Abstract
Ocean acidification (OA) and warming related to the anthropogenic increase in atmospheric CO2 have been shown to have detrimental effects on several marine organisms, especially those with calcium carbonate structures such as corals. In this study, we evaluate the response of two Mediterranean shallow-water azooxanthellate corals to the projected pH and seawater temperature (ST) scenarios for the end of this century. The colonial coral Astroides calycularis and the solitary Leptopsammia pruvoti were grown in aquaria over a year under two fixed pH conditions, control (8.05 pHT units) and low (7.72 pHT units), and simulating two annual ST cycles, natural and high (+3 °C). The organic matter (OM), lipid and protein content of the tissue and the skeletal microdensity of A. calycularis were not affected by the stress conditions (low pH, high ST), but the species exhibited a mean 25 % decrease in calcification rate at high-ST conditions at the end of the warm period and a mean 10 % increase in skeletal porosity under the acidified treatment after a full year cycle. Conversely, an absence of effects on calcification and skeletal microdensity of L. pruvoti exposed to low-pH and high-ST treatments contrasted with a significant decrease in the OM, lipid and protein content of the tissue at high-ST conditions and a 13 % mean increase in the skeletal porosity under low-pH conditions following a full year of exposure. This species-specific response suggests that different internal self-regulation strategies for energy reallocation may allow certain shallow-water azooxanthellate corals to cope more successfully than others with global environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The response of marine ecosystems to global environmental changes is one of the major challenges facing modern society. It is well known that increases in atmospheric CO2 concentration are leading to changes in the carbonate chemistry and temperature of the ocean (Gattuso and Hansson 2011; Khatiwala et al. 2013). The impact of these processes on marine ecosystems will depend on the ability of organisms to cope with such changes (Ries et al. 2009; Byrne 2011; Wicks and Roberts 2012; Kroeker et al. 2013; Parker et al. 2013). Hence, numerous experiments have investigated the potential effects of ocean acidification (OA) and warming on different marine species (e.g., Hoegh-Guldberg et al. 2007; Guinotte and Fabry 2008; Doney et al. 2009, 2012; Ries et al. 2009; Wicks and Roberts 2012). Among them, coral reef communities have attracted special attention because their calcifying organisms may be severely affected by these two global stressors in the near future, particularly under the more pessimistic range of scenarios projected by the Intergovernmental Panel on Climate Change (IPCC 2013). Skeletal records from the Great Barrier Reef show, for instance, that coral calcification has dropped over the last few decades (Cooper et al. 2008; De’ath et al. 2009) and, although the causes of this decline remain unknown, increasing seawater temperature (ST) and declining pH are among the most likely drivers (e.g., Brown 1997; Hoegh-Guldberg 1999; Anthony et al. 2007; Fabricius et al. 2011). Since both stressors act simultaneously, a growing number of studies have evaluated the combined effect of increased ST and reduced pH on reef zooxanthellate corals. However, outcomes have revealed diverse and often contrasting responses as a result of the interaction between ST and pH, with warming counteracting the adverse effects of acidification in some instances (e.g., Muehllehner and Edmunds 2008; McCulloch et al. 2012) or leading to a synergy of the two stressors in others (e.g., Reynaud et al. 2003; Anthony et al. 2008).
