells et al. 2017). Sex ratios can be influential when multiple mating by females depends on the frequency of encountering suitable males (Uller and Olsson 2008), resulting in higher levels of multiple paternity in populations with male-biased versus female-biased sex ratios (Barry et al. 1992). Female population age- or size structure is also likely to be important (Sorin 2004). Age structure, and concomitant variation in mating experience, may be influential in situations where cross-seasonal sperm storage occurs, with opportunities for offspring to be sired by males from previous as well as current mating seasons (Gist and Congdon 1998). Higher levels of multiple paternity may, therefore, exist in experienced versus neophyte females (Pearse et al. 2002), due to long-term sperm storage, but would depend on the extent of sperm depletion and loss of sperm viability over time (Uller et al. 2013).
The potential for multiple paternity and associated fitness benefits can have consequences for the development of mate choice strategies in both males and females. Selection on mating strategies is often expected to be greater for females, given the greater cost of offspring production by females (Bateman 1948), but it is increasingly recognised that energy costs of sperm production cannot be disregarded (Olsson et al. 1997; Wedell et al. 2002). Fitness advantages of polyandry to females and their offspring are expected under the assumption that better quality males produce better quality sperm that successfully compete with sperm of lesser quality males for paternity (Simons 2005). This can include increased success in fertilisation or embryonic development, and increased offspring size (Banger 2012; Squires et al. 2012), which is often an advantageous trait particularly during dispersal phases (Janzen et al. 2000; Gyuris 2000). Increased phenotypic variability among offspring in multiple paternity broods compared to single paternity broods may also increase population persistence in variable environments (Calsbeek and Sinervo 2004). The combination and interaction of demography and mate choice patterns, in the context of fitness benefits, can potentially produce varied population-level outcomes for levels of multiple paternity.
Marine turtles are particularly interesting for studying MP because it is ubiquitous but variable in degree among, and often within, species (Jensen et al. 2013). For instance, the highest levels (> 90%) have been recorded in populations of loggerhead (Caretta caretta) (Zbinden et al. 2007) and olive ridley turtles (Lepidochelys olivacea) (Jensen et al. 2006) and some of the lowest values (20–23%) have also been observed in these two species (Hoekert et al. 2002; Nielsen 2010). Explanations for this diversity have included variation in female size structure (Zbinden et al. 2007), operational sex ratio (OSR)—the ratio of sexually active females to males in a population (Westneat and Sherman 1997; Stewart and Dutton 2011; Wright et al. 2012; Lasala et al. 2013), population size or the density of breeding turtles at breeding grounds (Ireland et al. 2003; Jensen et al. 2006; Lasala et al. 2013; Duran et al. 2015) and sex-biased population structure (Wright et al. 2012). Variation in MP may also occur through population differences in female response to persistent male courtship behaviour (Frick et al. 2000; Lee and Hays 2004), or in the capacity of males to choose more fecund, larger or experienced females (Zbinden et al. 2007; Lasala et al. 2013; Wright et al. 2013). Understanding marine turtle mating systems is becoming increasingly important as adult sex ratios are expected to eventually change following increased feminisation of hatchlings due to climate change and temperature-dependent sex determination (Fuentes et al. 2010; Hays et al. 2010).
Here, we examine multiple paternity in an Australian loggerhead turtle population to assess attributes of females in relation to the production of multiply sired clutches and make inferences about mate choice strategies and the potential for cross-seasonal sperm storage, and, secondly, determine outcomes for clutches and hatchlings to infer fitness benefits of multiple paternity. Multiple paternity was previously studied in this population using allozymes, finding that 33% of clutches were multiply sired (Harry and Briscoe 1988), but there was low statistical power to detect multiple paternity, and no morphological or fitness data were analysed. We combine DNA data with a rare opportunity afforded by extensive field data and laparoscopic examinations to categorise females as experienced breeders or first-time nesters (neophytes) to ask if multiple paternity is related to female breeding experience, body size or fecundity. If large or experienced females have higher multiple paternity levels than first-time breeders, it would suggest that males preferentially mate with larger, or more experienced females, or that these females are more promiscuous. We test for correlations between female breeding success, breeding history, and female size with levels of multiple paternity. We explore the possibility that cross-seasonal sperm storage within a female’s oviduct increases multiple paternity rates in experienced females. If cross-seasonal sperm storage occurs, then experienced females would be expected to have a higher proportion of multiple paternity clutches than neophytes (regardless of body size), and a higher number of fathers per clutch if the viability of older sperm is maintained (Pearse et al. 2001). We examine if multiple paternity has the potential to increase offspring fitness via higher fertilisation or hatching success or the production of clutches with larger or more morphologically variable offspring. Finally, we determine the OSR based on our results and compare this to known population sex ratios for this population and compare these ratios to other studies to consider the impact of climate change on marine turtle mating systems.
Materials and methods
Sample collection and field methods
Nesting loggerhead turtles at Mon Repos Beach (24°48°S, 152°27°E) in south eastern Queensland (Fig. 1) have been studied intensively since 1969 (Limpus 1985). All nesting females have been individually flipper tagged and since 1983 the nesting status and history of approximately 100 (Limpus unpubl data) newly recruiting females were determined using laparoscopy (Limpus and Limpus 2003a). The laparoscopic data indicate that at this beach approximately 98% of the untagged females are neophytes (nesting for their first season) (Limpus unpubl data). Additional data on nesting histories were known from tagging females at several other rookeries in the region, and at feeding grounds where laparoscopy identified maturity status of some females prior to nesting for the first time (Limpus 1985; Limpus and Limpus 2003a). The combination of individual tagging histories that span decades and the use of laparoscopic examination has created a unique situation where breeding outcomes of experienced versus neophyte breeders can be compared.
During the 2011–2012 season, Mon Repos Beach was patrolled nightly for nesting loggerhead turtles to identify females by their flipper tags, and select those that were either neophytes or were nesting for at least their fourth season. Nesting turtles were measured for curved carapace length (CCL) using a fibreglass tape measure (± 1 mm). Immediately after a female completed nesting, a tissue sample was taken by removing a small piece of skin (approx. 0.5 cm2) from between the scales on a flipper and stored in a buffer (20% dimethyl sulfoxide (DMSO), saturated with NaCl). Nest locations were flagged with the female’s identification number and nest date, and distances to permanent marker posts noted.
Prior to expected hatchling emergence, enclosures made from plastic gutter guard were placed around the nest site and checked every 30 min during the night for signs of hatchling emergence. Hatchlings were collected from the enclosure once all were fully emerged from the nest. Twenty hatchlings were selected at random using methods described in Whiting et al. (2008) and two measurements were recorded: minimum straight carapace length (SCL min) was measured using digital vernal callipers (± 0.1 mm) between the posterior end of the nuchal scales and the post-vertebral notch, and weight was measured using digital scales (± 0.5 g) (Bolten 1999). Hatchling tissue samples were collected by removing a sliver of tissue (approx. 1 × 4 mm) from one of the rear marginal scutes of the carapace and stored as above. After processing, hatchlings were released in groups at the top of the beach slope to make a natural journey to the ocean. Following nest emergence, nests were excavated to determine hatching success and whether observable embryonic development had occurred in unhatched eggs. Broken egg shells that constituted more than half of a whole egg were counted as a hatched egg. Unhatched eggs with evidence of development, including eye spots, capillaries or more developed embryos (Miller 1985), were counted as developed but unhatched and those with no evidence of these traits were deemed to be undeveloped. Using these counts, female fecundity (total number of eggs), fertility (proportion of developed eggs) and embryo success (proportion of developed eggs that hatched) were determined.
