Abstract
Individual foraging consistency is commonly seen in wild populations, even in species considered generalists and allows individuals to forage more efficiently. It may, therefore, have important consequences on ecological processes, for individuals and populations. Within seabirds, data on timescales over which consistency is maintained is lacking, despite its potential to determine how adaptable individuals and populations are to face environmental changes. Little penguins (Eudyptula minor) were tracked at two colonies in south-eastern Australia during 5 years, using GPS data loggers and dive recorders. This study investigated the presence of behavioural consistency, its persistence through time and the influence of extrinsic and intrinsic factors on behaviour and consistency. Individual consistency was compared between colonies, among consecutive foraging trips, among different breeding stages/clutches and years. Individuals showed high plasticity, with foraging metrics influenced by site, year, stage/clutch. Low to moderate short-term consistency in foraging metrics was highlighted, except for bearing. Over larger timescales, no consistency in these metrics was detected. Mass and morphology are known to influence foraging behaviour and consistency, but seemed not to affect behavioural consistency, which varied with year and site instead. This further highlights the plasticity of animals foraging on prey highly spatially and temporally variable in their distribution. We emphasize the importance of taking timescale into account when assessing behavioural consistency. Finally, mechanisms other than behavioural consistency seem to allow little penguins to find mobile food in the water column (e.g. group foraging, and switching from short to long trips at specific times of the breeding season).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individual consistency in foraging, leading to specialisations, is widespread in wild populations, and is thought to help foragers avoid competition with conspecifics and increase their foraging efficiency (Bolnick et al. 2003; Araújo et al. 2011; Ceia and Ramos 2015). Recent research has brought to light the fact that even populations that are usually considered generalists can in fact be composed of individual specialists (Araújo et al. 2011; Loxdale et al. 2011; Matich et al. 2011; Layman and Allgeier 2012; Fodrie et al. 2015). As top predators, at the top or near the top of foodwebs, have the potential to affect prey populations and induce trophic cascades, it is important to understand their pattern of specialisations (Matich et al. 2011). Quantitative approaches to document their magnitude in populations in different contexts are necessary to understand how ecological interactions may influence the amount of among-individual variation, and how the amount of variations affects ecological dynamics (Araújo et al. 2011).
While this knowledge gap is increasingly being filled, few studies have focused on quantifying timescales over which individual specialisations are maintained. Failure to account for timescale in studies of individual specialisations likely results in inconsistent predictions regarding the effects of intraspecific variation on predator–prey interactions (Kernaléguen et al. 2015b; Novak and Tinker 2015). Studying timescales over which behavioural or dietary specialisations are maintained can help predict how adaptable individuals can be when faced with environmental changes (Hamer et al. 2007; Harris et al. 2014). Also, if maintained over long timescales, it is suggested that these specialisations can subject to natural selection and lead to species diversification (Bolnick et al. 2003; Knudsen et al. 2010; Harris et al. 2014). Such specialisations have been shown, in a few studies in marine animals, to be maintained over timescales ranging from days to years, but with decreasing consistency over long time spans (Woo et al. 2008; Harris et al. 2014).
Seabirds are top predators that face the challenge of finding food in complex and dynamic environments (Grémillet and Charmantier 2010; Cook et al. 2013). In their review, Ceia and Ramos (2015) suggested that the incidence of individual specialisations is potentially widespread, but may fluctuate spatio-temporally among/within species and populations due to the frequency of specialists, predictability of resources or environmental conditions. Within this group, some studies report on strong individual specialisations in foraging behaviour and in diet, which can sometimes be maintained over several weeks and years within the same individuals (Wanless and Harris 1993; Cook et al. 2006; Elliott et al. 2009; Ratcliffe et al. 2013; Harris et al. 2014).
Seabirds are ideal to study consistency in behaviour as they generally nest in colonies. They are central place foragers during the breeding season and often display a high level of nest fidelity, which allows the repetitive access necessary for longitudinal studies (Camprasse et al. 2017). In addition, collecting data from multiple members of the same colony allows the level of variation in behaviour between individuals that arises from specialisation to be determined since animals have access to the same resources and are exposed to the same environmental conditions (Ratcliffe et al. 2013; Ceia and Ramos 2015; Camprasse et al. 2017).
We chose to study little penguins (Eudyptula minor) as they are considered generalists but have been suggested to exhibit fidelity to specific dive depths (Ropert-Coudert et al. 2003; Kowalczyk et al. 2014). This inshore forager relies mostly on small pelagic schooling prey such as Clupeiformes (Reilly 1974; Stahel et al. 1987; Hobday 1991; Cullen et al. 1992; Chiaradia et al. 2007b; Hoskins et al. 2008; Saraux et al. 2011). Little penguins, being limited in their range and restricted to hunting during daylight hours (Reilly 1974; Dann and Norman 2006; Chiaradia et al. 2007a; Ropert-Coudert et al. 2009; Kowalczyk et al. 2015), have to find ways to increase their foraging effort, which warrant further investigation. Therefore, the aims of the present study were to: (1) determine the factors influencing foraging behaviour; (2) quantify the magnitude of behavioural consistency and assess timescales over which it is maintained; and (3) investigate the influence of various intrinsic and extrinsic factors on behavioural consistency.
