Abstract
The non-indigenous European green crab (Carcinus maenas) has well-documented impacts on many native species. In the Atlantic Canada region, the green crab distribution is increasingly overlapping with the distribution of mud crabs (primarily Dyspanopeus sayi), a prominent native species. Despite the potential for antagonistic interactions, the relationship between the two species has not been examined, particularly in the context of the diversity of habitats available in the region. This study used observational beach seine data collected between 2009 and 2013 from the southern Gulf of St. Lawrence to explore the temporal and spatial relationships between mud crabs and green crabs, and detected a negative relationship between these species. Twenty-four-hour laboratory experiments examined their predator–prey interactions and assessed the influence of habitat complexity on the outcomes of these interactions. Mud crabs and similar-sized green crabs collected during July and August of 2010 and 2011 were used as prey for large green crab. These predators consumed almost twice as many mud crab compared with juvenile green crab in the two less structured habitats (no substrate or sandy substrate), but predation rates were statistically similar in oyster bed habitat. In that particular habitat, mud crab mortality dropped by ~65 %, whereas the generally lower mortality affecting juvenile green crabs was unaffected by habitat. These results suggest that complex habitats mediate predator–prey interactions and dampen the effect of green crab prey preference. As green crab continues to invade areas dominated by mud crabs, they may threaten the sustainability of this native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation plays a major role in the regulation and structuring of prey populations and communities (Connell 1961; Luppi et al. 2001). On sedimentary bottoms, most predators and prey are mobile and have developed behavioral abilities to seek prey and avoid predators, respectively. Decapod crustaceans, in particular crabs, are an interesting group of predators given their broad range of interactions and the consequences of these interactions for other species. For example, some predatory crabs have been shown to affect size structure of bivalve prey (Peterson 1982), whereas others structure communities (Botto and Iribarne 1999) or modify the distribution or behavior of individual prey, including decapod species (McDonald et al. 2001). It is well established that the habitat in which predators and prey interact has a strong influence on the outcome of these interactions (Diehl 1992; Ebersole and Kennedy 1995; Finke and Denno 2002; Hill and Weissburg 2013). In complex habitats (sea grass, seaweed, or bivalve beds, for example), prey may seek refuge from predators more easily than in structurally simple habitats such as muddy or sandy sediments. Meanwhile, predators may become less efficient at foraging for prey (Crowder and Cooper 1982).
The spread of marine non-indigenous predators provides an opportunity to study predator–prey interactions in relation to habitat (Sih et al. 2010). Native species may be naïve or unprepared to avoid or overcome mortality due to new predators, or these predators may disrupt well-established interactions that often allow native species to persist (Ricciardi and Atkinson 2004; Paolucci et al. 2013). In many examples, the introduction of a new predator has also displaced or caused local extinctions of native prey, in some cases leading to declines in overall biodiversity (Mills et al. 1993; Cohen et al. 1995; Grosholz and Ruiz 1996). In this context, it is important to gain insight into how different factors, including new predators and habitat complexity, interact to affect the outcome of predator–prey interactions.
The European green crab (Carcinus maenas Linnaeus, 1758) is an invasive species in various parts of the world including North America, South Africa, Australia, South America, and Asia. Green crabs first invaded the eastern American coast in 1817 and expanded northward to Maine by the early 1900s (Audet et al. 2003). Over the next 50 years, green crabs continued their northward colonization up to the Bay of Fundy in Canada, reaching Prince Edward Island by 1997 (Audet et al. 2003). The green crab is a voracious predator that feeds on an array of small bivalves and small crustaceans, including younger life stages of its own species (Baeta et al. 2006). Previous research indicates that native prey survival when facing a predator like the green crab will be higher in habitats that are structurally more complex (Crowder and Cooper 1982; Fernández et al. 1993; Fernández 1999; Hill and Weissburg 2013; Hernández Cordero and Seitz 2014). However, it does not elucidate how habitat will affect green crab interspecific and intraspecific prey preference and predation rates.
Several studies have shown that green crabs prey upon or displace native crustaceans from their habitat (McDonald et al. 2001; Jensen et al. 2002; Rossong et al. 2006; Williams et al. 2009). This may become the case for mud crabs (Dyspanopeus sayi Smith, 1869), a small species native to Canada that is well established in habitats like sandy and muddy sediments and oyster beds. Because mud crabs and green crabs use some of the same habitats, it has been suggested that these species may interact negatively as green crab populations continue to grow and spread (Breen and Metaxas 2009). Our own observations (unpublished) and observations of others (Lloyd 1968; Cushing 1991) also suggest that adult green crabs are predators of mud crabs and juvenile green crabs, which raises two related questions: is there evidence of a spatial/temporal negative relationship between these species? And if so, are predator–prey interactions a mechanism mediating this relationship?
In this study, we assessed abundance data from a large dataset of beach seine surveys conducted in multiple estuaries in the southern Gulf of St Lawrence over the course of five years to explore potential negative relationships between green crabs and mud crabs. We then conducted experiments that manipulate habitat complexity to investigate how habitat influences green crab predation rates and prey preferences when presented with small native mud crabs and juvenile green crabs. In addition to mortality, we assessed injury rates in order to examine the incidence of sublethal effects. Our null hypothesis was that prey mortality and injury levels would be similar between prey species (mud crab and green crab) regardless of habitat type (no substrate, sandy sediments, and oyster bed mimics). However, based on previous studies that examined the effects of habitat complexity on predator–prey relationships, we expected prey mortality to decrease with increasing habitat complexity (Crowder and Cooper 1982; Fernández et al. 1993; Fernández 1999; Hill and Weissburg 2013; Hernández Cordero and Seitz 2014).