In contrast to the large number of studies including shallow-water tropical species, only a handful of experiments have assessed the OA response of corals inhabiting temperate regions (e.g., Ries et al. 2010; Holcomb et al. 2010, 2012) and, among them, only five have been conducted with Mediterranean species so far. These five Mediterranean studies have also revealed high variability between and within species in their response to a range of acidified conditions. While some of this variability appears to be due to different methodological approaches, reported results vary from a complete dissolution of the skeleton in Oculina patagonica reared in aquaria at pH 7.4 (Fine and Tchernov 2007), to a decrease in calcification rate up to ~35 % in Cladocora caespitosa maintained in aquaria at pH 7.8 (Movilla et al. 2012) and ~18 % in Balanophyllia europaea transplanted along a natural CO2 gradient at in situ pH 7.7 (Fantazzini et al. 2015). When the response to low pH was further assessed in combination with warming, no detectable effects on the calcification of C. caespitosa (Rodolfo-Metalpa et al. 2010) or B. europaea (Rodolfo-Metalpa et al. 2011) were found at in situ pH levels similar to those expected by year 2100 (7.8 pH units). All these previous studies with temperate species have targeted zooxanthellate specimens (i.e., most tropical species), in which the complexity of the combined effects of pH and ST on coral calcification can result, in part, from the tight relationship between photosynthesis and calcification (Gattuso et al. 1999). In this context, an advantage of working on asymbiotic corals, as we do in the present study, is that the direct impacts of OA and ST on the calcification process should be better discerned, with no confounding effects of photosynthesis and symbiotic processes. Furthermore, it is important to point out that all these previous studies assessed the responses of the organisms in terms of calcification rate, photosynthesis and respiration, yet did not assess how the two stressors may affect the biochemical composition of corals. It is still uncertain what the effects may be of the increase in energy consumption apparently required by these organisms to calcify under adverse conditions (Cohen and Holcomb 2009; Allemand et al. 2011) and whether ST and pH stress may affect other physiological and biological processes (Hoegh-Guldberg et al. 2007; Brewer and Peltzer 2009; Pelejero et al. 2010). Therefore, studies assessing the effect of decreasing pH and increasing ST in both calcification and metabolic balance are critical to fully understand the potential response of temperate corals in a changing environment.
The present study investigates the cumulative effects of acidification and warming on the calcification rate, skeleton properties (microdensity and porosity) and biochemical composition of the tissue [organic matter (OM), lipid and protein content] in two temperate azooxanthellate corals from the Mediterranean, Astroides calycularis and Leptopsammia pruvoti, by simulating an annual temperature cycle in an aquarium experiment. The bright orange scleractinian A. calycularis is a colonial coral with a limited geographic distribution, being present along the Atlantic coasts of Morocco and Spain, in the Mediterranean Iberian Peninsula from the Strait of Gibraltar to Cape Palos, in the south Alboran Sea to the Algeria and Tunisian waters and in the south-central part of the Western Mediterranean Sea (Zibrowius 1980, 1995; Bianchi 2007), with some recent recordings in northern areas of the Tyrrhenian and Adriatic Seas (Bianchi and Morri 1994; Kružić et al. 2002; Grubelić et al. 2004; Casellato et al. 2007). The sunset cup coral L. pruvoti is a solitary species distributed in the Mediterranean basin and along the eastern Atlantic coast from Portugal to southern England (Zibrowius 1980). These two non-symbiotic scleractinian corals typically inhabit low irradiance habitats (i.e., vertical walls, overhangs and caves) at depths from 0 to 50 m (Rossi 1971; Zibrowius 1980; Cebrian and Ballesteros 2004; Goffredo et al. 2006). To our knowledge, this is the first multi-seasonal assessment using these two coral species, where the potential response to future acidification and to the regional variation in ST across the Western Mediterranean Sea is investigated.
Materials and methods
Specimen collection
In January 2011, 15 colonies of A. calycularis and 60 individuals of L. pruvoti were collected by scuba diving between 6 and 12 m depth in Cartagena (SE Spain, 37°33′N, 0°58′W) and in L’Estartit (NE Spain, 42°03′N, 3°13′E), respectively. Coral specimens were immediately transported to the Experimental Aquarium Zone at the Institute of Marine Sciences (ICM-CSIC) in Barcelona and placed in a 225-L tank with 50 μm filtered running natural seawater. Shortly after arrival, four fragments (~3 cm in diameter) harvested from each of the 15 collected colonies of A. calycularis and the 60 individuals of L. pruvoti were carefully cleaned of epiphytes and sediment, and glued onto labeled methacrylate plates with an inert mastic compound. The specimens of L. pruvoti were randomly distributed in 12 experimental aquaria (five specimens per aquarium), whereas specimens of A. calycularis were distributed in these aquaria such that there was at least one representative from each colony in each treatment. Seawater renewal rate in the 11 L experimental aquaria was 20 times per day. Light intensity was adjusted to ~9 μmol photons m−2 s−1 (measured with a Li-cor Li-1935B; Lincoln, NE; USA) in a 12:12 light:dark cycle, thus simulating the low irradiance habitats commonly inhabited by these azooxanthellate coral species (Weinberg 1979). A mixed diet including freeze-dried Tetraselmis sp. and Mysidacea (Ocean Nutrition™) and fresh Artemia salina nauplii was supplied three times per week.