Materials and methods
Data collection
Data loggers, which allow the characterisation of foraging behaviour, including GPS trackers and dive recorders, were deployed on little penguins at two colonies in south-eastern Australia, sometimes over multiple consecutive trips, in five breeding seasons (2011/2012–2015/2016; all breeding seasons are referred to as the year they started in), including different breeding stages and/or clutches. Fieldwork was conducted at two sites in south-eastern Australia, London Bridge (LB, 38°62′S, 142°93′E), a small mainland colony (ca 70–80 nests) (Berlincourt and Arnould 2014) and Gabo Island (GI, 37°56′S, 149°91′E), a large insular colony (ca 35,000 nests) (Fullagar et al. 1995) during multiple breeding seasons (2011/2012–2015/2016), either at early chick-rearing (guard stage), late chick-rearing (post-guard stage), or both, in clutch 1, clutch 2, or both. Depending on the year in which instrumentation occurred, either one or multiple consecutive trips were obtained (see Table 1 for sample sizes). GPS data loggers (I-gotU GT120, Mobile Action, Taiwan; 44.5 × 28.5 × 13.0 mm, 22.0 g in air corresponding to ca 1.0% of mean body mass) were deployed in combination with time-depth recorders (TDR, LAT1800S, Lotek Wireless Inc.; 36.0 × 11.0 × 7.2 mm, 4.8 g in air corresponding to ca 0.2% of mean body mass). GPS loggers were programmed to sample positions every 1 min or every 2 min depending on sampling years and stages. The TDR units were set to record depth and temperature at 1 or 2 s intervals depending on sampling years and stages.
Individuals were captured at the colony in their burrows, weighed in a cloth bag using a suspension scale (±10 g, Salter, Bristol, UK), and microchipped for identification. The GPS loggers—removed from their housings and encased in heat shrink plastic for waterproofing—and the dive recorders were attached to the back feathers using waterproof tape (Tesa 4651, Germany) and cyanoacrylate glue (Loctite 401, Prism, Instant Adhesive, UK). Individuals were recaptured after one or multiple consecutive trips depending on sampling year, using the method previously described. The data loggers were removed and individuals were weighed again and morphometric measurements (bill length, bill width, bill depth, head length, flipper length) were taken with a Vernier calliper and metal ruler (±0.05 and 1 mm, respectively). Handling times ranged 5–10 min at deployment and 15–20 min at retrieval, during most of which the bird’s head was covered with a hood to reduce stress.
Data processing and analysis
All data analyses were conducted in the R Statistical Environment version 3.2.0 (R Core Team 2015). Summaries of dive parameters were obtained thanks to the diveMove package (Luque 2007). A depth threshold of 1 m was used to identify dive events. The diveMove package was also used to apply a speed filter to the GPS data to remove erroneous locations with a threshold of 1.5 m s−1. The GPS data was separated into individual trips thanks to a custom-made algorithm. GPS records were linearly interpolated to the 1 s intervals in the adehabitatLT package (Calenge 2006) to provide spatial information for the dive records.
From the GPS and TDR data obtained, four foraging metrics were calculated and used, in turn, as response variables in our models: bearings (bearings from the colony to the most distal points of tracks, one measure per trip, comprised between 0° and 360°) using the circular package (Agostinelli and Lund 2011), maximum distances from the colony (distances between the colony and the most distal points of tracks, one measure per trip) using the trip package (Sumner 2009), mean bottom depths using the diveMove package (one measure per dive within trips, hereafter dive depth), and total distances travelled per hour (cumulative vertical and horizontal distances travelled divided by the duration of foraging trips, one measure per trip). When multiple trips were obtained for the same deployment, the standard deviations for bearing (circular measure) and the coefficients of variation for the other three metrics were calculated. Linear and linear mixed effects models were run in the nlme package (Pinheiro et al. 2014), and whenever applicable, full models were dredged using the MuMIn package (Barton 2013) to determine the best fixed effects, based on the models’ AIC. Linear models were run when a single observation was available per individual, while linear mixed effects models were used when repeated data for each individual were available.