Methods
2009–2013 Crab monitoring in the southern Gulf of St. Lawrence
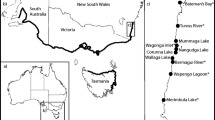
The relative abundance of crab species (including green crab and mud crab) was estimated in 29 estuaries of the southern Gulf of St. Lawrence using beach seine nets as part of the Community Aquatic Monitoring Program (CAMP) (Fig. 1). The CAMP program is a Citizen Science monitoring program that collects data about fish, macro-crustaceans (including crabs), and local physical parameters in estuaries of the Canadian Maritimes: New Brunswick (NB), Nova Scotia (NS), and Prince Edward Island (PEI). A detailed description of the study area and sampling methodology can be found in Weldon et al. (2005); briefly, six stations per estuary were sampled once per month during June, July, and August of each year by pulling a 30 × 2 m (6 mm mesh) beach seine in the shallow subtidal of each station. Sandy sediments and scattered oyster beds, two of the habitats represented in the laboratory experiments (see below) in addition to eelgrass beds and salt marshes, are prominent features on a large majority of these estuaries.
Outline of the study area and map of the 29 sample locations. Estuary codes are as follows: ANTI: Antigonish; BASH: Basin Head; BOUC: Bouctouche; BRUD: Brudenell; CHET: Cheticamp; COCA: Cocagne; DESA: Desable; HILL: Hillsborough; JOUR: Jourimain; LAME: Lameque; MABO: Mabou; MALP: Malpeque Bay; MIRA: Miramichi; MURR: Murray River; PELE: Cap Pelé; PICT: Pictou; PINE: Pinette; POKE: Pokemouche; PUGW: Pugwash; RICH: Richibouctou; SCOU: Scoudouc; SHED: Shediac; SHIP: Shippagan; SOUR: Souris; STLO: St. Louis de Kent; SUMM: Summerside; TABU: Tabusintac; TATA: Tatamagouche; TRAC: Tracadie
For each estuary, the number of crabs per month (regardless of size) was integrated into relative abundances of each species per summer season (year). Hence, relative abundances per species correspond to percentages over the total number of crabs (not the mean number of crabs) collected during that season. In the estuaries included in CAMP, mud crabs refer to members of the family Panopeidae. However, a large majority of these mud crabs (>95 %) belong to Dyspanopeus sayi, whereas the white-fingered mud crab Rhithropanopeus harrisii (Gould, 1841) was a considerably less common second species. In estuaries of southern PEI, where large number of samples of mud crabs were collected over three consecutive summers (2009–2011), D. sayi represented ~97 % of all the mud crabs collected (Pickering 2011).
Crab collection and tank setup for experimental work
During July and August of 2011, large adult green crabs (70–80 mm carapace width) were periodically collected from the Souris River estuary on the eastern shore of PEI (Fig. 1). In order to avoid biases associated with gender or the molting process, only intact (uninjured) males without signs of molting were retained and subsequently used as predators. Simultaneously, juvenile green crabs and adult D. sayi mud crabs (both 25–30 mm carapace width) were regularly collected from North River (Hillsborough estuarine system, southern PEI; Fig. 1). Only intact individuals of both species were retained and used as prey in the experiments described below. Habitats in which both species were collected included extensive sedimentary bottoms (particularly sandy sediments) associated with oyster, mussel, and eelgrass beds. These sites were also considerably similar in terms of water quality and tide regimes. Predators and prey were not “naïve” to each other or to the habitats used in the experiments. Experiments were run in glass tanks with dimensions 21.6 cm × 41 cm × 25 cm high, filled with prepared seawater made from chlorinated well water from the University of Prince Edward Island and Instant Ocean (25 ppt, 18–20 °C). Each tank had an oxygen stone, and its top and sides were covered to minimize external visual stimuli and prevent crab escape (Palacios and Ferraro 2003). Three distinct habitat mimics representing increasing habitat complexity were prepared in these tanks: no substrate (water only), sandy sediment habitat (tanks were fitted with a 3-cm layer of cleaned sandy sediments), and oyster bed habitat (tanks were fitted with a 3-cm layer of clean oyster shells). Although tanks with water were admittedly an artificial habitat, they provided the conditions in which prey could not physically hide from predators. As for the two more complex habitats, sandy sediments were originally collected from Brackley Bay, PEI (fine to medium sands, ~0.5–1.0 mm grain size), whereas oyster shells (Crassostrea virginica) were collected from North River (~2–4 cm SL). Before all experiments, both sandy sediments and oyster shells were repeatedly washed and filtered in order to remove any live organisms that could act as alternative prey. Water and substrate mimics were replaced after each individual experiment.
Experimental procedure
Two separate experimental designs aimed to assess predator feeding rates and preference for prey, both in relation to habitat. The first design assessed the effects of a predator (large green crab) on five small preys (either mud crabs or juvenile green crabs). The second design examined predator preference upon both prey species combined: three mud crabs and three juvenile green crabs in the same tank. For each design, 15 replicates per treatment were conducted. Individual predators were starved for 48 h prior to the experiment in order to standardize hunger levels (e.g., Mascaró and Seed 2001). In addition, new predators were used for each experiment to avoid the risk of biased results due to learning (Cunningham and Hughes 1984). Two response variables were measured: prey mortality rate (i.e., the number of individuals of each prey species that were found dead after 24 h) and prey injury rate (the number of individuals with missing claws or legs or signs of damaged carapace after 24 h). All the experiments were initiated in the morning (~10 am), and although they lasted 24 h, systematic observations were made after 0.5, 1, 2, 3, 4, 5, and 24 h in order to identify potential trends in timing or period of most intense foraging (e.g., Pickering and Quijón 2011). We used a natural light/dark cycle, but sides and top of each tank were covered to control for most light exposure. As expected, most predation occurred during the night hours, and given the lack of any consistent trends during the first 5 h, results are reported (and statistically analyzed) for the 24-h period only.