Molecular analyses
DNA was extracted from tissue samples using a standard salting out procedure (Theissinger et al. 2009). DNA quality and quantity were assessed on 1% agarose gels and SyBr green dye (Life Technologies Corporation). Three PCR multiplex reactions using fluorescent tags (see Real et al. 2009) were used to amplify six microsatellite regions (Online Resource 1). Amplifications were conducted in 10 µL final volumes containing 1× reaction buffer, 2.0 mM MgCl2, 0.7 mM dNTPs, 0.1 µM tailed primer, 0.4 µM opposing primer, 0.4 µM fluorescent tag and 0.3 Units MyTaq DNA polymerase (Bioline). PCR conditions were as follows: initial denaturing at 95 °C (3 min) then 35 cycles of 95 °C (15 s) denaturing, annealing °C (20 s), standard or touchdown annealing (decreasing 1 °C every 2 cycles; Table 1), 72 °C (75 s) extension, and a final extension of 74 °C (10 min). PCR products were analysed using a 3130 Genetic Analyser (Applied Biosystems) and allele sizes were determined using Geneious 5.6.3 (Biomatters Ltd) against the GSLIZ500 ladder (Applied Biosystems).
Paternity analyses
Microsatellite variation was characterised across six loci in 40 adult females (including 29 females subsequently used in the parentage analyses), to provide baseline allele frequency distribution information needed for paternity analysis programs (see below). MICRO-CHECKER (Van Oosterhout et al. 2004) was used to assess the presence of scoring errors, allelic dropout or null alleles, none of which were detected for any loci. Tests for linkage disequilibrium and deviations from Hardy–Weinberg Equilibrium (HWE) were conducted in GENEPOP 4.1 (Rousset 2008). Allele frequencies, allelic diversity, observed and expected heterozygosity were estimated using GENEPOP 4.1 (Rousset 2008) (see Online Resource 2 for summary statistics).
To ensure that the suite of microsatellites provided sufficient power for paternity analysis, we calculated the probability of detecting multiple paternity using the larger data set of 40 females in PrDM (Neff and Pitcher 2002) under different scenarios: varying the number of fathers (2–5), their contribution to paternity (equal or skewed) and the number of hatchlings genotyped (14 or 20) (see Table 1 for variable combinations). Additionally, the probability of exclusion was calculated using the program GERUD Version 2.0 (Jones 2005) using all six loci, and again after removing the most informative (variable) locus to evaluate sensitivity to the dataset.
Hatchlings from the first observed clutches of 29 adult females (14 neophytes and 15 experienced) were assessed for paternity using three paternity analysis programs, each with different statistical approaches and assumptions to determine paternity: COLONY (Jones and Wang 2010), GERUD Version 2.0 (Jones 2005) and DADSHARE (W. Amos, available at http://www.zoo.cam.ac.uk/zoostaff/amos/). COLONY uses a maximum likelihood algorithm that incorporates the full pedigree of each individual to reconstruct paternal genotypes from maternal and offspring genotypes and assigns offspring to groups of full siblings. Genotyping errors from allelic dropout, null alleles and mutations were accounted for by selecting an error rate of 0.001. Each clutch was analysed separately under assumptions of polygamy, outbreeding, and 100% probability of the mother’s genotype. Runs were set as medium length, full likelihood, and multiple runs (up to 3) were conducted to check for consensus of results. GERUD Version 2.0 determines the minimum number of fathers necessary to account for a particular progeny array in a clutch, by removing maternal alleles from the offspring genotypes and reconstructing all possible paternal genotype combinations. Where multiple combinations were possible, each combination is assigned a likelihood based on Mendelian segregation and expected allele frequencies. DADSHARE builds a paternal genotype distance matrix and uses a UPGMA clustering method (Sokal and Michener 1958) to identify clusters of individuals with the same father. The input order of offspring can affect the clustering outcome; therefore, DADSHARE was run twice per clutch, and if clustering order differed, then offspring were reordered and the program rerun until a consensus was reached. The minimum number of fathers per clutch (henceforth “number of fathers”) was estimated from the consensus of at least two programs, or if there was no consensus, the average value was used. The possibility that a particular male fathered hatchlings from more than one clutch was tested by comparing all paternal genotypes generated by GERUD for each pair of clutches. Genotypes were compared at each locus separately and if there were matches, we assessed whether matches existed across all loci.
Breeding experience and female size effects
To determine if breeding history influenced multiple paternity, we applied a binomial generalised linear model (GLM) with the binomial response variable specifying if a clutch had single or multiple paternity, and explanatory variables of female breeding history (experienced or neophyte) and female CCL (normally distributed, hence no transformation was applied, Wilks–Shapiro test, W = 0.96, P > 0.4). Both explanatory variables were included to decouple the effects of breeding history and size, which are positively related (see results). In all GLMs throughout, model simplification involved the stepwise removal of insignificant interaction terms, followed by the main effect terms to arrive at the minimum adequate model. We compared the number of fathers for neophyte versus experienced females with a non-parametric Wilcoxon rank sum test due to non-normality of data and few data points for many categories.
For multiply sired clutches, we tested whether the proportion of hatchlings sired by the primary father (i.e. the male with most offspring in the clutch) varied according to female breeding experience and female size, using a binomial GLM.
Influence of multiple paternity on hatchling size and within-clutch variation
To determine if hatchlings from multiply versus singly sired clutches differed in size (weight and length), we conducted linear mixed models fitted by Restricted Maximum Likelihood (REML) with paternity class (singly or multiply sired clutches) as a fixed effect and clutch within paternity class as a random nested effect, as implemented in package ‘lme4’ (Bates et al. 2011). This method was chosen over a nested ANOVA because of an unbalanced experimental design and to provide greater statistical power (Crawley 2007). To determine the importance of the fixed effect, maximum likelihood models with and without paternity class were compared via a likelihood ratio test using the χ 2 statistic. To test whether within-clutch variation in hatchling weight or length was higher in multiple paternity clutches, the variances in weight and length within each clutch for the two paternity classes were compared (one-tailed t test).
Correlates of female breeding success
We assessed if measures of female breeding success were related to breeding history, level of multiple paternity or female body size. For each female, data from multiple clutches were combined to give a seasonal total of developed versus undeveloped eggs and for the developed eggs, the total number of hatched versus unhatched eggs. Genetic testing of paternity levels was conducted for a single clutch from each female; therefore, we assumed that a similar pattern of multiple paternity occurred across clutches for a given female in a season, as has been observed in other studies (Joseph and Shaw 2011; Wright et al. 2013). For each binomial dependent variable (number developed versus undeveloped eggs, and of those that developed, the number of hatched versus unhatched eggs), a GLM with main effects of breeding history (neophyte versus experienced), level of multiple paternity (consensus number of fathers for a single clutch from a given female), and female CCL, plus the three two-way and single three-way interactions was specified. Due to overdispersion in both cases, a quasibinomial error structure was applied. One individual (EXP008) had a very low number of developed eggs (136 hatched out of 646 total across 4 clutches) and was removed from the analysis as an outlier.
We tested whether average clutch size among all females that nested in the 2011–2012 breeding season was explained by female breeding history or female size using a Poisson GLM. Further, we tested whether the estimated total number of clutches laid by a female in the breeding season (a proxy for seasonal fecundity) was explained by these same variables using a Poisson GLM. Unobserved nesting events were added to the estimate if the time between sequential observations was twice the expected length of the re-nesting interval (13.9 ± 1.7 days; Limpus 2008). The number remains an underestimate of the actual number of clutches laid due to missed observations of nesting events either at Mon Repos or nearby beaches at the beginning and end of the season (Tucker 2010). Except where noted, all statistical analyses were conducted in R version 3.0.2 (R Core Team 2013), with functions in the base package of R or other R packages where specified.