To investigate the factors influencing the foraging behaviour of instrumented individuals, we used the ‘full’ dataset (i.e. data obtained from all individuals in both sites and from the 5 years of sampling, including all individuals for which GPS and TDR data covered at least one complete trip). We ran two different sets of models, one taking into account year, site, breeding stage, clutch, sex, and one taking into account mass, bill length and flipper length. Indeed, these explanatory variables could not all be used in a single model as sex and morphometrics measurements were collinear, as were mass, stages/clutches and years. Following Zuur et al. (2009), we started with models fitted with “REML”, which included all the explanatory variables considered, we compared models with and without the random effect associated with individual, and chose the models with the lowest AICs. Once we established whether a random effects structure was necessary, we inspected residuals for heterogeneity, and when necessary included a stage, clutch and year-specific variance structure. The best nesting structure, if any, was selected based on the comparisons of models with the full nested structure (individual nested within stage/clutch nested within year), all the way to models with individual only has a random effect, and all the combinations in-between. These models were compared with the anova function. Models were then refitted with “ML” to select the best appropriate fixed effects, comparing models with the anova function. Finally, models were refitted with “REML” to estimate model parameters.
To investigate whether little penguins exhibit consistency in foraging behaviour, and if so, over which timescales, four different datasets were used: “day-to-day” (i.e. data obtained on subsequent trips on the same individuals), “stage-to-stage” (i.e. data obtained in guard stage and then in post-guard stage of the same clutch, within the same year for the same individuals), “clutch-to-clutch” (i.e. data obtained either in guard or post-guard of the same clutch in clutch 1 and then in clutch 2 of the same year for the same individuals), and “year-to-year” (i.e. data obtained in the same stage and clutch in different years from the same individuals). Model selection was performed as described above. Once the optimal models were found, a variance component analysis was run following Ratcliffe et al. (2013) and Harris et al. (2014). An estimate of individual specialisation is given by the proportion of variance explained by the individual variance component (Bolnick et al. 2003; Dingemanse and Dochtermann 2013; Ratcliffe et al. 2013). In cases for which the inclusion of the random effect ‘individual’ did not improve the initial model, variance component analyses were not run as otherwise calculations are unreliable (Ratcliffe et al. 2013). For the short-term timescale, data analysis were run on the full “day-to-day” dataset [i.e. individuals for which at least two trips were obtained (n = 88 individuals and 288 trips)]. As described above, two sets of models (one for year, site, stage/clutch and sex, and for mass and morphometrics) were run to investigate what factors influence the consistency in foraging behaviour of the instrumented individuals.
Results
Factors influencing foraging behaviour
A total of 264 different individuals were sampled, as indicated in Table 1. The “full” dataset comprised 549 foraging trips (mean of 2.1 ± 1.7 trips per individual [1–13]). Little penguins stayed relatively close to the colonies and exploited shallow depths (Table 2).
Bearings to maximum distance were influenced by site, by year and by stage/clutch (linear model: F 1,539 = 7.6, P = 0.006, F 4,539 = 3.0, P = 0.02, F 3,539 = 5.1, P = 0.001, respectively). Likewise, maximum distances from the colony were influenced by site, by year and by stage/clutch (linear mixed effects model: df = 1, F = 7.3, P = 0.007, df = 4, F = 7.2, P < 0.0001, df = 3, F = 5.1, P = 0.002, respectively). Total distances per hour varied according to site, year, stage/clutch, and sex (linear model: df = 1, F = 7.5, P = 0.006, df = 4, F = 14.8, P < 0.0001, df = 3, F = 3.2, P = 0.02, df = 1, F = 5.4, P = 0.02, respectively). In the same way, dive depths varied according to site, year, stage/clutch, and sex (linear mixed effects model: df = 1, F = 127.6, P < 0.0001, df = 4, F = 9.2, P < 0.0001, df = 3, F = 17.9, P < 0.0001, df = 1, F = 10.9, P = 0.001, respectively). Bearings and maximum distances travelled were not influenced by mass and morphometric measurements.
Degree of individual consistency in behaviour and relevant timescales
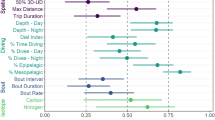
Short-term consistency in foraging behaviour over subsequent trips (“day-to-day” dataset) was investigated initially (Fig. 1). Variance component analyses were performed to determine the proportion of variance associated with the random effects, including the individual components, after model selection was performed to find out the best model for each of the four foraging metrics (Table 3). Overall, the proportion of variance associated with the individual components was low to moderate (from 3.0% for dive depths to 29.8% for bearings). When investigating stage-to-stage consistency, the models including “individual” in the random effects did not differ from the linear models for each of the four response variables of interest (all P > 0.2). The same pattern was present in the clutch-to-clutch dataset (all P > 1.0), as well as in the year-to-year dataset (all P > 1.0).