Statistical analyses
2009–2013 Crab monitoring in the southern Gulf of St. Lawrence
We explored the relationships among all crab species collected each year and from each of the 29 estuaries that had consistent records between 2009 and 2013. Data were analyzed using the multivariate method called “principal coordinate analysis” (PCoA) available in PRIMER version 6. routines (see Clarke and Gorley 2006; Anderson et al. 2008). The PCoA was used as a linear ordination of species annual relative abundances in the 29 estuaries of the southern Gulf of St. Lawrence. The PCoA creates a matrix of distances between points using the well-known Bray–Curtis similarity index based on square-root-transformed data. PCoA processes data like a principal component analysis (PCA) which entails linear combinations of the variance of multivariate data. However, unlike PCA the PCoA has the advantage of being able to use any distance measure (including Bray–Curtis) and not just Euclidean distance to identify correlations (see Anderson et al. 2008). These correlations among crab species and axes strengths are reported as Pearson’s correlations (r). The program also displays vectors that correspond to the increasing relative abundance of the crab species under analysis. The outcome of the analysis provides a visual picture of the relative similarity (spatial clustering) of data points and captures relevant between-year variation.
Mean annual abundance estimates of mud crab and green crab across the region were modeled over time using simple linear regression in Minitab 17 (2010) with the goal of identifying potential changes over time for the entire study area. In the analysis the mud crabs (Panopeidae Ortmann, 1893) species were pooled due to difficulties distinguishing them apart. Crab relative abundances were square root transformed to better meet the assumptions of linear models. For this analysis, only 21 (of the original 29) estuaries were considered, given that in eight of these estuaries there were no records of green crab invasion/establishment during the study period (i.e., for the regression analysis, n = 105). Regardless, the outcome of a parallel analysis including all 29 estuaries was virtually identical.
Laboratory experiments
For the first design (predator feeding rates on individual prey species), we used a two-way ANOVA model (two-sided test) to examine mortality and injury rates as response variables:
where Prey (mud crab or green crab) and Habitat (water, sediment, or oyster bed) were considered fixed factors. When the interaction term was significant, pairwise comparisons using Tukey’s honest significant differences were subsequently applied to elucidate the influence of each main factor separately. The test corrected P values for multiple comparisons.
For the second design (predator feeding rates on both prey species combined, hereafter referred as “preference”), we adapted a two-way ANOVA model (two-sided test) again using mortality and injury rates as response variables:
where Prey and Habitat were considered fixed factors and Tank (Habitat) was considered a random factor. In this model, tank was nested within habitat (given that not every habitat is in every tank) as was considered the unit of replication in order to avoid potential pseudoreplication. When the interaction term was significant (i.e., effect of prey type on mortality differed by habitat type), pairwise comparisons using Tukey’s honest significant differences were applied to elucidate the influence of each main factor. Statistically significant differences for all linear models, including field and experimental analysis, were defined as P ≤ 0.05.
Results
2009–2013 Crab monitoring in the southern Gulf of St. Lawrence
At least four crab species were identified from the beach seine samples: the invasive green crab and the native mud crab (primarily Dyspanopeus sayi, and potentially a small fraction of the white-fingered mud crab Rhithropanopeus harrisii), rock crab (Cancer irroratus Say, 1817), and lady crab (Ovalipes ocellatus Herbst, 1799). In the analysis of the relationships among crab species, the PCoA explained 99.8 % of the variance (Fig. 2). The first axis of the PCoA explained 75.7 % of the relative abundance in crab species and was primarily associated with the variation of green crab (r = 1.0), mud crab (r = −0.8), and rock crab (r = −0.7). All correlations in this analysis were statistically significant (P < 0.05, n = 145). The second axis explained 24.1 % of the variance and was associated with rock crab (r = −0.7) and mud crab (r = 0.6). The relative abundance of green crabs and mud crabs was negatively correlated (r = −0.8). Likewise, green crab and rock crab were negatively correlated (r = −0.7), and rock crabs and lady crabs were less strongly but positively correlated (r = 0.5) (Fig. 2). The main clustering of data points was observed across the first axis toward the right side, where green crabs were numerically dominant (Fig. 2). Toward the end of the green crab vector, a circle identifies an overlap of 60 data points, including all five annual samples collected from seven different estuaries: Antigonish, Basin Head, Cheticamp, Mabou, Murray River, Pictou, and Souris. Figure 2 also illustrates between-year variation in several estuaries. For instance, Bouctouche samples were dominated by mud crabs early during the sampling period (2009–2011) but are subsequently displaced toward the right side as green crabs enter the system and begin to dominate in abundance (Fig. 2). In contrast, samples from Malpeque Bay, an area not yet invaded by green crabs, remained associated with high densities of mud crabs, toward the left side of axis 1 (Fig. 2).