Experimental setup and treatment conditions
In situ ST was recorded hourly using an Onset HOBO Pendant Temperature Data Logger (UA-002–64, Onset Computer Corporation) placed at the two collection locations (Cartagena and L’Estartit) at 5 m depth over a 2-year period (July 2010 to June 2012). For each location, mean monthly STs were determined on the basis of averaged daily values. The ST regime at L’Estartit was selected as a reference for setting the experimental ST conditions.
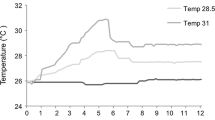
An acclimation phase simulating mean winter-ST conditions at the reference location (13.0 ± 0.5 °C) was conducted in the aquaria from January to March 2011, thereby ensuring a proper healing of the tissue following the transfer and mounting operations. Over the next 3 months (April to June 2011), coral specimens were exposed to the natural springtime ST rise from 13 to 20 °C, adjusted at 0.5 °C week−1 (Fig. 1). At this point (July 2011), pH and ST were gradually adjusted to the experimental conditions (at 0.03 pH units day−1 and 0.3 °C day−1, respectively) and maintained over an annual cycle (July 2011 to August 2012). The four experimental treatments crossed two fixed pH levels (control pH and low pH) with two annual cycle STs (control ST and high ST), using triplicate aquaria in each treatment. The mean pHT (total scale at the in situ ST) used in the control-pH conditions was 8.05 units, similar to the mean annual pHT measured at the reference location of L’Estartit (unpublished data). The decreased pHT used in the low-pH conditions was pH 7.72, following the ‘business-as-usual’ RCP8.5 (Representative Concentration Pathway, 8.5 W m−2) scenario by the year 2100 (IPCC 2013). The annual cycle of ST used in the control-ST conditions was adjusted weekly according to the mean monthly ST measured in situ at the reference location (L’Estartit), whereas the ST in the high-ST conditions was adjusted ~3 °C above the control ST, simulating a rise in ST as expected in the Mediterranean by the end of this century (Solomon et al. 2007; Sokolov et al. 2009).
Mean monthly seawater temperature (ST) variation over the study period (January 2011–August 2012) in the experimental control-ST and high-ST conditions (empty and solid circles, respectively). Gray squares indicate the mean monthly ST logged in situ at 5 m depth in the reference collection site (L’Estartit). The different buoyant weight sampling times (T−1 to T8) and the corresponding experimental phases (acclimation, basal calcification and exposure to pH and ST treatments) are indicated
ST was measured continuously in the two controlled-temperature baths in which the experimental aquaria were placed and temperature was controlled with two electronic Pt100 regulators (Delta Ohm HD9022) connected to a water cooler and 300 W heaters. Seawater pH was adjusted by bubbling CO2 into the overhead feed tanks and the pH levels were continuously monitored by glass electrodes (LL Ecotrode plus—Metrohm) connected to two pH controllers (Consort R362; Topac Inc., USA). For further details on the experimental setup, see Movilla et al. (2012) and Bramanti et al. (2013). Samples of seawater from each treatment were taken bimonthly for total alkalinity (TA) analyses by potentiometric titration (Perez and Fraga 1987; Perez et al. 2000) and pH by spectrophotometry (Clayton and Byrne 1993). At the same time, temperature and salinity of each treatment aquarium were also monitored using a YSI-30 M probe and used to calculate the rest of the parameters of the carbonate system in seawater using the CO2sys.xls software (version 2.1; Pelletier et al. 2007) with the carbonic acid dissociation constants of Mehrbach et al. (1973) refitted by Dickson and Millero (1987).
