Abstract
Rapana venosa is one of the most widespread species of muricid gastropod and lives on all types of substrata. Although common in Bohai Sea, relatively little is known about its ecology specially foraging behavior. We examined (1) prey selection by R. venosa when offered three prey species, Manila clams Ruditapes philippinarum, blue mussels Mytilus edulis and oysters Crassostrea gigas, (2) relative importance of various behavioral components (i.e., encounter, attack, capture and consumption) to the prey selection and (3) in detail the foraging behaviors of R. venosa in the laboratory. Predation rates, prey characteristics (shell strength, energy content per prey, handling time per prey), behavioral components (encounter probabilities, foraging time budget) and major behavioral transition frequencies were analyzed. R. venosa consumed more clams per day than mussels and oysters. Predation on clams was the most effective with a consumption rate of 0.75 clams day−1, although whelks spent only 1.23 % of their time searching for prey. In comparison, 0.54 mussels day−1 were consumed while spending 2.28 % of their time searching, and 0.29 oysters day−1 were consumed occupying 3.08 % of the time. With the preference for clams over mussels and oysters resulted from R. venosa active selection, the prey has a higher profitability. The probability of capture upon attack and consumption upon capture, reflected prey shell strength and morphology, was the two most important behavioral components to determine the prey selection for clams. Searching events took place more often when offered oysters than mussels and clams. Therefore, prey species selection by R. venosa appears to be determined by differences in prey vulnerability and accessibility, and active selection of prey with the highest profitability. Our results have implications for the provision of protective refuges for species of interest (i.e., oysters), such as in population enhancement operations and bottom aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation, which plays a key role in structuring ecological communities, is an important ecological process and that many activities of both predator and prey such as assemblage, distribution and population dynamics are often driven by predation. In addition, predation of seeded bivalves often causes mortality and limits the success of culture (Flagg and Malouf 1983; Halary et al. 1994; Barbeau et al. 1996). A good knowledge of predator–prey interactions is essential to understand the predation. How predators choose their diets and hunting efficiency are crucial to our understanding of predator–prey interactions, particularly with regard to potential stabilizing properties (Elner and Hughes 1978).
In the Bohai Sea, Rapana venosa is a widely distributed and commercially important species. It inhabits all types of substrata and forages mostly on bivalve prey (Harding and Mann 1999; Savini et al. 2002; Savini and Occhipinti-Ambrogi 2006; Kosyan 2015). R. venosa is harvested usually by SCUBA for human consumption. As local demand for sea products has increased, R. venosa is being considered as a potential candidate for molluscan aquaculture, although it has been colonizing aquatic ecosystems as an invader in the other ocean, like the Adriatic Sea (Ghisotti 1974), the Aegean Sea (Koutsoubas and Voultsiadou-Koukoura 1991) and the American Atlantic Sea/Chesapeake Bay (Harding and Mann 1999). Although it is one of the most common gastropods in the Bohai Sea, relatively little is known about its ecology. As the important fishery resource, the Manila clam Ruditapes philippinarum and the oyster Crassostrea gigas have become two of four main aquacultured shellfish in China. However, during the enhancement and culture process, predation by R. venosa is usually a major constraint to the production. Consequently, providing the target bivalve with a refuge from predation has been considered. The presence of an alternative prey species may provide such a refuge, as predator may choose the alternative prey and consume fewer target bivalve. Mussels Mytilus edulis are being considered as the alternative prey, since they are less commercially important. Therefore, it is urgent to know predation of R. venosa when offered less commercial prey and commercial prey individually or simultaneously.
Selection of prey by predators could be influenced by many factors, including detectability, accessibility, easy of capture, energy content of prey, time required to handle the prey and time required for non-predatory behavior (Hughes and Dunkin 1984). According to optimal foraging theory, predators actively select specific prey to maximize the net rate of energy intake per unit foraging time (Pyke et al. 1977; Hughes 1980). Underwood et al. (2004) argued that descriptions of diets of predators as the only evidence about preference are not enough and the existence of a preference requires (and implies) an outcome of behavior by an organism. A simple concept, termed the predation cycle, can be typically characterized predation events as a series of behaviors that include searching for, attacking, capturing and consuming prey (Holling 1966; O’Brien 1979; Barbeau and Scheibling 1994). Based on this cycle, predation rates are determined by a number of behavioral characteristics: time budget, encounter rate between the predator and prey, the probability of attack upon encounter, the probability of capture upon attack and the probability of consumption upon capture (Nadeau et al. 2009). Dissection of a predation event into its component parts provides direct information about the mechanisms underlying patterns of prey selection and clarifies the interactions between predator and prey (O’Brien 1979; Cooper et al. 1985). For example, the encounter rate between the predator and prey and searching time budget could reflect the detectability of predator and the ability for prey to escape. The probability of consumption upon capture and handling time budget may reflect the accessibility, profitability and vulnerability of prey to predator. Until recently, many studies of the predation cycle have focused on crustaceans and echinoderms (Barbeau and Scheibling 1994; Mistri 2004; Wong et al. 2010; Wong 2013), but rarely on gastropods. In addition, ethograms could examine sequences in the behaviors of predators, which include the comprehensive descriptions of the characteristic behavior and clearly show the behavior transitions (Himmelman et al. 2005; Nadeau et al. 2009).