Representative examples of short-term foraging area consistency in little penguins. A single individual is shown from each study site (Gabo Island, top 2 panels, and London Bridge, bottom 2 panels), depicting moderate consistency in distances travelled over subsequent trips (indicated by different colours) and how there was little consistency between breeding stages/years; PG1 post-guard of the first clutch, PG2 post-guard of the second clutch
Factors influencing individual consistency
We investigated the effects of site, year, stage/clutch and sex on the measures of consistency for the four variables of interest (Supplemental Table S1, S2). The standard deviation in bearings averaged 0.3 [0.0–3.0], and was lower in 2014 compared to 2013 (−0.2 ± 0.08, t = −2.7, P = 0.03). The coefficients of variation for maximum distances from the colony were, on average, 0.3 [0.0–1.2], and were lower in London Bridge compared to Gabo Island (−0.09 ± 0.04, t = −2.0, P = 0.04), and in 2014/2015 compared to 2011/2012 (0.2 ± 0.09, t = −2.7, P = 0.04). The coefficients of variation in the total distanced travelled per hour averaged 0.2 [0.0–1.0], and did not differ with site, year, stage/clutch, nor sex. The coefficients of variation for dive depths were on average 0.6 [0.3–1.2], and were higher in 2014 compared to 2013 (0.1 ± 0.03, t = 3.0, P = 0.01).
Second, we looked at the effects of mass and two morphometric measurements (bill and flipper lengths) on the coefficients of variation of the variables previously mentioned (Supplemental Table S3). The best models, as judged by AIC, for bearings, maximum distances from the colony, total distances travelled per hour, and bottom dive depths were the null models including a year and stage/clutch specific variance structure.
Discussion
This study investigated the factors influencing the foraging behaviour and behavioural consistency of little penguins, and the timescales over which foraging consistency is maintained, over five consecutive years and at two sites of different oceanographic regimes in northern Bass Strait, Australia. We confirmed that little penguins were shallow divers and inshore foragers at our study sites (Gales et al. 1990; Ropert-Coudert et al. 2003; Kato et al. 2008; Kowalczyk et al. 2015). We looked at the factors influencing foraging behaviour and found that underlying differences in certain parameters among years, breeding stages, clutches and sites were influential drivers. We investigated the timescales over which foraging consistency is maintained and found low to moderate short-term consistency (subsequent trips), but no consistency for greater timescales. Consistency in foraging behaviour was not related to intrinsic factors, but instead varied between sites, years and stages and clutches.
Factors influencing foraging behaviour
Our results suggest that the factors influencing foraging behaviour in little penguins include extrinsic factors, such as year, breeding stage and clutch, and site, and to a lesser extent, sex and body mass. This is consistent with other studies reporting high flexibility in many aspects of the species’ foraging behaviour, and in some years, of their diet (Chiaradia and Nisbet 2006; Hoskins et al. 2008; Chiaradia et al. 2010; Saraux et al. 2011; Berlincourt and Arnould 2015; Kowalczyk et al. 2015). While no data on prey availability, distribution or environmental conditions is available to explain these differences, this flexibility seems to be in response to variations in prey conditions associated with different environmental conditions (Chiaradia and Nisbet 2006; Hoskins et al. 2008; Berlincourt and Arnould 2015). For example, in years of reduced food availability, little penguins have been found to increase their foraging trip duration, a proxy for maximum distances from the colony (Saraux et al. 2011), instead of decreasing the mass of meals delivered to their chicks (Chiaradia and Nisbet 2006).
The diet of little penguins comprised mostly of Clupeiformes, including anchovy, pilchard, and sprat, and exhibits relatively little variation in prey type (Hobday 1991; Cullen et al. 1992; Chiaradia et al. 2010; Deagle et al. 2010; Sutton et al. 2015). Therefore, from our results, it seems likely that the differences in foraging behaviour across space and time arise from differences in the local distribution and abundance of Clupeiformes, thought to be associated with different environmental conditions, rather than from differences in prey types (Hoskins et al. 2008). As in the present study, the importance of foraging plasticity in coastal marine predators has been highlighted in other seabird species (Ishikawa and Watanuki 2002; Lescroël and Bost 2005; Deagle et al. 2008; Castillo-Guerrero et al. 2016).
Degree of individual consistency in behaviour and relevant timescales
Ropert-Coudert et al. (2003) suggested individual little penguins maintain consistent dive depths; indeed, in their study 12 individuals were observed to be either very shallow divers consistently over two to three consecutive trips (1.9 ± 1.7 m), or consistently shallow divers (8.1 ± 4.7 m). In the present study, in contrast, we found low short-term consistency in dive depths. Similarly, there was a lack of short-term consistency in bearings to maximum distance, indicating that over subsequent days, little penguins foraged in different locations within their home range. These findings confirm that little penguins are highly plastic (Cullen et al. 1992; Chiaradia et al. 2010; Deagle et al. 2010; Sutton et al. 2015). In contrast, 20–25% of the variance in maximum distances from the colony and total distances travelled per hour was explained by the individual. This indicates that despite capturing prey at different depths and different locations each day, over the short-term little penguins remain moderately consistent in their foraging effort. This is consistent with previous studies which have shown foraging trip duration, which correlates with distances travelled, is correlated with body condition (Numata et al. 2000; Kato et al. 2008; Saraux et al. 2011).