Results
Molecular analyses and paternity assessment
A total of 552 hatchlings (14–20 hatchlings per clutch for 29 clutches) were genotyped at six microsatellite loci to determine paternity. The probability of exclusion was 0.99 and remained high (0.98) after removing the most variable locus (Cc7), the later representing the worst case scenario for paternity exclusion. The probability of detecting multiple paternity was high (> 95%) (Table 1). Clutch sizes ranged from 93 to 163 eggs (average = 126.4 ± 4.2), giving a sampling effort ranging from 11.1 to 20.0% (average = 15.4%). Tests for linkage disequilibrium and deviations from Hardy–Weinberg Equilibrium (HWE) demonstrated independence of loci (Bonferroni adjusted α/15 = 0.003, all P > 0.05), and no significant deviations from HWE (Bonferroni adjusted α/6 = 0.008, all P > 0.05), respectively; hence, all loci were kept for analysis. Of the 29 clutches analysed, COLONY detected multiple paternity in 12 clutches, GERUD in 20 clutches and DADSHARE in 19 clutches (Table 2). Using a consensus approach, 19 of the 29 clutches (65.5%) displayed multiple paternity, eight of which (42.1%) were deemed to have more than two fathers. There was complete agreement among the three programs in nine of ten cases where a single father was identified. It is possible that the same male fathered four hatchlings in clutch Neo113 and two hatchlings in clutch Exp403; however, several other potential paternal genotypes were considered more likely for clutch Exp403.
Breeding experience and female size effects on multiple paternity
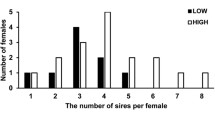
Female breeding experience was positively related to size; for all females that nested in the 2011–2012 season (one-tailed t test: t = 5.230, df = 190, P < 0.00001, average CCL ± SD, experienced: 96.3 ± 4.3 versus neophyte: 93.4 ± 4.2), and for the set of females used in paternity analysis (one-tailed t test, t = 3.401, df = 25, P < 0.0011, average CCL ± SD, experienced: 98.8 ± 3.7 versus CCL neophyte: 92.8 ± 5.0). Paternity status of a clutch (single versus multiple) was not explained by breeding history, female size, or the interaction of those terms (binomial GLM, all P values > 0.2, sequential removal of non-significant terms did not result in significance of any term). The number of fathers per clutch did not differ according to breeding history (Wilcoxon rank sum test, W = 81, P = 0.28, neophyte average ± SD: 1.7 ± 0.63; experienced: 2.3 ± 1.24). When considering only multiply sired clutches, experienced female clutches had a higher number of fathers than those of neophytes (Wilcoxon rank sum test, W = 22, P = 0.03, neophyte average ± SD: 2.14 ± 0.34; experienced: 2.93 ± 1.0). The proportion of hatchlings sired by a primary male was higher for neophytes than experienced females, and positively related to CCL for both experience classes (Table 3; Fig. 2).
Proportion of loggerhead turtle offspring sired by a primary father versus all other fathers, within each of 29 clutches. Lines show the model fit of the binomial GLM (from Table 5) for neophyte and experienced females
Influence of multiple paternity on hatchling size and within-clutch variation
Across 577 hatchlings, 29 clutches and 2 paternity classes, a linear mixed effect model found paternity class explained 36.6 and 58.5% of variation in hatchling length and weight, respectively (Table 4), with hatchlings from single paternity clutches tending to be smaller. However, the difference between maximum likelihood models with and without paternity class as a fixed effect fell short of significance (χ 2 = 3.19, df = 1, P = 0.07 for hatchling length and χ 2 = 3.12, df = 1, P = 0.08 for hatchling weight). Additionally, the t tests to determine whether there were differences in variance within clutches in hatchling weight or length depending on paternity class were non-significant (weight variance t = 1.38, df = 21.3, P = 0.18; length variance t = − 0.87, df = 9.1, P = 0.41).
Correlates of female breeding success
The proportion of developed to undeveloped eggs in a clutch was positively related to the number of fathers (binomial GLM: t = 2.15, P < 0.04) (Fig. 3). The other explanatory variables (breeding experience and female size) and all interactions were insignificant. The level of hatching success of developed eggs, however, was not significantly related to any of the variables tested (breeding experience, number of fathers per clutch or female size).
Among all females that nested in the 2011–2012 season, variation in number of clutches observed across the breeding season was significantly higher for experienced versus neophyte females, while average clutch size was positively related to female size (Table 5a, b). When the analysis was repeated for only those nesting females that laid at least three clutches, similar effects were seen though the effect of breeding history fell marginally short of significance (Table 5c, d).
Operational and population sex ratios
An estimated 59 males sired the offspring of 29 females, providing a male bias to parentage (2.0:1). This is higher than the adult male bias of 1.3:1 and 1.6:1 observed at two primary feeding grounds for this population (Limpus et al. 1994; Limpus and Limpus 2003a). We found only three additional populations for which OSR estimates from paternity studies, and levels of multiple paternity, can be compared to demographic data from foraging grounds used by each population (Table 6). Of the four comparisons across populations the trend suggests that increased male bias in adults on feeding grounds is related to the higher levels of MP and increased male bias to clutch parentage (Fig. 4).
Proportion of adult marine turtles that are males as estimated from demographic studies at feeding grounds in comparison to (1) Prop males with paternity: the estimated proportion of males involved in breeding based on parentage analyses and (2) Prop multiple paternity clutches: the proportion of clutches displaying multiple paternity. Number codes for species and nesting location: 1 = Green turtles from Heron Island, Queensland, Australia; 2 = Loggerhead turtles from three sites in Western Australia, Australia; 3 = Loggerhead turtles from Mon Repos, Queensland, Australia (this study); 4 = Green turtles from Tortuguero, Costa Rica (see Table 6 for references). The dashed line represents a 1:1 relationship, and where estimates of proportion of males were available from multiple feeding grounds, standard error bars around mean values are shown
Discussion
Our paternity study of a Pacific Ocean loggerhead turtle lineage does not support the notion that mate choice preferences (whether by males or females) result in more experienced or larger females gaining matings, as two expectations were not realised: larger, more experienced females were not more likely to produce multiply fathered clutches and nor did they have more fathers contributing to their clutches. However, more subtle patterns emerged that suggest differences in the dynamics of multiple paternity between experienced and neophyte females. These differences indicate potential for cross-seasonal sperm storage, a role for sperm competition, variation in mating behaviour and possible fitness benefits of multiple paternity in marine turtles.
Female mate choice, breeding history and sperm storage
Multiple paternity occurred at a similar rate in neophyte and experienced females, but multiple paternity clutches of experienced females had a higher number of fathers with a lower proportion of the offspring sired by a primary father. These results could reflect differences in the timing or frequency of multiple mating events, sperm competition, cross-seasonal sperm storage, or a combination of these three factors. The limited data on marine turtle mating behaviour suggest that females are receptive to mating during a relatively short interval (~1 week) prior to the start of the nesting season (Comuzzie and Owens 1990), and that non-receptive females can reject mating behaviour by males (Booth and Peters 1972; Sakaoka et al. 2007). The higher number of fathers seen in experienced female MP clutches could be explained by having a longer receptive period and/or selection of breeding sites frequented by more males; however, there is no evidence to date that either of these features occurs in marine turtles.