We predicted that R. venosa would select a particular prey species, since clams, mussels and oysters would differ in prey characteristics (shell strength, energy content and profitability). Specifically, we predicted that R. venosa would active select clams over mussels and oysters, resulting in clams being highest in profitability and oysters being lowest. We predicted that encounter rate would not determine the prey selection, since all of three prey species cannot escape when preying by R. venosa. We predicted that the encounter behaviors would show different relative importance in determine prey selection. Furthermore, we predicted that the probability of consumption upon capture would be lowest for oysters. Also, handling time would be highest, resulting in oysters being lowest in profitability and highest in shell strength.
Materials and methods
Experimental materials
The experiments were conducted in the Ecology Laboratory of the Institute of Oceanology, Chinese Academy of Sciences, from August to September 2015. All experimental organisms (R. venosa: 70–94 mm shell height; R. philippinarum: 21–43 mm; M. edulis: 19–48 mm; C. gigas: 20–46 mm shell length) were collected from the Laizhou Bay, Bohai Sea. R. venosa was collected by a SCUBA diver, transported to the laboratory and acclimated in individual glass aquaria for 2 weeks with seawater (26 ± 1 °C; 30 ‰ salinity) sand-filtered at 1 mm. Manila clams R. philippinarum and oysters C. gigas were collected from the coastal beach and sub-tidal rocks by ourselves, respectively. Blue mussels M. edulis were purchased from a local market, and they were collected by a local fisherman. Whelks were fed twice daily with sufficient fresh clams Scapharca subcrenata (purchased from a local aquatic market) before being starved for 6 days prior to the experiment, to standardize hunger level and predation experience level on experimental prey. Only healthy and whelks with intact shells were used. Whelks were measured as the maximum dimension from spire to canal (Table 1). The three prey species were measured for shell length and shell height (Table 1) and held in separate aquaria (50 cm long × 40 cm wide × 30 cm high; 60 L) for 1 week with seawater conditions as described above for the whelks. The photoperiod was set to 14 h light: 10 h dark. The water in the aquaria was aerated and changed regularly (>50 % volume every 2 days).
Experimental design
Prey selection, active selection and predator foraging behavior were examined in three-prey, two-prey (choice) and single-prey (no choice) experiments. To examine prey selection, three-prey experiments were designed and individual whelks were offered six clams plus six mussels and six oysters, represented by RCMO (Table 2). This treatment was replicated four times and lasted 4 days. A single R. venosa was randomly allocated to a circular experimental aquarium (r = 0.5 m, h = 0.4 m; 314 L) 24 h prior to starting an experiment, after which the 18 prey were placed in the aquarium. Two additional aquaria containing the three prey types and no predator were used as controls to monitor natural prey mortality in each experiment (over 4 days, two clams and one mussel died).
To detect active selection and examine foraging behavior, two-prey and single-prey experiments were designed. In two-prey experiments, individual whelks were offered three prey-treatment combinations: nine clams plus nine mussels, nine clams plus nine oysters and nine mussels plus nine oysters, represented by RCM, RCO and RMO (Table 2). Each treatment was replicated four times and lasted 4 days. A single R. venosa was randomly allocated to a circular experimental aquarium 24 h prior to starting an experiment, after which the 18 prey were placed in the aquarium. Two additional aquaria containing the two prey types and no predator were used as controls to monitor natural prey mortality in each experiment (over 4 days, one clam died in the experiment with nine clams plus nine mussels). In single-prey experiment, a video camera was used to record R. venosa behavior. There were three treatment combinations: the predator species with one of three prey species (clams, mussels or oysters), represented by RC, RM and RO, respectively (Table 2). Each treatment combination was replicated four times. A single R. venosa was randomly allocated to a circular experimental aquarium for 24 h prior to starting an experiment, after which 18 clams (or 18 mussels or 18 oysters) were placed in the aquarium. Each trial lasted 6 days, and the behavior of R. venosa was recorded for the first 96 h. Two aquaria without predators were used as controls to monitor natural prey mortality (no deaths occurred over 6 days).