Few previous studies have reported on the variance explained by the individual in foraging metrics of seabirds over the short-term. Interestingly, these values tend to be higher in benthic species than in species exhibiting mixed benthic and pelagic diving, and no other study reports on this in pelagic foragers. For benthic blue-eyed shags (Phalacrocorax georgianus, P. verrucosus, and P. atriceps), the proportion of variance in dive depths ranged from 41.5 to 85.1% (Ratcliffe et al. 2013; Harris et al. 2014; Camprasse et al. 2017). For species exhibiting mixed benthic and pelagic behaviour, such as thick-billed murres (Uria lomvia) and king cormorants (P. albiventer), these values dropped to 44 and 25%, respectively (Kato et al. 2000; Woo et al. 2008). While the proportion of variance in foraging metrics was not reported, two other species with mixed benthic and pelagic behaviour, Japanese (P. filamentosus) and pelagic cormorants (P. pelagicus), have been shown to display individually consistent patterns for depth usage and exhibit foraging site fidelity. These findings suggest that short-term individual consistency in foraging behaviour may be linked to the availability and distribution of prey; they are thought to be more predictable for benthic divers (Watanuki et al. 2004; Cook et al. 2006), which have an increased potential to rely on memory of seafloor features for navigation to re-visit specific areas (Davoren et al. 2003; Cook et al. 2006; Woo et al. 2008; Harris et al. 2014) compared to pelagic little penguins.
Only a few studies have followed the same individuals throughout the breeding season and between years to assess whether individual consistency throughout longer timescales is maintained (Woo et al. 2008; Elliott et al. 2009; Harris et al. 2014). All of these studies show that behavioural consistency decreased through time [see also Bell et al. (2009)]. In the present study, individual consistency in foraging metrics was only present over subsequent foraging trips, with no consistency detected for longer timescales. This suggests that little penguins feed on prey that is temporally unpredictable on timescales longer than multiple consecutive days. The fact that little penguins do not seem to exhibit long-term consistency in foraging behaviour might be beneficial in the face of rapidly changing climate, like the one experienced in the southern parts of Australia (Lima and Wethey 2012). Long-term fidelity in foraging areas and diving behaviour seem indeed to prevent individuals from adapting to rapid environmental change and from avoiding areas that have become unsuitable for foraging (Vander Zanden et al. 2016; McIntyre et al. 2017).
Our study highlights the importance of repeated sampling across various time scales, as short-term results have previously been shown to over-estimate individual consistency (Kernaléguen et al. 2015b). More trips could be obtained to confirm the degree of behavioural consistency exhibited in short time frames. For example, increasing the number of consecutive trips obtained could lead to an increased estimate of individual consistency if individuals take longer than a few days to re-visit areas where they had been successful before. Bigger samples sizes for timescales other than day-to-day also should be obtained to confirm the lack of consistency on longer timescales, as it would increase confidence in the estimates of the degree of consistency.
Factors influencing individual consistency
No effects of body mass or morphometric measurements were found on foraging behaviour consistency. In contrast, the consistency of the metrics studied was affected by the year of sampling and site. This suggests that little penguins are very plastic and respond to variations in prey availability and distribution determined by local environmental conditions instead of relying on set strategies based on individual morphology or body mass. Obtaining data on environmental conditions and prey abundance and distribution at a fine enough scale would be invaluable to be able to understand the interplay between environmental and prey conditions, time of sampling and behavioural consistency.
Strong behavioural and/or dietary consistency in seabirds is reported in species exhibiting important variations in foraging habitat and associated prey, as shown by large variations between individuals in both behaviour and diet (Cook et al. 2006; Ratcliffe et al. 2013; Harris et al. 2014). Therefore, the low consistency in foraging behaviour observed in little penguins could reflect low ecological opportunity (Hobday 1991; Cullen et al. 1992; Hoskins et al. 2008; Chiaradia et al. 2010; Sutton et al. 2015). Indeed, little penguins are less likely to have access to a wide array of prey or foraging habitats on which to specialise.
Individual consistency, leading to foraging specialisations have been suggested to help increase foraging efficiency by reducing competition with conspecifics and/or by focusing on prey individuals can easily find, handle or digest (Estes et al. 2003; Cook et al. 2006; Ceia et al. 2012; Harris et al. 2014; Kernaléguen et al. 2015a). This would seem particularly relevant for inshore and resident species, such as little penguins, with a limited foraging range (Cook et al. 2006; Ratcliffe et al. 2013; Harris et al. 2014), which could, for example, benefit from re-visiting areas where profitable prey have been encountered more easily than offshore foragers. As short-term consistency is low or moderate, little penguins appear to have other ways of increasing their foraging effort in this limited environment, such as group foraging and alternating long and short trips at specific times of the breeding season (Ropert-Coudert et al. 2004; Sutton et al. 2015).