Results of the principal coordinate analysis (PCoA) of four crab species in 29 estuaries of the southern Gulf of St. Lawrence from 2009 to 2013 (shortened to two digits). Years were removed from the encircled area due to the overlap of 60 data points, including all the points from ANTI, BASH, CHET, MABO, MURR, PICT, and SOUR and most (4 out of 5) data points from BRUD, DESA and HILL. Estuary codes are similar to those shown in Fig. 1. Mud crab refers to Dyspanopeus sayi (and to a much lesser extent to Rhithropanopeus harrisii), Rock crab refers to Cancer irroratus, Lady crab refers to Ovalipes ocellatus, and Green crab refers to Carcinus maenas
The annual green crab abundance per estuary ranged from 0 to 1048 individuals (the highest abundance for the study period was recorded in Basin Head in 2013). For mud crabs, annual abundances ranged from 0 to 1084 crabs, with the highest estimate recorded in Bouctouche in the 2010 season. Temporal trends for green crabs and mud crabs from a subset of 21 estuaries in which both species occur (Fig. 3) showed a significant increase in the number of green crabs over time (r 2 = 0.8, F 1,3 = 13.8, P = 0.034). Relative number of green crabs for the entire study area increased from 59 % to 82 %. Conversely, mud crabs did not significantly decrease over the same period (r 2 = 0.4, F 1,3 = 1.7, P = 0.279), although a slight negative trend was apparent (Fig. 3). Temporal trends using data from the full set of 29 estuaries (not shown) were virtually identical.
Green crab feeding rates and prey preference
For both mortality and injury rates, the results of the two-way ANOVA model indicated that habitat type, prey species, and their interactions were all significant (P < 0.05, Fig. 4; Table 1). Post hoc Tukey’s honest significant difference tests showed that levels of mortality and injury for mud crabs were almost twice those for green crabs, except in oyster shell habitat, where they were not significantly different (Fig. 4; Table 1). Mortality and injury rates were similar across habitat types for juvenile green crabs. With regard to experiments assessing preference (Fig. 5), the results of the two-way ANOVA model indicated that for both mortality and injury rates, habitat type and prey species were significant and that their interaction was significant for mortality (Table 1). Post hoc Tukey’s honest significant difference tests showed that in general, mortality and injury values for mud crabs were twice those for green crabs (Fig. 5; Table 1). Mortality and injury values were similar across habitat types for juvenile green crabs.
Mean (±SE) mortality and injury rates for trial run with combined prey species (three mud crabs and three green crabs) in three different habitat types (n = 15). Bars with different letters indicate a statistically significant difference (P < 0.05). For injury rates, the interaction term was nonsignificant, so letters refer to significant differences among habitats (prey species was significant on each habitat)
Discussion
Patterns in estuaries of the Canadian maritimes
Green crab populations continue to spread and grow in some areas of the Canadian Atlantic region (e.g., PEI and Newfoundland; Audet et al. 2003; Blakeslee et al. 2010). It is in these areas where green crab populations have been hypothesized to be most aggressive (Rossong et al. 2012) and therefore most likely to negatively interact with native species. The large CAMP dataset spanning 5 years and 29 estuaries provides comprehensive evidence of a negative relationship between this invasive species and at least two native crab species, including the mud crabs (primarily D. sayi), the focus of this study. Such negative relationship, of course, may not be causally related to green crab aggressiveness or their predation on mud crabs. In fact, for a spatial scale like the one studied here, a negative relationship is most likely linked to multiple factors, related or not to predation. Negative relationships between green crabs and rock crabs have already been partially addressed in a few laboratory and field studies (Bélair and Miron 2009; Gregory and Quijón 2011; Matheson and Gagnon 2012). However, the strongest negative correlation detected here, between green crabs and mud crabs, has not previously been studied in detail. Several possible mechanisms could explain this negative relationship including but not restricted to physiological tolerance, habitat variation, competition for food or other resources, and predation.
Differences in physiological tolerance would easily segregate coastal populations and species (Hunt and Behrens Yamada 2003). However, such differences are unlikely to drive a negative relationship between these two species. Mud crab and green crab temperature, salinity, and depth tolerances are both quite broad and fairly similar to each other (Breen and Metaxas 2009). In addition, CAMP sampling sites are purposely located within a range of salinities to ensure samples from each estuary come from brackish waters and not from freshwater and marine conditions (Weldon et al. 2005). Alternatively, spatial or temporal variations in productivity or the availability of good quality habitat may easily contribute to the variation in crab abundances. For instance, species like mud crabs and green crabs use habitats like oyster, mussel, or eelgrass beds recurrently (Kneib et al. 1999), so their presence and distribution are likely critical for some stage of the crabs life cycle (e.g., Thiel and Dernedde 1994). As discussed below, oyster beds are highly structured in comparison with habitats like sandy flats and should be preferred habitat not only for the purpose of predator refuge (Grabowski 2004). Highly structured habitats embedded within more uniform sandy sediments reduce risk of desiccation during low tide conditions and may enhance local food availability (Thiel and Dernedde 1994). Oyster beds and sandy sediments are widespread in the southern Gulf of St. Lawrence. Nonetheless, it is difficult to quantify their area or availability in the 29 estuaries included in this study due to the lack of consistent reports. Most likely, variations in these habitat characteristics (presence, area, distribution) would interact with slight changes in water characteristics and influence crab distribution and dynamics.
Negative mud crab–green crab interactions may be also associated with competition for food or habitats as those identified above. These interactions are common among crustaceans (e.g., Hunt and Behrens Yamada 2003) including green crabs (Rossong et al. 2006). In addition to the shallow subtidal, mud crabs thrive on mid-low intertidal oyster, mussel, and seaweed beds that are also a preferred substrate of juvenile green crabs (Day and Lawton 1988; Hedvall et al. 1998). It follows that potential interactions between small crabs of both species can occur if such beds are small, patchy, or limited in number. The likely consequence of these interactions would be displacement or local exclusion of one of the species. The same applies to potential migrations of either type of small crab into deeper waters or upper intertidal areas (beyond seine net reach) as a response to competition. A fourth mechanism to explain the negative relationship between green crabs and mud crabs is the one further addressed in this study: predation. Predator–prey interactions were expected to operate in this system given the obvious differences in size between large and small crabs (potential predator and prey), the aggressive nature of adult green crabs (Rossong et al. 2006), and anecdotic evidence suggesting a green crab preference for mobile prey such as small crabs (P Quijon, unpublished). As addressed in detail below, it is also plausible that habitat and the occurrence of green crab cannibalism events (Moksnes 2004; Almeida et al. 2011) play a role in these interactions, and that was the reason to assess both factors in our experiments.