Collection and statistical analysis of data
Predation and selectivity data
In all experiments, each aquarium was surveyed twice daily (at 08:00 a.m. and 20:00 p.m.) to count the number of prey eaten by the predator. The consumed prey was replaced with a live individual of the same species. In the single-prey experiments, the predation rate for each replicate was calculated as the number of prey animals eaten per day per predator. In the two-prey and three-prey experiments, the total numbers of each prey species eaten by the predator were counted to calculate the selection index, as follows:
where α i is the selection index for prey type i, r is the number of prey eaten by the predator, n is the total number of prey in the aquarium, and q is the total number of prey types in the environment (Chesson 1978).
The number of each prey type eaten in the two-prey experiment (choice situation) was designated as the observed frequency; the number of prey eaten in the single-prey experiment (non-choice situation) was designated as the expected frequency. If prey selection was observed, active selection was examined by comparing observed frequencies to expected frequencies using the Chi-square test with Yates’s correction for continuity or Fisher’s exact test (Liszka and Underwood 1990; Zar 1996). Expected frequencies were calculated as:
where E i and E m are the expected numbers of prey type i and prey type m eaten, respectively, in predator i–m combination; R is the number of prey type i plus the number of prey type m eaten when presented together; and S i and S m are the numbers of prey type i and prey type m eaten when presented alone, respectively (Liszka and Underwood 1990). Active selection was evident when the Chi-square value was significant.
Collection of behavioral data
Behavioral data for R. venosa during the first 96 h were acquired using a video recorder (model DS-2CD864-EI3, China) fixed 1 m above the water surface and stored in the recorder (Hikvision, DS-7604 N, China) for further analysis. Predator behaviors were quantified during continuous 96-h periods for each treatment. Predator foraging behaviors included searching for and handling the prey (Barbeau and Scheibling 1994; Wong and Barbeau 2003; Sun et al. 2015). The proportions of searching and handling time were calculated as: total searching time/total observation time, and total handling time/total observation time. In this study, searching time was estimated as the total time from predator search beginning to encounter with a prey animal; the handling time was estimated as the total time taken by the predator to manipulate and consume a single prey animal, from encounter to consumption ending. We also quantified the individual prey-handling times in each treatment.
For R. venosa, there were four outcomes that were commonly observed after an encounter: avoidance, pre-capture rejection, rejection (post-capture) and consumption. Encounters between predator and prey, and the subsequent outcomes of the encounters, were quantified (Wong and Barbeau 2003). In this study, encounter rate was calculated as the total number of encounters/total searching time (h−1). The probability of attack upon encounter, Pr{attack | encounter}, was calculated as [(encounters − avoidances)/encounters]; the probability of capture upon attack, Pr{capture | attack}, was calculated as [(rejections + consumptions)/(pre-capture rejections + rejections + consumptions)]; the probability of consumption upon capture, Pr{consumption | capture}, was calculated as [consumptions/(rejections + consumptions)]. Individual prey-handling times of R. venosa in each treatment were counted on the video recording. The foraging behavior of R. venosa is described in more detail in Results section.
Predator behavior sequences were examined using ethograms, specifically considering the states: (1) stationary, (2) moving and not foraging, (3) searching and (4) prey-handling (Nadeau et al. 2009; Sun et al. 2015). Relative frequencies of transition were calculated from the number of transitions between two behaviors divided by the total number of transitions. This analysis and the associated kinematic graphs were performed for each R. venosa–clam, R. venosa–mussel and R. venosa–oyster combination. The kinematic graphs were drawn used Microsoft Office Visio 2003 software to assist the behavior analysis.
Collection of prey characteristics data
To better understand the mechanisms underlying observed prey selection, prey characteristics (energy content, handling time per prey, prey profitability, shell strength) were measured (Wong and Barbeau 2005). To quantify energy content per prey, tissue samples from clams, mussels and oysters (n = 30) were dried for 48 h at 80 °C. Mean dry weight per prey item was calculated from the number of prey items divided by the total dry weight. Mean energy per unit dry weight was measured using an oxygen bomb calorimeter (Parr Instrument Company). The energy content per prey item was calculated as the mean dry tissue weight multiplied by the mean energy content per unit dry weight. Handling time per prey item was measured during video analysis. Prey profitability was calculated from its mean energy content divided by mean handling time per prey item (Stephens and Krebs 1986). A Shore hardness tester was used to measure the shell strength of the three prey species.
Statistical analysis
The selectivity indices were analyzed using an independent sample T test. Predation rates, behavioral datum (proportions of searching time, proportions of handling time, encounter rates, probabilities of attack upon encounter, probabilities of capture upon attack and probabilities of consumption upon capture), transitions between the four major behaviors, searching events (stationary to searching, moving to searching and handling to searching) and prey characteristics (energy content, handling time per prey, prey profitability and shell strength) were analyzed using one-way ANOVA. Levene’s test was used to test the assumption of homogeneity of variances. Square root transformation of the probability of attack upon capture was performed to obtain homogeneity of variance. For multiple comparisons, Duncan’s test was used to compare the means. Probabilities of transition in the ethograms for predator–clam, predator–mussel and predator–oyster combinations were compared using the independence test (Chi-square test). All data are reported as mean values ± standard deviation (mean ± SD). Statistical analyses were performed using SPSS 22.0 software.