In summary, low to moderate consistency in foraging behaviour was observed over the short-term but not over longer timescales. Both foraging behaviour and its consistency varied extensively across sites, years, breeding stages and clutches, showing that little penguins are highly plastic, which might be necessary for such small, pelagic predators with limited ranges to forage successfully. Such strategy seems to have the potential to help little penguins cope with their rapidly changing environment, as behavioural adaptations could limit their ability to do so, although this warrants further investigation. One caveat of this study, however, is the relatively low sample sizes for timescales other than short-term consistency, and limited number of trips obtained, which could have affected the estimate of individual specialisations. This study should be replicated on more pelagic divers and species generally considered generalists to see if the patterns highlighted here are widespread within this group.
References
Agostinelli C, Lund U (2011) R package circular: circular statistics (version 0.4-3). http://CRAN.R-project.org/package=circular
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14:948–958. doi:10.1111/j.1461-0248.2011.01662.x
Barton K (2013) R package MuMIn: Multi-model inference (version 1.9.5). http://CRAN.R-project.org/package=MuMIn
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Berlincourt M, Arnould JPY (2014) At-sea associations in foraging little penguins. PLoS One 9:e105065
Berlincourt M, Arnould JPY (2015) Influence of environmental conditions on foraging behaviour and its consequences on reproductive performance in Little penguins. Mar Biol 162:1485–1501
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. doi:10.1016/j.ecolmodel.2006.03.017
Camprasse ECM, Cherel Y, Arnould JPY, Hoskins AJ, Bost CA (2017a) Combined bio-logging and stable isotopes reveal individual specialisations in a benthic coastal seabird, the Kerguelen shag. PLoS One 12:e0172278
Camprasse ECM, Cherel Y, Bustamante P, Arnould JPY, Bost CA (2017b) Intra- and inter-individual variation in the foraging ecology of a generalist subantarctic seabird, the Gentoo penguin. Mar Ecol Prog Ser. doi:10.3354/meps12151
Castillo-Guerrero JA, Lerma M, Mellink E, Suazo-Guillén E, Peñaloza-Padilla EA (2016) Environmentally-mediated flexible foraging strategies in Brown boobies in the Gulf of California. Ardea 104:33–47
Ceia FR, Ramos JA (2015) Individual specialization in the foraging and feeding strategies of seabirds: a review. Mar Biol 162:1923–1938
Ceia FR, Phillips RA, Ramos JA, Cherel Y, Vieira RP, Richard P, Xavier JC (2012) Short- and long-term consistency in the foraging niche of Wandering albatrosses. Mar Biol 159:1581–1591. doi:10.1007/s00227-012-1946-1
Chiaradia A, Nisbet ICT (2006) Plasticity in parental provisioning and chick growth in little penguins Eudyptula minor in years of high and low breeding success. Ardea 94:257–270
Chiaradia A, McBride J, Murray T, Dann P (2007a) Effect of fog on the arrival time of little penguins Eudyptula minor: a clue for visual orientation? J Ornithol 148:229–233
Chiaradia A, Ropert-Coudert Y, Kato A, Mattern T, Yorke J (2007b) Diving behaviour of Little penguins from four colonies across their whole distribution range: bathymetry affecting diving effort and fledging success. Mar Biol 151:1535–1542
Chiaradia A, Forero MG, Hobson KA, Cullen JM (2010) Changes in diet and trophic position of a top predator 10 years after a mass mortality of a key prey. ICES J Mar Sci 67:1710–1720
Cook TR, Cherel Y, Tremblay Y (2006) Foraging tactics of chick-rearing Crozet shags: individuals display repetitive activity and diving patterns over time. Polar Biol 29:562–569. doi:10.1007/s00300-005-0089-y
Cook TR, Lescroël A, Cherel Y, Kato A, Bost CA (2013) Can foraging ecology drive the evolution of body size in a diving endotherm? PLoS One 8:e56297. doi:10.1371/journal.pone.0056297
Cullen JM, Montague TL, Hull C (1992) Food of little penguins Eudyptula minor in Victoria: comparison of three localities between 1985 and 1988. Emu 91:318–341
Dann P, Norman F (2006) Population regulation in little penguins Eudyptula minor: the role of intraspecific competition for nesting sites and food during breeding. Emu 106:289–296
Davoren GK, Montevecchi WA, Anderson JT (2003) Search strategies of a pursuit-diving marine bird and the persistence of prey patches. Ecol Monogr 73:463–481
Deagle BE, Gales NJ, Hindell MA (2008) Variability in foraging behaviour of chick-rearing Macaroni penguins Eudyptes chrysolophus and its relation to diet. Mar Ecol Prog Ser 359:295–309