Sperm competition could play a role in the higher number of fathers and more even paternal contribution seen in clutches of experienced females. The partitioning of paternal success depends on the ability of sperm to occupy the oviduct storage areas and compete with sperm from other males. Under a scramble mating system with quick succession between matings (Jessop et al. 1999), viable sperm from multiple males would be mixed within oviducal storage areas (Gist and Congdon 1998). However, with sufficient time between the first and successive matings, sperm from the first male could fill oviducal storage areas and have a competitive advantage over from sperm of secondary males. If experienced females mate in quick succession compared to neophytes, then this might explain both the higher number of fathers and lower proportion of hatchlings sired by the primary male in their MP clutches. However, again, there is no evidence to suggest that experienced female loggerhead turtles tend more towards a scramble mating system than neophytes. Furthermore, evidence from captive loggerhead turtles suggests that any role of sperm competition is likely to be complex, with failure of paternity prediction based on duration or time of mating, and the observation that multiple matings do not necessarily lead to multiply sired clutches (Sakaoka et al. 2011).
A higher number of fathers in multiply sired clutches of experienced versus neophyte females may result from cross-seasonal sperm storage. Within-season sperm storage has been documented in all marine turtle species examined to date (FitzSimmons 1998; Kichler et al. 1999; Crim et al. 2002; Theissinger et al. 2009; Zbinden et al. 2007; Phillips et al. 2013), but evidence for cross-seasonal sperm storage is limited and circumstantial. Wright et al. (2013) suggested cross-seasonal sperm storage as an explanation for lower levels of multiple paternity in presumed neophyte (first-time tagged females) versus experienced female green turtles, though breeding history was not validated by laparoscopy in that study. Definitive demonstration of cross-seasonal sperm storage in turtles has been shown for freshwater species (Palmer et al. 1998; Pearse and Avise 2001) where sperm can remain viable for up to 4 years (Ewing 1943), and where long-term storage appears to occur in separate locations to those areas used for within-breeding season sperm storage (Sarkar et al. 2003; Xiangkun et al. 2008; Chen et al. 2015). Similar studies on sperm viability times and sperm storage areas of marine turtles are lacking; however, the remigration intervals of experienced turtles used in our study (8 of 12 nested 2 years earlier, and 2 had nested 3 years earlier) are within plausible storage times for viable sperm in Testudines. Similar to Wright et al. (2013), our findings are consistent with capacity for cross-seasonal paternal contributions, and we argue that it is a more parsimonious explanation for the patterns of paternity found than the within-season behavioural differences required for explanations outlined above.
Female body size and male mate choice
We found no strong evidence to suggest that males selectively mate with larger females. This is consistent with previous studies in green turtles (Wright et al. 2013; Lee and Hays 2004), but contrasts with findings of Zbinden et al. (2007) for a loggerhead turtle population in Greece. However, in this latter study female size and breeding history were conflated. The only effect of female size observed in our study was that the proportion of hatchlings sired by a primary male tended to increase with female size for both neophytes and experienced females. This was not a particularly strong effect, but one possible explanation is that males may remain mounted on larger females for a longer duration, providing sperm greater access to oviducal storage areas and increasing primacy of paternity. Regardless of the specific mechanism, the advantage to males of siring a high proportion of young when mated to a larger female likely lies in the positive relationship between female size and one aspect of fecundity, the number of eggs per clutch. This positive relationship was observed in our study, and has been observed in previous years in the Mon Repos population (Limpus 1985) and in other loggerhead populations worldwide (van Buskirk and Crowder 1994; Tiwari and Bjorndal 2000; LeBlanc et al. 2014).
A second indicator of fecundity, the number of clutches per season, was not related to female size per se, rather experienced females laid more clutches in the season than neophytes. Other marine turtle studies show increased fecundity in larger (more eggs/clutch) or more experienced (more clutches/season) females (Van Buskirk and Crowder 1994; Broderick et al. 2003). In Mon Repos loggerhead turtles, females in their second and third breeding seasons increase their seasonal egg production by 1.5 and 1.6 times, mostly via an increase number of clutches laid rather than an increase in eggs per clutch (Limpus 1996). Although the females in our two experience classes differed in size on average, there is no tight relationship between age in years and size in loggerhead females (Limpus 1985). Therefore, males are unlikely to be able to use size as a cue for total fecundity, due in part to the relationships between egg size, clutch size and clutch frequency and their interactive influences on fecundity (Tucker and Frazer 1991; Tiwari and Bjorndal 2000). Additionally, the quality of the foraging habitat influences growth rates and body size at maturity, as well as clutch size (Cardona et al. 2014; Hatase et al. 2015). Use of tagging and telemetry data would be valuable for linking feeding ground locations to female body size and total fecundity.
Multiple paternity and correlates of female breeding success
We found support for a fitness advantage of multiple matings with a positive relationship between the number of fathers and the proportion of developed eggs within a clutch, which is a finding consistent with sperm competition. However, the advantages of multiple paternity did not appear to carry through to hatching success, although this is perhaps not surprising given the large range of environmental factors that influence hatch success relating to moisture, salinity, gas exchange, temperature, microbial infection and predation (Miller et al. 2003).
Other proposed advantages of multiple paternity, such as increased size of offspring and increased phenotypic variation within clutches, were not observed. However, hatchling size is influenced by several factors including female body size, egg size, sand temperature and incubation duration (Pinckney 1990; Booth et al. 2005; Read et al. 2013) and these may have impacted our results. In green turtles, no relationship was found between levels of multiple paternity and fertilisation success, hatching success, emergence success or hatchling size (Wright et al. 2013), but in loggerhead turtles there was a positive relationship between hatching success and the number of fathers (Zbinden et al. 2007). Understanding the dynamics of relationships between multiple paternity and offspring outcomes would be best explored using a large controlled experiment where it would be possible to select mating partners, positively identify the paternity of all hatchlings and embryos and reduce environmental variation during incubation.
Multiple paternity and operational sex ratio
Intraspecific variation in levels of multiple paternity has primarily been attributed to variation in female abundance or density (Jensen et al. 2006), or variation in operational sex ratios (OSR) at breeding grounds (Lodé et al. 2005; Weir et al. 2011). In loggerhead turtles, the density of breeders at breeding grounds may be more important in determining rates of MP than female population size per se, and this density may depend on the geographic extent of breeding grounds used by the population (Zbinden et al. 2007; Schofield et al. 2010; Tedeschi et al. 2014). The nesting population at Mon Repos is part of the southwest Pacific loggerhead population, which uses breeding grounds spread across the southern Great Barrier Reef and adjacent coastal areas where observations of mating pairs are limited and the size or density of breeding aggregations is unquantified (Limpus 1985; Limpus and Limpus 2003b). Hence, it is not currently possible to determine how density differences may contribute to the level of MP in this study versus other loggerhead populations.
In contrast, multiple sources of information suggest that a highly male-biased operational sex ratio on the breeding grounds of eastern Australian loggerhead turtles contributed to the relatively high level of multiple paternity observed in this study. Understanding the dynamics of operational sex ratios (OSR) in marine turtle populations requires consideration of adult sex ratios in the population, breeding frequency differences across seasons between males and females (Wibbels et al. 1990; Limpus 1993; Hays et al. 2010) and variation in OSR within a breeding season due to differences in arrival and departure times of males and females at breeding grounds (Limpus 1993; Schofield et al. 2010; Hays et al. 2010). At eastern Australian feeding grounds, the adult loggerhead population is strongly male-biased at locations commonly used by females nesting at the Mon Repos rookery (Limpus 1985; Limpus et al. 1994). Additionally, the seasonal breeding frequency is higher for males than females in this population, with most adult males thought to breed every year in comparison to females that have remigration intervals of 3.8 (± 1.8) years (Wibbels et al. 1990; Limpus 2008). Differences in breeding ground activity are also likely with female receptivity to mating likely to be measured in days (Comuzzie and Owens 1990), compared to up to 2 months of mating activity for males (Wibbels et al. 1990). This combination of male-biased sex ratio, increased seasonal breeding frequency of males and longer duration of breeding within a season, would result in a highly male-biased operational sex ratio at breeding grounds and contribute to the relatively high rate of multiple paternity observed.