Results
Prey characteristics
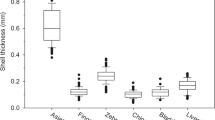
The energy contents, handling times per prey item, profitability and shell strengths of clams, mussels and oysters are shown in Table 3. There were significant differences among the three prey species in energy content (MS t = 20.83, MS e = 0.046, F 2,6 = 438.079, P < 0.001). The mean energy content per clam was significantly higher than per mussel, and the mean energy content per mussel was significantly higher than per oyster. There were no significant differences among the handling times of clams, mussels and oysters (MS t = 12.717, MS e = 4.218, F 2,7 = 3.015, P = 0.114). For profitability, there were significant differences among the three prey species (MS t = 4.121, MS e = 0.114, F 2,7 = 36.289, P < 0.001). Profitability was significantly higher for clams than for mussels and oysters, but there was no significant difference between mussels and oysters. There were significant differences among the three prey species in shell strength (MS t = 1869.62, MS e = 14.08, F = 132.787, P < 0.001). It was significantly lower for mussels than for clams and oysters, and the shell strength of oysters was significantly higher than that of clams.
Predation rates and prey selection
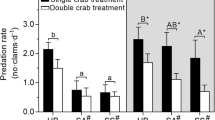
In the single-prey experiments, the predation rates of R. venosa on clams, mussels and oysters were 0.75 ± 0.21, 0.54 ± 0.25 and 0.29 ± 0.25 day−1, respectively (Fig. 1). There was a statistically significant difference among clams, mussels and oysters consumed by R. venosa (MS t = 0.210, MS e = 0.043, F 2,9 = 4.857, P = 0.037). The predation rate on clams was significantly higher than on oysters, but the differences between clams and mussels, and between mussels and oysters, were not significant. In the three-prey experiment, no oysters were consumed by R. venosa and the predation rates on clams and mussels were 1.25 ± 0.35 and 0.13 ± 0.14 day−1.
The prey selectivity indices in the RCO combination are shown in Fig. 2a. The clam selectivity index was 1, and the oyster selectivity index was 0; that is, only clams were consumed by R. venosa in this combination. In the RCM combination (Fig. 2b), the clam selectivity index (0.83 ± 0.12) was significantly higher than the mussel selectivity index (0.17 ± 0.12) (t 6 = 7.979, P < 0.001). In the RMO combination (Fig. 2c), when mussels and oysters were presented together, only mussels were consumed; that is, in this combination, the mussel selectivity index was 1 and the oyster selectivity index was 0. In the RCMO combinations (Fig. 2d), the oyster selectivity index was 0 and the clam selectivity index (0.92 ± 0.10) was significantly higher than the mussel selectivity index (0.08 ± 0.10) (t 6 = 12.001, P < 0.001). R. venosa strongly selected clams over mussels and oysters in all experiments.
Active selection was detected underlying observed selection, and the results are shown in Table 4. The numbers of clams, mussels and oysters consumed in the two-prey experiments differed significantly from the expected numbers calculated from the single-prey experiments, indicating that R. venosa actively selected one type of prey when given a choice. When clams were presented together with oysters or mussels, R. venosa actively selected clams. In the presence of oysters and mussels, R. venosa actively selected mussels.
Foraging behavior
Behavioral observations
When not foraging, R. venosa remained quiescent on the bottom of the aquarium or on the wall near the water surface, or moved in a circular route. The foraging behavior was similar for the three bivalves used in this study. R. venosa searched for prey by probing the bottom and moving slowly with the foot. The search path changed with the position of the prey. Actual contact between R. venosa and a prey animal was considered an encounter. Upon encountering a prey, R. venosa attacked with the foot flipping over the prey or pushing the shell of the prey. Escape behavior was not observed by any of the prey species in this study. Avoidance by R. venosa was considered to have occurred when its foot or shell lightly touched the edge of the shell of an encountered prey, and it moved away. Capture was deemed to have occurred when the prey was covered by the foot of the whelk. Handling of the prey began when the whelk completely enclosed the prey and ended when the whelk rejected or consumed the prey. In this study, the whelk consumed prey by boring into the edges of the shell valves or by smothering the prey, and then digesting its tissues.
Encounter rate between R. venosa and prey
Encounter rates with clams, mussels and oysters are shown in Fig. 3 (MS t = 50.927, MS e = 6.610, F 2,9 = 7.705, P = 0.011). The values were 5.08 ± 1.87, 8.91 ± 3.38 and 12.2 ± 2.22 prey items per search time for clams, mussels and oysters, respectively. The encounter rate with oysters was significantly higher than with clams; there were no significant differences between mussels and clams, or between mussels and oysters. R. venosa’s search efficiency was higher when offered oysters than offered clams.