Calcification rates
Skeletal calcification rate of coral specimens was assessed by means of the buoyant weight (BW) technique (Jokiel et al. 1978, Davies 1989), using a 0.1 mg resolution balance (Mettler Toledo AB204 SFACT). An initial measurement of the BW was taken at the end of the acclimation phase (April 2011, T−1), a second right before setting the experimental pH and ST conditions (July 2011, T0), and subsequent weighing was conducted throughout the annual experiment (July 2011 to August 2012, at 20, 49, 91, 143, 200, 258, 307 and 369 days; T1 to T8, respectively; Fig. 1). The BW of the corals was transformed to dry weight (DW) using the seawater density (δ SW) and the specific value of the aragonite skeleton microdensity (δ ar) determined for each species (see the next section), using the function:
Calcification rate was normalized to the initial skeletal DW of coral specimens and expressed as mg CaCO3 g−1 d−1 using the exponential growth function:
where G is the net calcification rate, DW0 is the initial dry weight, DW n is the dry weight after n days, and n is the time interval (in days) since the beginning of the exposure (e.g., Reynaud et al. 2007; Maier et al. 2013). The basal coral calcification rate in the aquaria was calculated during the spring season (April–July 2011; T−1 to T0) right before setting the experimental treatments. During the experimental stage, coral calcification rate was calculated both for the summer period (T0 to T3) and over the whole annual cycle (T0 to T8).
Skeletal microdensity and porosity
For each coral species, three specimens of each treatment (one specimen per individual replicate aquarium) were randomly selected at the end of the experiment to estimate skeleton microdensity and porosity following the Bucher et al. (1998) technique. Coral specimens were submerged in sodium hypochlorite for 48 h to remove the OM and washed with distilled water. BW and DW of each specimen were recorded before and after their coating in molten paraffin wax (105–110 °C) to form a watertight barrier. The BW was measured in distilled water at 20 °C with a specific gravity of ~1.00 g cm−3. Total enclosed volume, skeleton matrix volume and bulk density were also calculated for each specimen using the equations described in Bucher et al. (1998).
Biochemical composition of the tissue
For each coral species, six specimens of each treatment (two specimens per individual replicate aquarium) were randomly selected at the end of the experiment to estimate potential changes on the biochemical composition of the coral tissue (OM, protein and lipid content). Samples were frozen at −80 °C, subsequently freeze-dried, crushed with a mortar and pestle (including tissue and skeleton) and then stored frozen at −20 °C until biochemical analyses were conducted. Approximately 250 mg from each sample were heated at 80 °C for 48 h and weighed to obtain the DW, then combusted at 450 °C for 5 h and weighed again to obtain the ash weight (AW). The OM content was calculated as the difference between DW and AW and expressed as a percentage with respect to the DW (Slattery et al. 1995). For protein extraction, ~75 mg from each sample were homogenized in NaOH (1 N) and heated to 90 °C for 30 min. The protein content was quantified colorimetrically using the BCA Assay Kit (Interchim) with bovine serum albumin as standard and measured using a multimode microplate reader Tecan Infinite M200 spectrophotometer. For lipid extraction, ~75 mg from each sample were homogenized in 3 mL of chloroform–methanol (2:1). The lipid content was quantified colorimetrically according to the method of Barnes and Blackstock (1973), using cholesterol as standard, and measured with a Varian Cary 100 UV–Vis spectrophotometer. Protein and lipid contents were standardized by the OM content and expressed as µg protein or lipid (mg OM)−1.