Conservation implications
Population sex ratios of adult are a fundamental parameter of population growth, and associated changes away from optimal OSR values can lead to reduced population size (Steifetlen and Dale 2006; Lehikoinen et al. 2008). Estimation of the average male to female parentage ratio obtained from mating studies of marine turtles has been used as a surrogate measure of OSR (Stewart and Dutton 2011; Wright et al. 2012) and has also been considered as a means of estimating population sex ratios (Theissinger et al. 2009; Lasala et al. 2013). However, the extent to which OSR estimates are related to population adult sex ratios has not been verified in marine turtles and it is likely to be a complex relationship (Payne et al. 2011). Studies of paternity across years would be informative, as yearly variation in the male to female parentage ratios has been observed in the few studies that have provided multi-year data (Wright et al. 2012; Lasala et al. 2013). Our study is one of the few of marine turtles in which sex ratio data are available from clutch parentage analyses as well as from demographic data at feeding grounds used by this population. Initial comparisons to other studies suggest positive correlations exist between population sex ratios, multiple paternity and sex ratios derived from parentage analyses.
Mating systems of marine turtles have particular conservation relevance in relation to climate change due to the operation of temperature-dependent sex determination, because future increases in temperature will lead to greater female bias within populations and at breeding grounds (Fuentes et al. 2010; Dalleau et al. 2012; Woolgar et al. 2013). This will occur unless there is a shift in nesting phenology to cooler months or nest site changes to cooler locations in response to selective pressures associated with high temperatures and embryonic death (Hawkes et al. 2007; Boyle et al. 2014). Several marine turtle populations have female-biased sex ratios, in which the higher frequency of male breeding is hypothesised to provide a buffer against the impact of climate change on adult sex ratios (Hays et al. 2010; Wright et al. 2012). However, if the increased frequency in male breeding does in fact provide a buffer against climate change, then we would expect to see that the sex ratio of adult males derived from parentage analyses would be higher than that observed at feeding grounds, and that is not consistent with the available data shown in Fig. 4.
A more comprehensive understanding of how an entire population functions across rookeries, foraging areas, and breeding grounds is needed to address conservation issues. Protection of multiple rookeries of the southwest Pacific loggerhead population is critical because mainland beaches, such as Mon Repos, have darker sand beaches that produce mostly female hatchlings (Chu et al. 2008), while male hatchlings are mostly produced on the reflective white sand beaches of offshore islands used by this population (Limpus and Limpus 2003b). Nesting in this population is spread widely over the summer months, so there is an evolutionary potential for a temporal shift of nesting to cooler spring or autumn months in response to rising temperatures, as well as a potential for spatial shifts to cooler beaches either offshore or further south that have low density nesting activity (Limpus 2008). Ultimately though, if climate change results in a strongly female-biased population, as suggested for green turtles in the northern Great Barrier Reef (Fuentes et al. 2010), then the capacity of males to fertilise the eggs of multiple females may determine population viability (Boyle et al. 2014) and influence effective population size (Pearse and Anderson 2009). Cross-seasonal sperm storage may become more important for ensuring fertilisation and maintaining genetic diversity within clutches. Marine turtle mating systems need to be resilient and flexible to adapt to changes in population size and sex ratios as influenced by changing climates and environments, which may influence mating behaviour including mate searching, competition and mate choice (Jessop et al. 1999; Weir et al. 2011) through changes in OSR (Shuster 2009). Climate change will add to the threats facing marine turtles and continued efforts are needed to monitor changes in demography and behaviour.
References
Adams EM, Jones AG, Arnold SJ (2005) Multiple paternity in a natural population of a salamander with long-term sperm storage. Mol Ecol 14:1803–1810. https://doi.org/10.1111/j.1365-294X.2005.02539.x
Alfaro-Núñez A, Jensen MP, Abreu-Grobois FA (2015) Does polyandry really pay off? The effects of multiple mating and number of fathers on morphological traits and survival in clutches of nesting green turtles at Tortuguero. PeerJ. https://doi.org/10.7717/peerj.880
Banger N (2012) Consequences of multiple paternity for female fitness in an Ontario population of northern map turtles, Graptemys geographica. Master Thesis. University of Ottawa, Ottawa
Barry F, Weatherhead P, Philipp D (1992) Multiple paternity in a wild population of northern water snakes, Nerodia sipedon. Behav Ecol Sociobiol 30:193–199. https://doi.org/10.1007/BF00166703
Bateman AJ (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368. https://doi.org/10.1038/hdy.1948.21
Bates D, Maechler M, Bolker B (2011) Lme4: linear mixed-effect models using s4 classes. http://cran.R-project.org/package=lme4. R package version 0.999375-42
Birkhead TR, Moller AP (1993) Sexual selection and the temporal separation of reproductive events-sperm storage data from reptiles, birds and mammals. Biol J Linn Soc 50:295–311. https://doi.org/10.1111/j.1095-8312.1993.tb00933.x
Birkhead TR, Moller AP (1998) Sperm competition and sexual selection. Academic Press, San Diego
Bolten AB (1999) Techniques for measuring turtles. In: Donnelly M, Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group, Washington, DC, pp 110–114
Booth J, Peters JA (1972) Behavioural studies on the green turtle (Chelonia mydas) in the sea. Anim Behav 20:808–812. https://doi.org/10.1016/S0003-3472(72)80155-6
Booth DT, Burgess E, McCosker J, Lanyon JM (2005) The influence of incubation temperature on post-hatching fitness characteristics of turtles. Int Congr Ser 1275:226–233. https://doi.org/10.1016/j.ics.2004.08.057
Boyle M, Hone J, Schwanz LE, Georges A (2014) Under what circumstances do climate-driven sex ratios enhance versus diminish population persistence? Ecol Evol 4:4522–4533. https://doi.org/10.1002/ece3.1316
Broderick AC, Glen F, Godley BJ, Hays GC (2003) Variation in reproductive output of marine turtles. J Exp Mar Biol Ecol 288:95–109. https://doi.org/10.1016/S0022-0981(03)00003-0
Calsbeek R, Sinervo B (2004) Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. J Evol Biol 17:464–470. https://doi.org/10.1046/j.1420-9101.2003.00665.x
Campbell CL, Lagueux CJ (2005) Survival probability estimates for large juvenile and adult green turtles (Chelonia mydas) exposed to an artesanal marine turtle fishery in the Western Caribbean. Herpetologica 61:91–103. https://doi.org/10.1655/04-26