Encounter behaviors between R. venosa and prey animals
In the single-prey experiments, the probabilities of attack upon encounter of R. venosa with clams, mussels or oysters were 72 ± 33, 62 ± 15 and 50 ± 12 %, respectively (Fig. 4a), and there were no significant differences among these groups (MS t = 0.017, MS e = 0.020, F 2,9 = 0.811, P = 0.475). This behavior component appeared not to be an important component of the observed selection.
The probabilities of capture upon attack by R. venosa on clams, mussels or oysters were 100, 84 ± 21 and 46 ± 18 %, respectively (Fig. 4b) (MS t = 0.306, MS e = 0.025, F 2,9 = 12.401, P = 0.003). All attacked clams were captured by R. venosa, but about half attacked oysters were rejected. The probabilities of capture upon attack for clams and mussels were significantly higher than for oysters. The probability of capture upon attack for clams was not significantly different than for mussels. This behavior component was the main mechanisms determining observed selection of R. venosa for three prey species.
The probabilities of consumption upon capture of clams, mussels or oysters were 80 ± 14, 35 ± 16 and 23 ± 5 %, respectively (Fig. 4c) (MS t = 0.0362, MS e = 0.016, F 2,9 = 22.286, P < 0.001). R. venosa rejected captured oysters relatively often (1–23 % of captures), but consumed almost all captured clams. The probability of consumption upon capture was significantly higher for clams than for mussels and oysters. There was no significant difference between the mussels and the oysters. This behavior component was the main mechanisms determining observed selection of R. venosa for three prey species.
R. venosa foraging time budget
Rapana venosa spent 1.23 ± 0.52, 2.28 ± 1.23 and 3.08 ± 0.35 % of their time searching for clams, mussels and oysters, respectively (Fig. 5a) (MS t = 3.5 × 10−4, MS e = 0.6 × 10−4, F 2,9 = 5.461, P = 0.028). The time budget of searching for oysters was significantly greater than for clams; there were no significant differences between mussels and clams, or between mussels and oysters.
The relative handling times for the different prey are shown in Fig. 5b (MS t = 0.073, MS e = 0.014, F 2,9 = 5.130, P = 0.033). R. venosa spent 35.97 ± 15.23 % of their time budget handling mussels, which was significantly higher than handling clams (about 9.05 ± 3.25 %). They spent 20.52 ± 13.56 % of their time budget handling oysters, which was not significantly different from clams or oysters. Handling time budgets were inconsistent among the three prey species. This variability may be related to the different methods used to open the bivalve prey by R. venosa.
Ethograms of R. venosa foraging clams, mussels and oysters
Ethograms for R. venosa preying on clams, mussels and oysters are shown in Fig. 6. The main transitions in the ethograms were between stationary state, moving and searching. The most frequent transitions were from moving to being stationary (24–32.4 %) and from being stationary to moving (19.1–29.3 %). The third and fourth most frequent change were from being stationary to searching (12.9–15.6 %) and searching to being stationary (7.0–8.9 %). In addition, handling behavior only transitioned from searching behavior. Predation behavior was linear and rarely by-passed the main sequence. Transitions between the four major behaviors in the R. venosa–oyster system (124 ± 18.0) were significantly higher than in the R. venosa–clam system (56 ± 23.8) and R. venosa–mussel system (64 ± 16.2) (MS t = 5500.08, MS e = 385.19, F 2,9 = 14.279, P = 0.002) (Fig. 7a). There were no significant differences among the three kinematic graphs (Table 5), although there were significantly more searching events (stationary to searching, moving to searching, handling to searching) for oysters (31.5 ± 8.3) than for clams (9.8 ± 3.4) or mussels (15.3 ± 5.9) (MS t = 511.58, MS e = 38.50, F 2,9 = 13.288, P = 0.002) (Fig. 7b). It suggests that R. venosa would be more active when preyed on oysters than on clams and mussels.
Discussion
Prey selection and foraging behavior
Rapana venosa strongly selected clams (R. philippinarum) when all three prey species were offered concurrently. R. venosa actively selected particular prey species when offered two species of prey concurrently. Clams were always selected when presented together with mussels (M. edulis) or oysters (C. gigas), and R. venosa selected mussels when offered mussels and oysters concurrently. In single-prey experiments, clams were generally consumed in the greatest number by R. venosa. These results imply that R. venosa preferred clams, followed by mussels and oysters. We predicted that R. venosa would select clams over mussels and oysters and our predictions were supported. In our study, handling time per prey did not provide a useful framework to understand selection since it did not differ much among clams, mussels and oysters; differences in energy content resulted differences in profitability (Table 3). From energy analyses, prey selection of R. venosa may be based on selection of prey with the highest energy content or profitability. The selection of clams is consistent with the optimal foraging theory (Hughes 1980) that predators actively select prey to maximize the rate of energy intake. In addition, proportion of time spent searching for clams was lower than mussels and oysters. These results confirm previous study made by Savini and Occhipinti-Ambrogi (2006), who tested prey preference of R. venosa in the Northern Adriatic Sea. They found that R. venosa behaved as a “pursuer” searched and consumed for specific prey (S. inaequivalvis), thus maximizing its net rate of energy intake.