Deagle BE, Chiaradia A, McInnes J, Jarman SN (2010) Pyrosequencing faecal DNA to determine diet of little penguins: is what goes in what comes out? Conserv Genet 11:2039–2048
Dingemanse NJ, Dochtermann NA (2013) Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol 82:39–54
Elliott KH, Woo KJ, Gaston AJ (2009) Specialization in murres: the story of eight specialists. Waterbirds 32:491–506. doi:10.1675/063.032.0402
Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE (2003) Individual variation in prey selection by Sea otters: patterns, causes and implications. J Anim Ecol 72:144–155. doi:10.1046/j.1365-2656.2003.00690.x
Fodrie FJ, Yeager LA, Grabowski JH, Layman CA, Sherwood GD, Kenworthy MD (2015) Measuring individuality in habitat use across complex landscapes: approaches, constraints, and implications for assessing resource specialization. Oecologia 178:75–87
Fullagar PJ, Heyligers PC, Crowley MA, Klomp NI (1995) Gabo Island penguin survey November 1994. MI Partners Report No 4. Moruya, NSW
Gales R, Williams C, Ritz D (1990) Foraging behaviour of the little penguin Eudyptula minor: initial results and assessment of instrument effect. J Zool 220:61–85
Grémillet D, Charmantier A (2010) Shifts in phenotypic plasticity constrain the value of seabirds as ecological indicators of marine ecosystems. Ecol Appl 20:1498–1503
Hamer K, Humphreys E, Garthe S, Hennicke J, Peters G, Grémillet D, Phillips R, Harris M, Wanless S (2007) Annual variation in diets, feeding locations and foraging behaviour of gannets in the North Sea: flexibility, consistency and constraint. Mar Ecol Prog Ser 338:295–305
Harris S, Raya Rey A, Zavalaga C, Quintana F (2014) Strong temporal consistency in the individual foraging behaviour of Imperial shags Phalacrocorax atriceps. Ibis 156:523–533. doi:10.1111/ibi.12159
Hobday DK (1991) Abundance and distribution of pilchard and Australian anchovy as prey species for the Little penguin Eudyptula minor at Phillip Island, Victoria. Emu 91:342–354
Hoskins AJ, Dann P, Ropert-Coudert Y, Kato A, Chiaradia A, Costa DP, Arnould JPY (2008) Foraging behaviour and habitat selection of the Little penguin Eudyptula minor during early chick rearing in Bass Strait, Australia. Mar Ecol Prog Ser 366:293–303. doi:10.3354/meps07507
Ishikawa K, Watanuki Y (2002) Sex and individual differences in foraging behavior of Japanese cormorants in years of different prey availability. J Ethol 20:49–54. doi:10.1007/s10164-002-0053-z
Kato A, Watanuki Y, Nishiumi I, Kuroki M, Shaughnessy P, Naito Y (2000) Variation in foraging and parental behavior of King cormorants. Auk 117:718–730. doi:10.1642/0004-8038(2000)117[0718:VIFAPB]2.0.CO;2
Kato A, Ropert-Coudert Y, Chiaradia A (2008) Regulation of trip duration by an inshore forager, the Little penguin Eudyptula minor, during incubation. Auk 125:588–593
Kernaléguen L, Arnould JPY, Guinet C, Cherel Y (2015a) Determinants of individual foraging specialization in large marine vertebrates, the Antarctic and Subantarctic fur seals. J Anim Ecol 84:1081–1092. doi:10.1111/1365-2656.12347
Kernaléguen L, Dorville N, Ierodiaconou D, Hoskins AJ, Baylis AMM, Hindell MA, Semmens J, Abernathy K, Marshall GJ, Cherel Y, Arnould JPY (2015b) From video recordings to whisker stable isotopes: a critical evaluation of timescale in assessing individual foraging specialisation in Australian fur seals. Oecologia 180:657–670. doi:10.1007/s00442-015-3407-2
Knudsen R, Primicerio R, Amundsen PA, Klemetsen A (2010) Temporal stability of individual feeding specialization may promote speciation. J Anim Ecol 79:161–168. doi:10.1111/j.1365-2656.2009.01625.x
Kowalczyk ND, Chiaradia A, Preston TJ, Reina RD (2014) Linking dietary shifts and reproductive failure in seabirds: a stable isotope approach. Funct Ecol 28:755–765
Kowalczyk ND, Reina RD, Preston TJ, Chiaradia A (2015) Environmental variability drives shifts in the foraging behaviour and reproductive success of an inshore seabird. Oecologia 178:967–979
Layman CA, Allgeier JE (2012) Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in The Bahamas. Mar Ecol Prog Ser 448:131–141
Lescroël A, Bost CA (2005) Foraging under contrasting oceanographic conditions: the Gentoo penguin at Kerguelen Archipelago. Mar Ecol Prog Ser 302:245–261
Lima FP, Wethey DS (2012) Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun 3:704
Loxdale HD, Lushai G, Harvey JA (2011) The evolutionary improbability of ‘generalism’ in nature, with special reference to insects. Biol J Linn Soc 103:1–18
Luque SP (2007) Diving behaviour analysis in R. R news 7:8–14