Statistical analyses
Data were tested for assumptions of normality and homoscedasticity using the Kolmogorov–Smirnov and Levene tests, respectively. A root square or arctan transformation was applied when normality was not fulfilled. For each coral species, the variation in calcification rate at two sampling points (summer period from T0 to T3, and annual cycle from T0 to T8), in skeletal properties (microdensity and porosity) and biochemical composition of the tissue (OM, protein and lipid contents) at the end of the experiment (T8), was compared among treatments using two-way ANOVAs with pH and ST as fixed factors. All results are expressed as mean ± standard error of the mean (SE). Statistical analyses were performed using the JMP 9.0.1 software (SAS Institute Inc., Cary, NC, USA).
Results
Carbonate system and temperature of seawater in the aquaria
Our experimental setup allowed a precise regulation of the selected pH conditions, which were maintained at 8.045 ± 0.004 and 7.717 ± 0.006 pHT units for control and low-pH conditions, respectively (Table 1). The mean calculated partial pressure of CO2 (pCO2) and ΩA for the control-pH conditions were ~430 μatm and 3.1, respectively. In the low-pH conditions, these values changed to ~1030 μatm and 1.6, respectively. TA and salinity values were similar in all treatments and remained constant throughout the whole experiment, with average values of ~2530 μmol kg−1 seawater (SW) and 38, respectively. Average values of other calculated parameters of the carbonate system are summarized in Table 1.
Over the whole experiment (January 2011 to August 2012), the mean monthly ST in the control-ST conditions was similar to that logged at the reference location of L’Estartit over a 2-year period (July 2010 to June 2012; Fig. 1). Mean monthly ST in the control-ST conditions ranged from 12.4 °C in winter to 22.5 °C in summer, whereas the high-ST conditions were, on average, 2.9 ± 0.3 °C above the control (ranging from 15.2 to 24.9 °C; Fig. 1). During the summer period (July–September 2011; T0 to T3), mean monthly ST was 21.2 ± 0.1 and 23.7 ± 0.1 °C for the control-ST and the high-ST conditions, respectively. Over the same period, mean monthly ST logged at the sampling location for A. calycularis (Cartagena) was evenly higher than ST in the high-ST experimental conditions, with a mean value during the summer period of 26.4 °C and reaching a peak of 27.1 °C.
Calcification rates
During the previous phase to the adjustment of the experimental conditions (T−1 to T0), the two species showed similar mean basal calcification rates (0.75 ± 0.04 and 0.88 ± 0.08 mg CaCO3 g−1 day−1 for A. calycularis and L. pruvoti, respectively), coinciding with the 13–20 °C natural ST rise associated with the spring season. During this preliminary phase (T−1 to T0), no significant differences were detected between treatments in specimens of each species (Table 2). Over the experimental stage, the specimens from both species reared under control-pH and control-ST conditions displayed similar values of calcification rate throughout the summer period (T0 to T3; Fig. 2a). However, when the whole annual cycle (T0 to T8) was considered, the mean calcification rate in control-pH and control-ST conditions decreased by 36–42 % (0.47 ± 0.06 and 0.52 ± 0.10 mg CaCO3 g−1 day−1 for A. calycularis and L. pruvoti, respectively; N = 15 for each; Fig. 2b). This indicates that about two-third of the annual skeletal growth occurred during the late spring–early fall period.
Regarding the response of A. calycularis under different experimental conditions, the effect of high ST caused a ~25 % significant reduction in its calcification rate when compared to that in control ST at the end of the summer period (T0–T3), while no effect was observed under low pH or the combination of low-pH and high-ST conditions (Table 2; Fig. 2a). Conversely, calcification rate of L. pruvoti during the summer period did not differ between ST or pH conditions (Table 2; Fig. 2a). When the whole annual cycle was considered (T0–T8), the negative effect of high-ST conditions on A. calycularis calcification rate observed during the summer period was attenuated (Fig. 2b). Thus, no significant effect of ST, pH or the combination of both factors was observed in A. calycularis or in L. pruvoti by integrating a whole annual growth cycle, (Table 2). None of the coral specimens of either species showed any signs of mortality (denuded skeleton) during the experiment and no tank effect was detected between triplicate aquaria of each treatment in any of the analyses.