Active selection was detected since R. venosa always consumed significantly more clams than expected when given a choice of prey types than when not given a choice (Table 4), suggesting that predation events (encounter, attack, capture and consumption) may associated with active selection by the R. venosa. In our study, the probability of attack upon encounter did not vary among prey types and so did not help to explain predation patterns. Despite the lowest encounter rate between whelks and clams, predation rates on clams were the highest. Therefore, encounter rates between whelks and prey types were unlikely to be important in such small mesocosms as those we used. Prey selection by R. venosa appeared to be due to a combination of the probability of capture and the probability of consumption. Both the probability of capture upon attack and the probability of consumption upon capture were the highest for clams (Fig. 4). In other words, clams experienced the highest predation rates because they were easier to capture and consume than mussels and oysters. According to the literature data, juvenile and small-sized R. venosa are feeding by drilling through the bivalve shell, whereas large snails can attack and consume bivalves use two methods edge drilling and suffocation (Chukhchin 1984; Harding et al. 2007; Kosyan 2015). Rapa whelks were large enough to manipulate their prey with the latter two methods in our study. Clams with smooth and low strength shell were easy to be wrapped (capture) by the foot of whelks and to be opened by edge drilling (consume). Oysters have the highest shell strength; therefore, they are difficult to drill by R. venosa. In addition, difficulty also comes from that the shell of oysters always tightly closed (Dietl 2003). The lowest probability of capture and consumption and highest handling time for oysters mostly likely resulted from the anti-predatory behavior and characteristic of oysters. In other words, oysters are less vulnerable and accessible to the predation by R. venosa than clams and mussels.
The encounter rate between R. venosa and clams was significantly lower than for oysters although the predation rate on clams was significantly higher than on oysters (Figs. 1, 3). This result may be explained by satiation. Whelks offered clams quickly reached satiation, whereas those offered oysters did so more gradually. Hughes and Dunkin (1984) found that hungrier dogwhelks Nucella lapillus crawled faster in straighter paths and spent more time for searching and less hunger dogwhelks would tend to restrict their searching close to original site. In our study, whelks offered oysters spent more time searching for prey (Fig. 2a) and improved search efficiency (encounter rate) to regulate starvation. This finding suggests that R. venosa applies similar searching strategy with dogwhelks to regulate starvation. Based on our comparison of the ethograms of R. venosa in different prey environments, transitions between the four major behaviors in the R. venosa–oyster system were much more frequent than in the R venosa–clam system and R venosa–oyster system (Figs. 6, 7a). Searching events in the R. venosa–oyster system were also significantly more frequent than in the R. venosa–clam system and R. venosa–mussel system (Fig. 7b). These results indicated that foraging activities were reinforced to regulate starvation when offered oysters. We speculate that R. venosa would more active when preying on non-preferential prey than preferential prey.
Until recently, sequential behavioral analysis using ethograms has rarely been used with marine animals (but see Himmelman et al. 2005; Nadeau et al. 2009; Sun et al. 2015). This behavioral approach provides a synoptic overview of the major behavioral transitions that occur within a predator–prey system and could complement or support analyses resulting from simple behavioral observations (Nadeau et al. 2009). The predation patterns of R. venosa on three prey species were similar (Table 4). Transitions mainly took place between stationary, non-foraging displacement and searching. R. venosa predation behavior was linear and rarely by-passed the main sequence. This predation pattern was similar to that of sea stars studied by Nadeau et al. (2009). Rapa whelks and sea stars both move slowly (<10 cm min−1) and consequently have low searching efficiency and handle each prey animal slowly (3 h~), which may explain the similarity.
Implications for practice and benthic communities
Our study has applications for enhancement of commercial bivalves. In single-prey experiments, all three prey species were consumed, substantially confirming R. venosa’s broad dietary capabilities (Mann and Harding 2003). Our study suggests that if R. venosa is the main predator of seeded oysters, additional mussels could be seeded for protect oyster, since mussels have a lower economic interest than clams. Mussels have a higher profitability and lower shell strength than oysters, which could lead to R. venosa active select mussels. In addition, clumps of mussels should be separated, because of interactions with clusters of conspecifics, which inhibit movement of the predators. However, increased prey density may result in aggregations of predators (Taylor 1984). Also, a new prey species may draw into new predator species such as crabs and sea stars. These potential issues must be taken care. In our study, all three prey species were offered with no refuge (sediment) to R. venosa. Actually, whether there is a refuge may influence the selection of R. venosa. Munari and Mistri (2011) studied the effect of short-term hypoxia on R. venosa prey preference. They offered three prey species: ark shell Scapharca inaequivalvis and Manila clam Tapes philippinarum and cockle Cerastoderma glaucum, and found that R. venosa preferred S. inaequivalvis under normoxia, while preferred T. philippinarum after short-term hypoxia, since clams migrated vertically into the sediment, to regulate hypoxia, becoming more vulnerable to the predator. So, further study with the sediment or specific study in the filed should be implemented.