Matich P, Heithaus MR, Layman CA (2011) Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J Anim Ecol 80:294–305. doi:10.1111/j.1365-2656.2010.01753.x
McIntyre T, Bester MN, Bornemann H, Tosh CA, de Bruyn PJN (2017) Slow to change? Individual fidelity to three-dimensional foraging habitats in Southern elephant seals Mirounga leonina. Anim Behav 127:91–99
Novak M, Tinker MT (2015) Timescales alter the inferred strength and temporal consistency of intraspecific diet specialization. Oecologia 178:61–74
Numata M, Davis LS, Renner M (2000) Prolonged foraging trips and egg desertion in little penguins Eudyptula minor. N Z J Zool 27:277–289. doi:10.1080/03014223.2000.9518236
Pinheiro JC, Bates DM, DebRoy S, Sarkar D (2014) R package nlme: linear and nonlinear mixed effects models (version 3.1-117). http://CRAN.R-project.org/package=nlme
Ratcliffe N, Takahashi A, O’Sullivan C, Adlard S, Trathan PN, Harris MP, Wanless S (2013) The roles of sex, mass and individual specialisation in partitioning foraging-depth niches of a pursuit-diving predator. PLoS One 8:e79107
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reilly PN (1974) Breeding of little penguins along the Great Australian Bight. Emu 74:198–200
Ropert-Coudert Y, Kato A, Naito Y, Cannell BL (2003) Individual diving strategies in the Little penguin. Waterbirds 26:403–408
Ropert-Coudert Y, Wilson RP, Daunt F, Kato A (2004) Patterns of energy acquisition by a central place forager: benefits of alternating short and long foraging trips. Behav Ecol 15:824–830
Ropert-Coudert Y, Kato A, Chiaradia A (2009) Impact of small-scale environmental perturbations on local marine food resources: a case study of a predator, the Little penguin. Proc Biol Sci 276:4105–4109. doi:10.1098/rspb.2009.1399
Saraux C, Robinson-Laverick SM, Le Maho Y, Ropert-Coudert Y, Chiaradia A (2011) Plasticity in foraging strategies of inshore birds: how little penguins maintain body reserves while feeding offspring. Ecology 92:1909–1916
Stahel C, Gales R, Burrell J (1987) Little penguin: Fairy penguins in Australia. New South Wales University Press, Kensington
Sumner MD (2009) Trip: tools for the analysis of animal track data (R package version 1.1-17). http://CRAN.R-project.org/package=trip
Sutton GJ, Hoskins AJ, Arnould JPY (2015) Benefits of group foraging depend on prey type in a small marine predator, the Little penguin. PLoS One 10:e0144297
Vander Zanden HB, Bolten AB, Tucker AD, Hart KM, Lamont MM, Fujisaki I, Reich KJ, Addison DS, Mansfield KL, Phillips KF (2016) Biomarkers reveal sea turtles remained in oiled areas following the deepwater horizon oil spill. Ecol Appl 26:2145–2155
Wanless S, Harris MP (1993) Use of mutually exclusive foraging areas by adjacent colonies of Blue-eyed shags Phalacrocorax atriceps at South Georgia. Colon Waterbirds 16:176–182
Watanuki Y, Ishikawa K, Takahashi A, Kato A (2004) Foraging behavior of a generalist marine top predator, Japanese cormorants Phalacrocorax filamentosus, in years of demersal versus epipelagic prey. Mar Biol 145:427–434. doi:10.1007/s00227-004-1345-3
Woo KJ, Elliott KH, Davidson M, Gaston AJ, Davoren GK (2008) Individual specialization in diet by a generalist marine predator reflects specialization in foraging behaviour. J Anim Ecol 77:1082–1091. doi:10.1111/j.1365-2656.2008.01429.x
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Statistics for biology and health. In: Gail M, Krickeberg K, Samet JM, Tsiatis a, Wong W (eds) Mixed effects models and extensions in ecology with R. Springer, New York, NY
Acknowledgements
This study was approved by the Deakin University ethics committee and therefore meets ethics guidelines. The assistance of numerous volunteers in data collection, including Selina Kent, Lauren Angel and Melanie Wells, is gratefully acknowledged. The authors would like to thank Norman Ratcliffe for providing advice on statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Deakin University ethics committee, which approved the study. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Funding
The study was funded in part by the Holsworth Wildlife Research Endowment and the Winifred Violet Scott Trust.
Additional information
Responsible Editor: V. H. Paiva.
Reviewed by K. Shiomi and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Camprasse, E.C.M., Sutton, G.J., Berlincourt, M. et al. Changing with the times: little penguins exhibit flexibility in foraging behaviour and low behavioural consistency. Mar Biol 164, 169 (2017). https://doi.org/10.1007/s00227-017-3193-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3193-y