The results of PCoA and regression analyses do not necessarily imply that predation (or for that purpose competition or habitat variation) is the factor driving the dissimilar patterns of green crab and mud crab populations. These analyses provide further evidence of the growth and expansion of green crabs in the southern Gulf of St. Lawrence (Audet et al. 2003; Blakeslee et al. 2010): While the regression shows a significant increase in the relative abundance of green crabs, the PCoA provides local evidence of changes in crab composition as a result of the recent invasion of green crabs. The changes associated with this expansion are reflected in the negative relationship with mud crabs, particularly along the prominent mud crab–green crab axis of the PCoA. However, we must be cautious with regard to the interpretation of these numeric changes: Although we provide experimental evidence of one of the mechanisms by which green crab can affect mud crabs (see below), the temporal decline in mud crab relative abundance was not significant.
Predation as a mechanism to explain negative relationships between species
Our results did not support the null hypothesis that prey mortality and injury rates would be similar between species and among habitats. Large green crabs consumed and injured significantly more mud crabs than juvenile green crabs when presented with a single type of prey and when given the choice between both species. Furthermore, we detected clear mortality and injury differences among habitat types for the native mud crab.
The influence of prey
Prior studies examining brachyuran crabs as prey have already identified some of the possible mechanisms behind preference and feeding rates. Kuroda et al. (2005) suggested that differences in predation rates upon two preys were related to their different burrowing abilities to escape predation. Similarly, Kneib et al. (1999) suggested that the less preferred prey in their study was quicker and more difficult to capture than the alternative (preferred) prey. Alternative mechanisms include differences in prey palatability, caloric value, predator’s search time, and prey defensive capabilities (Ellis et al. 2012). There is no published evidence comparing palatability and caloric values of mud crabs vs. juvenile green crabs, and it would be unwise to consider them similar simply because they are of similar size. However, we do suggest that there are differences associated with predator search time and prey profitability in relation to habitat that could explain some of the preferences in our experiments (see below). Studies on the ability of prey to escape or defend themselves are uncommon in decapods, but Ellis et al. (2012) found that these two factors explained seagulls’ preference for Jonah crab (Cancer borealis Stimpson, 1859) over green crab and rock crab.
Green crabs have been found to be less susceptible to predation than other species because of their cryptic coloration against dark backgrounds like mussel beds (Dumas 1993). Although there are no studies comparing green crabs with mud crabs, the fact that green crabs are skillful at hiding (using coloration or other mechanisms) may confer an advantage. Greater difficulty in detecting prey generally translates into higher energy costs for the searching and potentially handling of that prey. If mud crabs are less skillful at hiding than green crabs, optimal foraging theory (Pyke et al. 1977) and the concept of profitability (Norberg 1977) would explain the preferences observed.
Another intuitive explanation for our results relates to inclusive fitness (sensu Schausberger 2003). In general, predators are expected to prey upon heterospecific prey rather than upon conspecifics (cannibalism). Inclusive fitness reduces the likelihood of killing related individuals (Schausberger 2003), and this should be advantageous for species undergoing population growth, as invasive species are. In our experiments, juvenile green crab mortality and injury rates were low in the “no sediment” trials where they had no possibility to hide or disguise themselves, which suggests that avoidance of cannibalism may be occurring. Cannibalism has been recorded in green crabs at low rates (Baeta et al. 2005; Ropes 1968), which suggests that cannibalism avoidance may be another possible mechanism to explain the preference and feeding rates observed in this study.
The influence of habitat
Mud crab mortality and injury rates decreased with increasing habitat complexity. This result is consistent with a well-developed body of evidence that suggests that increasing habitat complexity reduces prey mortality (e.g., Fernández et al. 1993; Dittel et al. 1996; Fernández 1999; Langellotto and Denno 2006; Stoner et al. 2010; Hill and Weissburg 2013; Hernández Cordero and Seitz 2014). The most common mechanism to explain the influence of habitat complexity is a decrease in the rate of predator–prey encounters. In complex habitats, prey may seek refuge from predators more easily, or predators may be less mobile or efficient at finding and catching prey compared with less structured habitats (Crowder and Cooper 1982; Grabowski 2004).
Surprisingly, the pattern was quite different for the other prey species; juvenile green crab mortality and injury rates were unaffected by habitat complexity. We hypothesize two opposing mechanisms that could explain this result. First, juvenile green crabs are vulnerable to predation like any other small decapod seeking refuge in complex coastal habitats (Ellis et al. 2012). However, unlike most native species, juvenile green crabs may be as good at escaping predation in less structured habitats as they are in complex habitat. A second mechanism to explain why juvenile green crab mortality levels were unaffected by habitat is the exact opposite; juvenile green crabs may lack the ability to effectively use complex habitat to escape predation. Long et al. (2015) suggested that responding to predator presence by engaging in cryptic behavior (i.e., hiding in complex habitat) may be a learned behavior, and in some crab species, refuge-seeking behavior is known to develop with size and age (Johnson et al. 2008; Stoner et al. 2010; Pirtle et al. 2012). Under this hypothesis, we might expect that ‘naïve’ juvenile mortality would indeed be similar across habitat types, while the mud crabs (already adults in our experiment) would be expected to be more experienced and have a greater affinity for hiding in complex environments. Although both mechanisms are plausible and consistent with the mortality and injury rates reported in our study, we do not have direct evidence for either, so we call for further experiments to elucidate these mechanisms.