The interaction between predator and prey likely influences the abundance and distribution of predator and prey species. It is interesting that natural populations of mussels are almost absent from Laizhou Bay (can only be found on coastal rocky bottom). This may be attributed to the predation pressure from R. venosa due to a preference for this bivalve species. Conversely, no preference for oysters by R. venosa might explain the large numbers of this prey species. Sun et al. (2016) showed that natural Manila clams not only escape from predation by burrowing into sand but also hiding behind rock. Obviously, the latter way that clams use to avoid whelk predation has failed, since clams cannot be found in the rocky bottom community. This interaction might explain the distribution of clams that this species only abundant on the sandy bottom in the Laizhou Bay. Classical ecological theory suggests that prey share common enemies are unstable, leading to one species being eliminated from the interaction (Munari and Mistri 2011). The reason is often due to the different susceptibility of prey species to enemy, which results in one species “winning” and the other “losing” in the indirect interaction (Bonsall and Hassell 1997). The winning of the oyster in our study is determined by its higher shell strength and anti-predatory behavior. These prey characteristics make the oyster distribute on almost all rocky bottom in the Laizhou Bay. As the most abundant benthic predator, the large numbers of R. venosa may due to this species are probably capable of attacking virtually all components of the Bohai Sea invertebrate fauna.
References
Barbeau M, Scheibling RE (1994) Behavioural mechanisms of prey size selection by sea stars (Asterias vulgaris Verrill) and crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus Gmelin). J Exp Mar Biol Ecol 180:103–136
Barbeau MA, Hatcher BG, Scheibling RE, Hennigar AW, Taylor LH, Risk AC (1996) Dynamics of juvenile sea scallop (Placopecten magellanicus) and their predators in bottom seeding trials in Lunenburg Bay, Nova Scotia. Can J Fish Aquat Sci 53:2494–2512
Bonsall MB, Hassell MP (1997) Apparent competition structures ecological assemblages. Nature 388:371–373
Chesson J (1978) Measuring preference in selective predation. Ecology 59:211–215
Chukhchin VD (1984) Ecology of the gastropod molluscs of the Black Sea. Acad Sc USSR Kiev Naukova Dumka 1984:175
Cooper SD, Smith DW, Bence JR (1985) Prey selection by freshwater predators with different foraging strategies. Can J Fish Aquat Sci 42(11):1720–1732
Dietl GP (2003) Coevolution of a marine gastropod predator and its dangerous bivalve prey. Biol J Linn Soc 80:409–436
Elner RW, Hughes RN (1978) Energy maximization in the diet of the short crab, Carcinus maenas. J Anim Ecol 47:103–116
Flagg PJ, Malouf RE (1983) Experimental plantings of juveniles of the hard clam Mercenaria mercenaria (Linne) in the waters of Long Island, New York. J Shellfish Res 3:19–27
Ghisotti F (1974) Rapana venosa (Valenciennes), nuova ospite Adriatica? Conchiglie, Milano 10:125–126
Halary C, Royer Y, Corlouer JP, Dao JC (1994) Effects of predation and competition on scallop, Pecten maximus, seabed cultivation in Saint Brieuc Bay: preliminary results. Proceedings on the 9th International Pectinid Workshop, Nanaimo, BC, Canada 1993:39–49
Harding JM, Mann R (1999) Observations on the biology of the veined Rapa Whelk, Rapana venosa, (Valenciennes, 1846) in the Chesapeake Bay. J Shellfish Res 18:9–17
Harding JM, Kingsley-Smith P, Savini D, Mann R (2007) Comparison of predation signatures left by Atlantic oyster drills (Urosalpinx cinerea Say, Muricidae) and veined rapa whelks (Rapana venosa Valenciennes, Muricidae) in bivalve prey. J Exp Mar Biol Ecol 352:1–11
Himmelman JH, Dutil C, Gaymer CF (2005) Foraging behavior and activity budgets of sea stars on a subtidal sediment bottom community. J Exp Mar Biol Ecol 322:153–165
Holling CS (1966) The functional response of invertebrate predators to prey density. Mem Entomol Soc Can 48:1–87
Hughes RN (1980) Optimal foraging theory in the marine context. Oceanogr Mar Bio Ann Rev 18:423–481
Hughes RN, Dunkin SDB (1984) Behavioural components of prey selection by dogwhelks, Nucella lapillus (L.), feeding on mussels, Mytilus edulis L., in the laboratory. J Exp Mar Biol Ecol 77(1):45–68