Cardona L, Clusa M, Eder E, Demetropoullos A, Margaritoulis D, Rees A, Hamza AA, Khali M, Levy Y, Tükozan O, Marín I, Auilar A (2014) Distribution patterns and foraging ground productivity determine clutch size in Mediterranean loggerhead turtles. Mar Ecol Prog Ser 497:229–241. https://doi.org/10.3354/meps10595
Chen S, Zhang L, Le Y, Waqas Y, Chen W, Zhang Q, Ullah S, Liu T, Hu L, Li Q, Yang P (2015) Sperm storage and spermatozoa interaction with epithelial cells in oviduct of Chinese soft-shelled turtles, Pelodiscus sinensis. Ecol Evol 5:3023–3030. https://doi.org/10.1002/ece3.1575
Chu CT, Booth DT, Limpus CJ (2008) Estimating the sex ratio of loggerhead turtle hathclings at Mon Repos rookery (Australia) from nest temperatures. Aust J Zool 56:57–64. https://doi.org/10.1071/ZO08004
Coleman SW, Jones AG (2011) Patterns of multiple paternity and maternity in fishes. Biol J Linn Soc 103:735–760. https://doi.org/10.1111/j.1095-8312.2011.01673.x
Comuzzie DKC, Owens DW (1990) A quantitative analysis of courtship behavior in captive green sea turtles (Chelonia mydas). Herpetologica 46:195–202. http://www.jstor.org/stable/3892904
Crawley MJ (2007) The R book. Wiley, Chichester
Crim JL, Spotila LD, Spotila JR, O’Connor M, Reina R, Williams CJ, Paladino FV (2002) The leatherback turtle, Dermochelys coriacea, exhibits both polyandry and polygyny. Mol Ecol 11:2097–2106. https://doi.org/10.1046/j.1365-294X.2002.01591.x
Dalleau M, Ciccione S, Mortimer JA, Garnier J, Benhamou S, Bourjea J (2012) Nesting phenology of marine turtles: insights from a regional comparative analysis on green turtle (Chelonia mydas). PLoS One 7:e46920. https://doi.org/10.1371/journal.pone.0046920
Duran N, Dunbar SG, Escobar RA III, Standish TG (2015) High frequency of multiple paternity in a solitary population of olive ridley sea turtles in Honduras. J Exp Mar Biol Ecol 463:63–71. https://doi.org/10.1016/j.jembe.2014.10.023
Emlen ST, Oring LW (1977) Ecology, sexual selection, and evolution of mating systems. Science 197:215–223. https://doi.org/10.1126/science.327542
Ewing HE (1943) Continued fertility in female box turtle following mating. Copeia 1943:112–114. http://www.jstor/stable/1437776
FitzSimmons NN (1998) Single paternity of clutches and sperm storage in the promiscuous green turtle (Chelonia mydas). Mol Ecol 7:575–584. https://doi.org/10.1046/j.1365-294x.1998.00355.x
FitzSimmons NN, Moritz C, Moore SS (1995) Conservation and dynamics of microsatellite loci over 300-million years of marine turtle evolution. Mol Biol Evol 12:432–440. https://doi.org/10.1093/oxfordjournals.molbev.a040218
FitzSimmons NN, Limpus CJ, Norman JA, Goldizen AR, Miller JD, Moritz C (1997) Philopatry of male marine turtles inferred from mitochondrial DNA markers. Proc Nat Acad Sci USA 94:8912–8917. https://doi.org/10.1073/pnas.94.16.8912
Frick MG, Slay CK, Quinn CA, Windham-Reid A, Duley PA, Ryder CM, Morse LJ (2000) Observations of courtship behavior in loggerhead sea turtles (Caretta caretta) from southeastern Georgia and northwestern Florida. J Herpetol 34:153–158. http://www.jstor.org/stable/1565255
Fuentes MMPB, Hamann M, Limpus CJ (2010) Past, current and future thermal profiles of green turtle nesting grounds: implications from climate change. J Exp Mar Biol Ecol 383:56–64. https://doi.org/10.1016/j.jembe.2009.11.003
Gist DH, Congdon JD (1998) Oviductal sperm storage as a reproductive tactic of turtles. J Exp Zool 282:526–534. https://doi.org/10.1002/(SICI)1097-010X(199811/12)282:4/53.3.CO;2-Q
Gyuris E (2000) The relationship between body size and predation rates of hatchling green turtles (Chelonia mydas): Is bigger better? In: Pilcher N, Ismail G (eds) Sea turtles of the Indo-Pacific: research management and conservation. Academic Press, New York, pp 143–147
Harry JL, Briscoe DA (1988) Multiple paternity in the loggerhead turtle (Caretta caretta). J Hered 79:96–99. https://doi.org/10.1093/oxfordjournals.jhered.a110480
Hatase H, Omuta K, Komatsu T (2015) Loggerhead turtle (Caretta caretta) offspring size does not vary with maternal alternative foraging behaviors: support for their phenotypic plasticity. Mar Biol 162:1567–1578. https://doi.org/10.1007/s00227-015-2693-x
Hawkes LA, Broderick AC, Godfrey MH, Godley BJ (2007) Investigating the potential impacts of climate change on a marine turtle population. Glob Chang Biol 13:923–932. https://doi.org/10.1111/j.1365-2486.2006.01320.x
Hays GC, Fossette S, Katselidis KA, Schofield G, Gravenor MB (2010) Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv Biol 24:1636–1643. https://doi.org/10.1111/j.1523-1739.2010.01531.x
Heithaus MR, Frid A, Wirsing AJ, Bejder Dill LM (2005) Biology of sea turtles under risk from tiger sharks at a foraging ground. Mar Ecol Prog Ser 288:285–294. https://doi.org/10.3354/meps288285
Hoekert WEJ, Neufeglise H, Schouten AD, Menken SBJ (2002) Multiple paternity and female-biased mutation at a microsatellite locus in the olive ridley sea turtle (Lepidochelys olivacea). Heredity 89:107–113. https://doi.org/10.1038/sj.hdy.6800103
Ireland JS, Broderick AC, Glen F, Godley BJ, Hays GC, Lee PLM, Skibinski DOF (2003) Multiple paternity assessed using microsatellite markers, in green turtles Chelonia mydas (Linnaeus, 1758) of Ascension Island, South Atlantic. J Exp Mar Biol Ecol 291:149–160. https://doi.org/10.1016/S0022-0981(03)00118-7
Janzen FJ, Tucker JK, Paukstis GL (2000) Experimental analysis of an early life-history stage: selection on size of hatchling turtles. Ecology 81:2290–2304. https://doi.org/10.1890/0012-9658(2000)081[2290:EAOAEL]2.0.CO;2
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64. https://doi.org/10.1111/j.1469-185X.1999.tb00040.x
Jensen MP, Abreu-Grobois FA, FrydHayenberg J, Loeschcke V (2006) Microsatellites provide insight into contrasting mating patterns in arribada vs. Non-arribada olive ridley sea turtle rookeries. Mol Ecol 15:2567–2575. https://doi.org/10.1111/j.1365-294X.2006.02951.x
Jensen MP, FitzSimmons NN, Dutton PH (2013) Molecular genetics of sea turtles. In: Wyneken J, Lohmann KJ, Musick JA (eds) Biology of the sea turtles, vol 3. CRC Press, Boca Raton, pp 135–161
Jessop TS, FitzSimmons NN, Limpus CJ, Whittier JM (1999) Interactions between behavior and plasma steriods within the scramble mating system of the promiscuous green turtle, Chelonia mydas. Horm Behav 36:86–97. https://doi.org/10.1006/hbeh.1999.1527
Jones AG (2005) Gerud2.0: a computer program for the reconstruction of parental genotypes from half-sib progeny arrays with known or unknown parents. Mol Ecol Note 5:708–711. https://doi.org/10.1111/j.1471-8286.2005.01029.x
Jones OR, Wang J (2010) Colony: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 10:551–555. https://doi.org/10.1111/j.1755-0998.2009.02787.x
Joseph J, Shaw PW (2011) Multiple paternity in egg clutches of hawksbill turtles (Eretmochelys imbricata). Conserv Genet 12:601–605. https://doi.org/10.1007/s10592-010-0168-7
Kichler K, Holder MT, Davis SK, Marquez R, Owens DW (1999) Detection of multiple paternity in the Kemp’s ridley sea turtle with limited sampling. Mol Ecol 8:819–830. https://doi.org/10.1046/j.1365-294X.1999.00635.x