A major implication of this study is the potential detrimental effects of the loss of complex habitats, which, according to our results, would have worse effects on native mud crabs than on juvenile green crabs. Two factors that have had adverse effects on numerous habitats in the east coast of North America, as well as other regions, are habitat destruction and invasion by the green crab. Green crabs predate upon bivalves (Palacios and Ferraro 2003; Miron et al. 2005; Pickering and Quijón 2011), including habitat-building species like oysters and mussels, leading to decreases in habitat complexity. Green crabs also uproot and graze on eelgrass (Malyshev and Quijón 2011), which has a detrimental effect on associated species and services (Heck et al. 2003). As green crabs continue to spread (Carlton and Cohen 2003), changes to native species, and in particular to habitats, will likely continue. This study documents a negative relationship between green crabs and mud crabs in the southern Gulf of St. Lawrence and provides evidence that predation is one of the several mechanisms that may explain the association. Our results suggest that if mud crabs are being displaced into habitat that is less structurally complex, their survival rates at the local scale may further decrease.
References
Almeida JA, González-Gordillo JI, Flores AAV, Queiroga H (2011) Cannibalism, post-settlement growth rate and size refuge in a recruitment-limited population of the shore crab Carcinus maenas. J Exp Mar Biol Ecol 410:72–79. doi:10.1016/j.jembe.2011.10.011
Anderson MJ, Gorley RN, Clarke KR (2008) Permanova + for PRIMER: Guide to software and statistical methods. PRIMER-E. Plymouth Marine Laboratory, Plymouth
Audet D, Davis D, Miron G, Moriyasu M (2003) Geographical expansion of a nonindigenous crab, Carcinus maenas (L.), along the Nova Scotian shore into the southeastern Gulf of St. Lawrence, Canada. J Shellfish Res 22:255–262
Baeta A, Cabral HN, Marques JC, Pardal MA (2006) Feeding ecology of the green crab, Carcinus maenas (L., 1758) in a temperature estuary, Portugal. Crustaceana 79:1181–1193. doi:10.1163/156854006778859506
Bélair M, Miron G (2009) Predation behaviour of Cancer irroratus and Carcinus maenas during conspecific and heterospecific challenges. Aquat Biol 6:41–49. doi:10.3354/ab00166
Blakeslee AMH, McKenzie CH, Darling JA, Byers JE, Pringle JM, Roman J (2010) A hitchhiker’s guide to the Maritimes: anthropogenic transport facilitates long-distance dispersal of an invasive marine crab to Newfoundland. Divers Distrib 16:879–891. doi:10.1111/j.1472-4642.2010.00703.x
Botto F, Iribarne O (1999) Effects of the SW Atlantic burrowing crab Chasmagnathus granulata on a Spartina salt marsh. Mar Ecol Prog Ser 178:79–88. doi:10.3354/meps178079
Breen E, Metaxas A (2009) Overlap in the distributions between indigenous and non-indigenous decapods in a brackish micro-tidal system. Aquat Biol 8:1–13. doi:10.3354/ab00195
Carlton JT, Cohen AN (2003) Episodic global dispersal in shallow water marine organisms: the case history of the European shore crabs Carcinus maenas and C. aestuarii. J Biogeogr 30:1809–1820. doi:10.1111/j.1365-2699.2003.00962.x
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial, PRIMER-E. Plymouth Marine Laboratory, Plymouth UK 192 pp
Cohen AN, Carlton JT, Fountain MC (1995) Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Fransciso Bay, California. Mar Biol 122:225–237. doi:10.1007/BF00348935
Connell J (1961) Effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecol Monogr 31:61–104. doi:10.2307/1950746
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interactions between bluegills and their prey. Ecology 63:1802–1813. doi:10.2307/1940122
Cunningham PN, Hughes RN (1984) Learning of predatory skills by shorecrabs Carcinus maenas feeding on mussels and dogwhelks. Mar Ecol 16:21–26. doi:10.3354/meps016021
Cushing JM (1991) A simple model of cannibalism. Math Biosci 107:47–71. doi:10.1016/0025-5564(91)90071-P
Day E, Lawton P (1988) Mud crab (Crustacea: Brachyura: Xanthidae) substrate preference and activity. J Shellfish Res 9:257–312. doi:10.2983/035.029.0302
Diehl S (1992) Fish predation and benthic community structure: the role of omnivory and habitat complexity. Ecology 73:1646–1661. doi:10.2307/1940017
Dittel A, Epifanio CE, Natunewicz C (1996) Predation on mud crab megalopae, Panopeus herbstii H. Milne Edwards: effect of habitat complexity, predator species and postlarval densities. J Exp Mar Bio Ecol 198:191–202. doi:10.1016/0022-0981(96)00003-2
Dumas JV (1993) Predation by Herring Gulls (Larus argentatus Coues) on two rocky intertidal crab species [Carcinus maenas (L.) & Cancer irroratus Say]. J Exp Mar Bio Ecol 169:89–101. doi:10.1016/0022-0981(93)90045-P
Ebersole EL, Kennedy VS (1995) Prey preferences of blue crabs Callinectes sapidus feeding on three bivalve species. Mar Ecol Prog Ser 118:167–178. doi:10.3354/meps118167