Kosyan A (2015) Predation mechanisms of Rapana venosa (Gastropoda: Muricidae) in different biotopes along the Black Sea coast. Mar Pollut Bull 102(2):265–270
Koutsoubas D, Voultsiadou-Koukoura E (1991) The occurrence of Rapana venosa (Valenciennes, 1846) (Gastropoda, Thaididae) in the Aegean Sea. Boll Malacol Milano 26:201–204
Liszka D, Underwood AJ (1990) An experimental design to determine preferences for gastropod shells by a hermit-crab. J Exp Mar Biol Ecol 137:47–62
Mann R, Harding JM (2003) Salinity tolerance of larval Rapana venosa: implications for dispersal and establishment of an invading predatory gastropod on the North American Atlantic coast. Biol Bull 204:96–103
Mistri M (2004) Effects of hypoxia on predator–prey interactions between juvenile Carcinus aestuarii and Musculista senhousia. Mar Ecol Prog Ser 275:211–217
Munari C, Mistri M (2011) Short-term hypoxia modulates Rapana venosa (Muricidae) prey preference in Adriatic lagoons. J Exp Mar Biol Ecol 407(2):166–170
Nadeau M, Barbeau MA, Brêthes JC (2009) Behavioural mechanisms of sea stars (Asterias vulgaris Verrill and Leptasterias polaris Müller) and crabs (Cancer irroratus Say and Hyas araneus Linnaeus) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)), and procedural effects of scallop tethering. J Exp Mar Biol Ecol 374(2):134–143
O’Brien WJ (1979) The predator–prey interaction of planktivorous fish and zooplankton. Am Sci 67:572–581
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52(2):137–154
Savini D, Occhipinti-Ambrogi A (2006) Consumption rates and prey preference of the invasive gastropod Rapana venosa in the Northern Adriatic Sea. Helgol Mar Res 60(2):153–159
Savini D, Harding JM, Mann R (2002) Rapa whelk Rapana venosa (Valenciennes, 1846) predation rates on hard clams Mercenaria mercenaria (Linnaeus, 1758). J Shellfish Res 21(2):777–779
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Sun Y, Wang F, Dong S (2015) A comparative study of the effect of starvation regimes on the foraging behavior of Portunus trituberculatus and Charybdis japonica. Physiol Behav 151:168–177
Sun Y, Wang F, Liu D, Dong S (2016) Behavioral mechanisms underlying the functional response of the swimming crab Portunus trituberculatus preying on the Manila clam Ruditapes philippinarum. Mar Biol 163(5):1–9
Taylor RJ (1984) Predation. Chapman and Hall, New York
Underwood AJ, Chapman MG, Crowe TP (2004) Identifying and understanding ecological preferences for habitat or prey. J Exp Mar Biol Ecol 300(1):161–187
Wong MC (2013) Green crab (Carcinus maenas (Linnaeus, 1758)) foraging on soft-shell clams (Mya arenaria Linnaeus, 1758) across seagrass complexity: behavioral mechanisms and a new habitat complexity index. J Exp Mar Biol Ecol 446:139–150
Wong MC, Barbeau MA (2003) Effects of substrate on interactions between juvenile sea scallops (Placopecten magellanicus Gmelin) and predatory sea stars (Asterias vulgaris Verrill) and rock crabs (Cancer irroratus Say). J Exp Mar Biol Ecol 287:155–178
Wong MC, Barbeau MA (2005) Prey selection and the functional response of sea stars (Asterias vulgaris Verrill) and rock crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus Gmelin) and blue mussels (Mytilus edulis Linnaeus). J Exp Mar Biol Ecol 327:1–21
Wong MC, Peterson CH, Kay J (2010) Prey size selection and bottom type influence multiple predator effects in a crab–bivalve system. Mar Ecol Prog Ser 409:143–156
Zar JH (1996) Biostatistical analysis. Prentice-Hall Inc., Englewood Cliffs NJ
Acknowledgments
We thank Dr. Yunfei Sun for experiment suggestions and valuable advice in writing the article. This work was supported by the Natural Science Foundation of China (Grant No. 31572636), the Agricultural Major Application Technology Innovation Project of Shandong Province, the NSFC-Shandong Joint Fund for Marine Science Research Centers (Grant No. U1406403).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that he/she has no conflict of interest.
Human and animal rights
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: F. Bulleri.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Hu, N., Wang, F., Zhang, T. et al. Prey selection and foraging behavior of the whelk Rapana venosa . Mar Biol 163, 233 (2016). https://doi.org/10.1007/s00227-016-3006-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-3006-8