Lasala JA, Harrison JS, Williams KL, Rostal DC (2013) Strong male-biased operational sex ratio in a breeding population of loggerhead turtles (Caretta caretta) inferred by paternal genotype reconstruction analysis. Ecol Evol 3:4736–4747. https://doi.org/10.1002/ece3.761
LeBlanc AM, Rostal DC, Drake KK, Williams KL, Frick MG, Robinette J, Barnard-Keinath DE (2014) The influence of maternal size on the eggs and hatchlings of loggerhead sea turtles. Southeast Nat 13:587–599. https://doi.org/10.1656/058.013.0318
Lee PLM, Hays GC (2004) Polyandry in a marine turtle: females make the best of a bad job. Proc Nat Acad Sci USA 101:6530–6535. https://doi.org/10.1073/pnas.0307982101
Lehikoinen A, Christensen TK, Öst KC, Kilpi M, Saurola P, Vattulainen A (2008) Large-scale change in sex ratio of a declining eider Somateria mollissima population. Wildl Biol 14:288–301. https://doi.org/10.2981/0909-6396(2008)14[288:LCITSR]2.0.CO;2
Limpus CJ (1985) A study of the loggerhead turtle, Caretta caretta, in Queensland. Dissertation. University of Queensland, Queensland
Limpus CJ (1993) The green turtle, Chelonia mydas, in Queensland: breeding males in the southern Great Barrier Reef. Wildl Res 20:513–523. https://doi.org/10.1071/WR9930513
Limpus CJ (1996) Changing fecundity with age in Queensland Caretta caretta. In: Keinath JA, Barnard DE, Musick JA, Bell CD (eds) Proceedings of the fifteenth annual symposium on sea turtle biology and conservation, NOAA technical memorandum NMFS-SEFSC-387, pp 167–169
Limpus CJ (2008) A biological review of Australian marine turtles species. 1. Loggerhead turtle, Caretta caretta (Linnaeus). State of Queensland, Environmental Protection Agency, Brisbane
Limpus CJ, Limpus DJ (2003a) Biology of the loggerhead turtle in western South Pacific Ocean foraging areas. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Institution, Washington, DC, pp 93–113
Limpus CJ, Limpus DJ (2003b) Loggerhead turtles in the equatorial and southern Pacific Ocean: a species in decline. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Institution, Washington, DC, pp 199–209
Limpus CJ, Couper PJ, Read MA (1994) The loggerhead turtle, Caretta caretta, in Queensland: population structure in a warm temperate feeding area. Mem Qld Mus 37:195–204
Lodé T, Holveck M-J, Lesbarréres D (2005) Asynchronous arrival pattern, operational sex ratio and occurrence of multiple paternities in a territorial breeding anuran, Rana dalmatina. Biol J Linn Soc 86:191–200. https://doi.org/10.1111/j.1095-8312.2005.00521.x
Magnhagen C (1991) Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186. https://doi.org/10.1016/0169-5347(91)90210-O
Miller JD (1985) Embryology of marine turtles. In: Gans C, Billett F, Maderson PFA (eds) Biology of the reptilia, vol 14. Academic Press, New York, pp 269–328
Miller JD, Limpus CJ, Godfrey MH (2003) Nest site selection, oviposition, eggs, development, hatching and emergence of loggerhead turtles. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Institution, Washington, DC, pp 125–143
Neff BD, Pitcher TE (2002) Assessing the statistical power of genetic analyses to detect multiple mating in fishes. J Fish Biol 61:739–750. https://doi.org/10.1111/j.1095-8649.2002.tb00908.x
Nielsen JT (2010) Population structure and the mating system of loggerhead turtles (Caretta caretta). Dissertations. University of Miami, Coral Gables
Olsson M, Madsen T, Shine R (1997) Is sperm really so cheap? Cost of reproduction in male adders, Vipera berus. Proc R Soc B Biol Sci 264:455–459. https://doi.org/10.1098/rspb.1997.0065
Palmer KS, Rostal DC, Grumbles JS, Mulvey M (1998) Long-term sperm storage in the desert tortoise (Gopherus agassizii). Copeia 1998:702–705. https://doi.org/10.2307/1447800
Payne NL, Gillanderts BM, Semmens J (2011) Breeding durations as estimators of adult sex ratios and population size. Oecologia 165:341–347. https://doi.org/10.1007/s00442-010-1729-7
Pearse DE, Anderson EC (2009) Multiple paternity increases effective population size. Mol Ecol 18:3124–3127. https://doi.org/10.1111/j.1365-294X.2009.04268.x
Pearse DE, Avise JC (2001) Turtle mating systems: behavior, sperm storage, and genetic paternity. J Hered 92:206–211. https://doi.org/10.1093/jhered/92.2.206
Pearse DE, Janzen FJ, Avise JC (2001) Genetic markers substantiate long-term storage and utilization of sperm by female painted turtles. Heredity 86:378–384. https://doi.org/10.1046/j.1365-2540.2001.00841.x
Pearse DE, Janzen FJ, Avise JC (2002) Multiple paternity, sperm storage, and reproductive success of female and male painted turtles (Chrysemys picta) in nature. Behav Ecol Sociobiol 51:164–171. https://doi.org/10.1007/s00265-001-0421-7
Phillips KP, Jorgensen TH, Jolliffe KG, Jolliffe S-M, Henwood J, Richardson DS (2013) Reconstructing paternal genotypes to infer patterns of sperm storage and sexual selection in the hawksbill turtle. Mol Ecol 22:2301–2312. https://doi.org/10.1111/mec.12235
Pinckney J (1990) Correlation analysis of adult female, egg and hatchling sizes in the loggerhead turtle, Caretta caretta (L), nesting at Kiawah Island, South Carolina, USA. Bull Mar Sci 47:670–679
Read T, Booth DT, Limpus CJ (2013) Effect of nest temperature on hatchling phenotype of loggerhead turtles (Caretta caretta) from two South Pacific rookeries, Mon Repos and La Roche Perc. Aust J Zool 60:402–411. https://doi.org/10.1071/ZO12079
Real KM, Schmidt DJ, Hughes JM (2009) Mogurnda adspersa microsatellite markers: multiplexing and multi-tailed primer tagging. Conserv Genet Resour 1:411–414. https://doi.org/10.1007/s12686-009-9095-7
Rousset F (2008) Genepop ‘ 007: a complete re-implementation of the genepop software for windows and linux. Mol Ecol Resour 8:103–106. https://doi.org/10.1111/j.1471-8286.2007.01931.x
Sakaoka K, Yoshii M, Nakamura H, Kureha K, Uchida I (2007) Quantitative analysis of reproductive behavior in captive loggerhead turtles (Caretta caretta). Herpetol Rev 38:395–399
Sakaoka K, Yoshii M, Okamoto H, Sakai F, Nagasawa K (2011) Sperm utilization patterns and reproductive success in captive loggerhead turtles (Caretta caretta). Chelonian Conserv Biol 10:62–72. https://doi.org/10.2744/CCB-0878.1
Sarkar S, Sarkar NK, Maiti BR (2003) Oviductal sperm storage structure and their changes during the seasonal (dissociated) reproductive cycle in the soft-shelled turtle Lissemys punctata punctata. J Exp Zool 295A:83–91. https://doi.org/10.1002/jez.a.10135
Schofield G, Lilley MKS, Bishop CM, Brown P, Katseidis KA, Dimopoulos P, Pantis JD, Hays GC (2010) Conservation hotspots: implications of intense spatial area use by breeding male and female loggerheads at the Mediterranean’s largest rookery. Endanger Species Res 10:191–202. https://doi.org/10.3354/esr00137
Shamblin BM, Faircloth BC, Dodd M, Wood-Jones A, Castleberry SB, Carroll JP, Nairn CJ (2007) Tetranucleotide microsatellites from the loggerhead sea turtle (Caretta caretta). Mol Ecol Note 7:784–787. https://doi.org/10.1111/j.1471-8286.2007.01701.x
Shuster SM (2009) Sexual selection and mating systems. Proc Nat Acad Sci USA 106:1009–1016. https://doi.org/10.1073/pnas.0901132106