Ellis JC, Allen KE, Rome MS, Shulman MJ (2012) Choosing among mobile prey species: Why do gulls prefer a rare subtidal crab over a highly abundant intertidal one? J Exp Mar Bio Ecol 416–417:84–91. doi:10.1016/j.jembe.2012.02.014
Fernández M (1999) Cannibalism in Dungeness crab Cancer magister: effects of predator–prey size ratio, density, and habitat type. Mar Ecol Prog Ser 182:221–230. doi:10.3354/meps182221
Fernández M, Iribarne O, Armstrong D (1993) Habitat selection by young-of-the-year Dungeness Crab Cancer magister and predation risk in intertidal habitats. Mar Ecol Prog Ser 92:171–177
Finke DL, Denno RF (2002) Intraguild predation diminished in complex-structured vegetation: implications for prey suppression. Ecology 83:643–652. doi:10.1890/0012-9658%282002%29083%5B0643:IPDICS%5D2.0.CO;2
Grabowski JH (2004) Habitat complexity disrupts predator–prey interactions but not the trophic cascade on oyster reefs. Ecology 85:995–1004. doi:10.1890/03-0067
Gregory GJ, Quijón PA (2011) The impact of a coastal invasive predator on infaunal communities: assessing the roles of density and a native counterpart. J Sea Res 66:181–186. doi:10.1016/j.seares.2011.05.009
Grosholz ED, Ruiz GM (1996) Predicting the impact of introduced marine species: lessons from the multiple invasions of the European Green Crab Carcinus maenas. Biol Conserv 3207:59–66. doi:10.1016/0006-3207(94)00018-2
Heck KL, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol Prog Ser 253:123–136. doi:10.3354/meps253123
Hedvall O, Moksnes PO, Pihl L (1998) Active habitat selection by megalopae and juvenile shore crabs Carcinus maenas: a laboratory study in an annular flume. Hydrobiologia 376:89–100. doi:10.1023/A:1017081611077
Hernández Cordero AL, Seitz RD (2014) Structured habitat provides a refuge from blue crab, Callinectes sapidus, predation for the bay scallop, Argopecten irradians concentricus (Say 1822). J Exp Mar Bio Ecol 460:100–108. doi:10.1016/j.jembe.2014.06.012
Hill JM, Weissburg MJ (2013) Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Mar Ecol Prog Ser 488:209–219. doi:10.3354/meps10386
Hunt CE, Behrens Yamada S (2003) Biotic resistance experienced by an invasive crustacean in a temperate estuary. Biol Invasions 5:33–43. doi:10.1023/A:1024011226799
Jensen G, McDonald P, Armstrong D (2002) East meets west: competitive interactions between green crab Carcinus maenas, and native and introduced shore crab Hemigrapsus spp. Mar Ecol Prog Ser 225:251–262. doi:10.3354/meps225251
Johnson EG, Hines AH, Kramer MA, Young AC (2008) Importance of season and size of release to stocking success for the blue crab in Chesapeake Bay. Rev Fish Sci 16:243–253. doi:10.1080/10641260701696837
Kneib RT, Lee SY, Kneib JP (1999) Adult–juvenile interactions in the crabs Sesarma (Perisesarma) bidens and S. (Holometopus) dehaani (Decapoda:Grapsidae) from intertidal mangrove habitats in Hong Kong. J Exp Mar Bio Ecol 234:255–273. doi:10.1016/S0022-0981(98)00149-X
Kuroda M, Wada K, Kamada M (2005) Factors influencing coexistence of two brachyuran crabs, Helice tridens and Parasesarma plicatum, in an estuarine salt marsh, Japan. J Crustacean Biol 25:146–153. doi:10.2307/1549938
Langellotto GA, Denno RF (2006) Refuge from cannibalism in complex-structured habitats: implications for the accumulation of invertebrate predators. Ecol Entomol 31:575–581. doi:10.1111/j.1365-2311.2006.00816.x
Lloyd M (1968) Self regulation of adult numbers by cannibalism in two laboratory strains of flour beetles (Tribolium castaneum). Ecology 49:245–259. doi:10.2307/1934453
Long WC, Van Sant SB, Haaga JA (2015) Habitat, predation, growth, and coexistence: Could interactions between juvenile red and blue king crabs limit blue king crab productivity? J Exp Mar Bio Ecol 464:58–67. doi:10.1016/j.jembe.2014.12.011
Luppi TA, Spivak ED, Anger K (2001) Experimental studies on predation and cannibalism of the settlers of Chasmagnathus granulata and Cyrtograpsus angulatus (Brachyura: Grapsidae). J Exp Mar Bio Ecol 265:29–48. doi:10.1016/S0022-0981(01)00322-7
Malyshev A, Quijón PA (2011) Disruption of essential habitat by a coastal invader: new evidence of the effects of green crabs on eelgrass beds. ICES J Mar Sci 68:1852–1856. doi:10.1093/icesjms/fsr126
Mascaró M, Seed R (2001) Choice of prey size and species in Carcinus maenas (L.) feeding on four bivalves of contrasting shell morphology. Hydrobiologia 449:159–170. doi:10.1023/A:1017569809818
Matheson K, Gagnon P (2012) Effects of temperature, body size, and chela loss on competition for a limited food resource between indigenous rock crab (Cancer irroratus Say) and recently introduced green crab (Carcinus maenas L.). J Exp Mar Bio Ecol 428:49–56. doi:10.1016/j.jembe.2012.06.003
McDonald PS, Jensen GC, Armstrong DA (2001) The competitive and predatory impacts of the nonindigenous crab Carcinus maenas (L.) on early benthic phase Dungeness crab Cancer magister Dana. J Exp Mar Bio Ecol 258:39–54. doi:10.1016/S0022-0981(00)00344-0