Skeletal microdensity and porosity
Skeletal microdensity was not significantly affected by low pH, high ST or the combination of both factors, for either A. calycularis or L. pruvoti at the end of the experiment (Table 3). The mean skeletal microdensity was 2.3 ± 0.1 g cm−3 for A. calycularis and 2.5 ± 0.1 g cm−3 for L. pruvoti (N = 12 for each species; Fig. 3a). For both tested species, skeletal porosity in control-pH and control-ST conditions was affected significantly by low pH, but not by high ST or the combination of the two factors at the end of the experiment (Table 3). Thus, the mean skeletal porosity of A. calycularis in low-pH conditions (63 ± 16 %; N = 6) was found to be 10 % higher when compared to that in control-pH conditions (58 ± 12 %, N = 6; Fig. 3b). Likewise, a 13 % increase in the mean skeletal porosity of L. pruvoti was detected in the low-pH conditions with respect to control-pH conditions (56 ± 2 and 50 ± 1 %, N = 6, respectively; Fig. 3b).
Biochemical composition of the tissue
A different pattern in the biochemical composition of the tissue (OM, protein and lipid content) was observed in the two species investigated. In A. calycularis, biochemical results were not significantly affected by low pH, high ST or the combination of the two factors at the end of the experiment (Table 3). Thus, the mean proportion of OM of the tissue was 12.9 ± 1.1 %, the mean protein content was 503 ± 43 µg protein (mg OM)−1 and the mean lipid content was 190 ± 17 µg lipid (mg OM)−1 (N = 24 for each parameter; Fig. 4). Conversely, specimens of L. pruvoti reared under high-ST conditions displayed a significant reduction in all the biochemical components of the tissue at the end of the experiment (Table 3). The mean percentage of OM of L. pruvoti in high-ST conditions (9.9 ± 0.4 %; N = 12) was found to be 28 % lower when compared to that in control-ST conditions (13.7 ± 1.5 %; N = 12; Fig. 4a). Similarly, the mean protein and lipid content in high-ST conditions [580 ± 43 µg protein (mg OM)−1 and 278 ± 28 µg protein (mg OM)−1; N = 12 for each parameter] were reduced by 24 and 40 %, respectively, when compared to that in control-ST conditions [762 ± 54 µg protein (mg OM)−1 and 467 ± 44 µg lipid (mg OM)−1; N = 12 for each parameter; Fig. 4b, c].
Biochemical composition of the tissue of Astroides calycularis and Leptopsammia pruvoti under the four experimental pH and ST treatments at the end of the annual cycle experiment. a Organic matter, b protein and c lipid content. Different letters indicate significant differences between treatments (p < 0.05)
Discussion
Effects of acidification and warming on calcification rates
Our results show that the calcification rate of the azooxanthellate coral species Astroides calycularis and Leptopsammia pruvoti is unaffected by the low-pH conditions expected by the year 2100. These results match the lack of response to acidified conditions observed in a previous study on another temperate coral, Cladocora caespitosa, although the simulated pH was slightly higher in that study (7.9 pH units; Rodolfo-Metalpa et al. 2010). However, further work has shown that the response in terms of calcification of temperate corals reared under low-pH seawater is quite variable. For example, the zooxanthellate C. caespitosa and Oculina patagonica reared in aquaria at pH of 7.8 and 20 °C exhibited a ~35 and 32 % decrease in calcification rate, respectively (Movilla et al. 2012). Colonies of C. caespitosa transplanted for three months along a natural CO2 gradient exhibited a ~80 % decrease in calcification rate at pH 7.8 and ~26 °C, and net calcification of this species became negative at pH 7.5 (Rodolfo-Metalpa et al. 2011). Along a similar pH gradient, Balanophyllia europaea was more resistant to low-pH conditions than C. caespitosa exhibiting a slight decrease in calcification rate at pH 7.8–7.7 (Rodolfo-Metalpa et al. 2011; Fantazzini et al. 2015) and up to ~50 % decrease at pH 7.5 (Rodolfo-Metalpa et al. 2011). Considering that all these previous studies with Mediterranean corals have included only zooxanthellate temperate species, our results therefore suggest that calcification in azooxanthellate shallow-water corals, similar to the response observed in most cold-water corals (e.g., Movilla et al. 2014 and references therein), presents greater resistance to acidified conditions than symbiotic species.