Simons LW (2005) The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol Syst 36:125–146. https://doi.org/10.1146/annurev.ecolsys.36.102403.112501
Sokal R, Michener C (1958) A statistical method for evaluating systematic relationships. University of Kansas Sci Bull 38:1409–1438
Sorin AB (2004) Paternity assignment for white-tailed deer (Odocoileus virginianus): mating across age classes and multiple paternity. J Mammal 85:356–362. https://doi.org/10.1644/1545-1542(2004)085<0356:PAFWDO>2.0.CO;2
Soulsbury CD (2010) Genetic patterns of paternity and testes size in mammals. PLoS One 5:A152–A157. https://doi.org/10.1371/journal.pone.0009581
Squires ZE, Wong BBM, Norman MD, Stuart-Fox D (2012) Multiple fitness benefits of polyandry in a cephalopod. PLoS One 7:e37074. https://doi.org/10.1371/journal.pone.0037074
Steifetlen O, Dale S (2006) Viability of an endangered population of ortolan buntings: the effect of a skewed operational sex ratio. Biol Conserv 132:88–97. https://doi.org/10.1016/j.biocon.2006.03.016
Stewart KR, Dutton PH (2011) Paternal genotype reconstruction reveals multiple paternity and sex ratios in a breeding population of leatherback turtles (Dermochelys coriacea). Conserv Genet 12:1101–1113. https://doi.org/10.1007/s10592-011-0212-2
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R.project.org/
Tedeschi JN, Mitchell NJ, Berry O, Whiting S, Meekan M, Kennington WJ (2014) Reconstructed paternal genotypes reveal variable rates of multiple paternity at three rookeries of loggerhead sea turtles (Caretta caretta) in Western Australia. Aust J Zool 62:454–462. https://doi.org/10.1071/ZO14076
Theissinger K, FitzSimmons NN, Limpus CJ, Parmenter CJ, Phillott AD (2009) Mating system, multiple paternity and effective population size in the endemic flatback turtle (Natator depressus) in Australia. Conserv Genet 10:329–346. https://doi.org/10.1007/s10592-008-9583-4
Tiwari M, Bjorndal KA (2000) Variation in morphology and reproduction in loggerheads, Caretta caretta, nesting in the United States, Brazil, and Greece. Herpetologica 56:343–356. http://www.jstor.org/stable/3893411
Tucker AD (2010) Nest site fidelity and clutch frequency of loggerhead turtles are better elucidated by satellite telemetry than by nocturnal tagging efforts: implications for stock estimation. J Exp Mar Biol Ecol 383:48–55. https://doi.org/10.1016/j.jembe.2009.11.009
Tucker AD, Frazer NB (1991) Reproductive variation in leatherback turtles, Dermochelys coriacea, at Culebra National Wildlife Refuge, Puerto Rico. Herpetologica 47:115–124. http://www.jstor.org/stable/3892822
Uller T, Olsson M (2008) Multiple paternity in reptiles: patterns and processes. Mol Ecol 17:2566–2580. https://doi.org/10.1111/j.1365-294X.2008.03772.x
Uller T, Schwartz T, Koglin T, Olsson M (2013) Sperm storage and sperm competition across ovarian cycles in the dragon lizard, Ctenophorus fordi. J Exp Zool Part A Ecol Genet Physiol 319:404–408. https://doi.org/10.1002/jez.1803
Van Buskirk J, Crowder LB (1994) Life-history variation in marine turtles. Copeia 1994:66–81. https://doi.org/10.2307/1446672
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Note 4:535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Wedell N, Gage MJG, Parker GA (2002) Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol 17:313–320. https://doi.org/10.1016/S0169-5347(02)02533-8
Weir LK, Grant JWA, Hutchings JA (2011) The influence of operational sex ratio on the intensity of competition for mates. Am Nat 177:167–176. https://doi.org/10.1086/657918
Wells CP, Tomalty KM, Floyd CH, McElreath MB, May BP, Van Vuren DH (2017) Determinants of multiple paternity in a fluctuating population of ground squirrels. Behav Ecol Sociobiol 71:42. https://doi.org/10.1007/s/00265-017-2270-z
Westneat DF, Sherman PW (1997) Density and extra-pair fertilizations in birds: a comparative analysis. Behav Ecol Sociobiol 41:205–215. https://doi.org/10.1007/s002650050381
Weston Glenn JL, Civitello DJ, Lance SL (2009) Multiple paternity and kinship in the gray fox (Urocyon cinereoargenteus). Mamm Biol Z Säugetierkd 74:394–402. https://doi.org/10.1016/j.mambio.2008.10.003
Whiting AU, Chaloupka M, Limpus CJ (2008) Sampling error for hatchling turtle measurements: probing a rule-of-thumb. Copeia 2008:889–896. https://doi.org/10.1643/CH-07-194
Wibbels T, Owens DW, Limpus CJ, Reed PC, Amoss MS (1990) Seasonal changes in serum gonadal steroids associated with migration, mating and nesting in loggerhead sea turtle (Caretta caretta). Gen Comp Endocrinol 79:154–164. https://doi.org/10.1016/0016-6480(90)90099-8
Woolgar L, Trocini S, Mitchell N (2013) Key parameters describing temperature-dependent sex determination in the southernmost population of loggerhead sea turtles. J Exp Mar Biol Ecol 449:77–84. https://doi.org/10.1016/j.jembe.2013.09.001
Wright LI, Stokes KL, Fuller WJ, Godley BJ, McGowan A, Snape R, Tregenza T, Broderick AC (2012) Turtle mating patterns buffer against disruptive effects of climate change. Proc R Soc B 279:2122–2127. https://doi.org/10.1098/rspb.2011.2285
Wright LI, Fuller WJ, Godley BJ, McGowan A, Tregenza T, Broderick AC (2013) No benefits of polyandry to female green turtles. Behav Ecol 24:1022–1029. https://doi.org/10.1093/beheco/art003
Xiangkun H, Li Z, Meiying L, Huijun B, Nainan H, Qiusheng C (2008) Seasonal changes of sperm storage and correlative structures in male and female soft-shelled turtles, Trionyx sinensis. Anim Reprod Sci 108:435–445. https://doi.org/10.1016/j.anireprosci.2007.09.011
Zbinden JA, Largiader AR, Leippert F, Margaritoulis D, Arlettaz R (2007) High frequency of multiple paternity in the largest rookery of mediterranean loggerhead sea turtles. Mol Ecol 16:3703–3711. https://doi.org/10.1111/j.1365-294X.2007.03426.x
Acknowledgements
We thank the many volunteers of the turtle research program and the staff at Mon Repos Conservation Park for helping with the considerable logistics of the fieldwork. In particular, thanks to Helen Twaddle, the Limpus family, Kate Winter, Katherine Roberston and John Sergeev. We thank the Queensland Department of Environment and Heritage Protection for supporting the marine turtle research and monitoring. For helpful advice and support in the lab we thank Sophie Olsson-Pons and Nick Clark. Support for the project was provided through the Griffith School of Environment and the Environmental Futures Centre. We thank the anonymous reviewers of this manuscript for their constructive criticisms and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal ethics
Animal use in this study was conducted in conjunction with research and monitoring by the Queensland Department of Environment and Heritage Protection under their wildlife use and animal ethics permit.
Additional information
Responsible Editor: P. Casale.
Reviewed by C. Carreras and B. I. González-Garza.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Howe, M., FitzSimmons, N.N., Limpus, C.J. et al. Multiple paternity in a Pacific marine turtle population: maternal attributes, offspring outcomes and demographic inferences. Mar Biol 165, 2 (2018). https://doi.org/10.1007/s00227-017-3258-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3258-y