Mills EL, Leach JH, Carlton JT, Secor CL (1993) Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. J Great Lakes Res 19:1–54. doi:10.1016/S0380-1330(93)71197-1
Miron G, Audet D, Landry T, Moriyasu M (2005) Predation potential of the invasive green crab (Carcinus maenas) and other common predators on commercial bivalve species found on Prince Edward Island. J Shellfish Res 24:579–586. doi:10.2983/0730-8000(2005)24
Moksnes PO (2004) Self-regulating mechanisms in cannibalistic populations of juvenile shore crabs Carcinus maenas. Ecology 85:1343–1354. doi:10.1890/02-0750
Norberg RA (1977) An ecological theory on foraging time and energetics and choice of optimal food-searching method. J Anim Ecol 46:511–529. doi:10.2307/3827
Palacios K, Ferraro SP (2003) Green crab (Carcinus maenas Linnaeus) consumption rates on and prey preference among four bivalve prey species. J Shellfish Res 22:865–871. doi:10.2983/035.029.0302
Paolucci E, MacIsaac H, Ricciardi A (2013) Origin matters: alien consumers inflect greater damage on prey populations than do native consumers. Divers Distrib 19:988–995. doi:10.1111/ddi.12073
Peterson CH (1982) The importance of predation and intra- and interspecific competition in the population biology of two infaunal suspension-feeding bivalves, Protothaca staminea and Chione undatella. Ecol Monogr 52:437–475. doi:10.2307/2937354
Pickering T (2011) Predator–prey interactions between the European green crab (Carcinus maenas) and bivalves native to Prince Edward Island. MSc thesis dissertation, University of Prince Edward Island, Charlottetown, 169 pp
Pickering T, Quijón PA (2011) Potential effects of a non-indigenous predator in its expanded range: assessing green crab, Carcinus maenas, prey preference in a productive coastal area of Atlantic Canada. Mar Biol 158:2065–2078. doi:10.1007/s00227-011-1713-8
Pirtle J, Eckert G, Stoner A (2012) Habitat structure influences the survival and predator–prey interactions of early juvenile red king crab Paralithodes camtschaticus. Mar Ecol Prog Ser 465:169–184. doi:10.3354/meps09883
Pyke G, Pulliam H, Charnov E (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154. doi:10.2307/2824020
Ricciardi A, Atkinson SK (2004) Distinctiveness magnifies the impact of biological invaders in aquatic ecosystems. Ecol Lett 7:781–784
Ropes JW (1968) The feeding habits of the green crab, Carcinus maenas (L.). Fish B-NOAA 67:183–203
Rossong MA, Williams PJ, Comeau M, Mitchell SC, Apaloo J (2006) Agonistic interactions between the invasive green crab, Carcinus maenas (Linnaeus) and juvenile American lobster, Homarus americanus (Milne Edwards). J Exp Mar Bio Ecol 329:281–288. doi:10.1016/j.jembe.2005.09.007
Rossong MA, Quijón PA, Snelgrove PVR, Barrett TJ, McKenzie CH, Locke A (2012) Regional differences in foraging behaviour of invasive green crab (Carcinus maenas) populations in Atlantic Canada. Biol Invasions 14:659–669. doi:10.1007/s10530-011-0107-7
Schausberger P (2003) Cannibalism among phytoseiid mites: a review. Exo Appl Acarol 29:173–191. doi:10.1023/A:1025839206394
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. doi:10.1111/j.1600-0706.2009.18039.x
Stoner AW, Ottmar ML, Haines SA (2010) Temperature and habitat complexity mediate cannibalism in red king crab: observations on activity, feeding, and prey defense mechanisms. J Shellfish Res 29:1005–1012. doi:10.2983/035.029.0401
Thiel M, Dernedde T (1994) Recruitment of shore crabs Carcinus maenas on tidal flats: mussel clumps as an important refuge for juveniles. Helgol Meeresunters 48:321–332
Weldon J, Garbary D, Courtenay S, Ritchie W, Godin C, Thériault M-H, Boudreau M, Lapenna A (2005) The Community Aquatic Monitoring Project (CAMP) for measuring marine environmental health in coastal waters of the southern Gulf of St. Lawrence: 2004 overview. Can Tech Rep Fish Aquat Sci 2624:viii-53
Williams PJ, MacSween C, Rossong M (2009) Competition between invasive green crab (Carcinus maenas) and American lobster (Homarus americanus). N Z J Mar Freshw Res 43:29–33. doi:10.1080/00288330909509979
Acknowledgments
Dr E. Paolucci and an anonymous reviewer provided valuable comments on a preliminary version of this manuscript. This study was funded by a NSERC Discovery grant to PAQ and a NSERC CGSM to HG. RC was funded by the Canada Excellence Research Chairs Program. The Community Aquatic Monitoring Program (CAMP) is jointly coordinated by Fisheries and Oceans Canada–Gulf Region and the Southern Gulf of St. Lawrence Coalition on Sustainability.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: E. Briski.
Reviewed by E. Paolucci and an Undisclosed expert.
This article is part of the Topical Collection on Invasive Species.
Rights and permissions
About this article
Cite this article
Gehrels, H., Knysh, K.M., Boudreau, M. et al. Hide and seek: habitat-mediated interactions between European green crabs and native mud crabs in Atlantic Canada. Mar Biol 163, 152 (2016). https://doi.org/10.1007/s00227-016-2927-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2927-6