On the other hand, the calcification rate of L. pruvoti was unaffected by high-ST conditions, which is in agreement with the high thermal tolerance previously described for this species (Goffredo et al. 2007; Caroselli et al. 2011, 2012). In contrast, the calcification rate of A. calycularis at high-ST experimental conditions during the summer period was 25 % lower than that observed at control-ST conditions. The growth of scleractinian corals in temperate seas has been described as temperature-dependent, being enhanced up to a sublethal ST threshold and declining thereafter (e.g., Rodolfo-Metalpa et al. 2008; Dimond and Carrington 2007). Previous studies with other Mediterranean coral species indicate that the exposure to positive ST anomalies may lead to physiological stresses such as decreased calcification (Rodolfo-Metalpa et al. 2006, 2008, 2014), increased respiration (Coma et al. 2002; Rodolfo-Metalpa et al. 2006), higher susceptibility to pathogens (Bally and Garrabou 2007), bleaching (Kushmaro et al. 1998) or tissue necrosis (Coma et al. 2006; Rodolfo-Metalpa et al. 2006). When these ST anomalies are maintained for an extended period, global warming has been linked to significant changes in marine coastal ecosystems, resulting in mass mortality outbreaks and changes in the geographic distribution of species (Garrabou et al. 2009; Coma et al. 2009; Coll et al. 2010; Doney et al. 2012; Trenberth 2012). In fact, a mass mortality event of A. calycularis has already been reported during summer 2009 at Ischia (Tyrrhenian Sea), when the shallow-water coral populations (up to 15 m) were affected by a thermal anomaly elevating surface ST to 28–29 °C (Gambi et al. 2010).
Interestingly, the high-ST conditions during the summer period in our experiment (23.7 °C) were still ~3 °C lower than those registered at the collection site of A. calycularis in Cartagena (26.4 °C). Thus, the negative effects of high-ST conditions reported in our study may be magnified for coral populations living in this area, located ~30 km southwestward from the northernmost limit of their distribution along the Spanish coast (Cape Palos, Balearic Sea, 37°38′N; Zibrowius 1995). The particular local conditions found in the eastern Iberian Peninsula, northward from Cape Palos and up to Ebro river delta (40°45′N), led to an unexpected increase in summer ST with increasing latitude (Marbà et al. 2015). We hypothesize that high ST during the summer period found north of Cape Palos may be constraining the upper limit of A. calycularis distribution in the Balearic Sea. This species has recently expanded its distribution northward in both the Tyrrhenian and Adriatic Seas (Bianchi and Morri 1994; Kružić et al. 2002; Grubelić et al. 2004). Several studies based on A. calycularis fossil records and biogeography have hypothesized that the recent expansion of its previously limited distribution, confined to the southwestern Mediterranean Sea between the 14 and 15 °C isotherms for February, could be related to the increasing winter ST due to surface water warming (Zibrowius 1995; Bianchi 2007; Casado-Amezúa et al. 2012). An alternative hypothesis suggested by Bianchi and collaborators (2007) is that these recent coral expansions could be related to an unusual inversion of surface currents in the Ionian Sea. Additional field and mesocosm experiments are needed to gain insight into the ST mortality thresholds that may constrain the A. calycularis geographic distribution under the distinct ST regimes found in the western Mediterranean Sea.
When the whole annual cycle was considered, the calcification rates of both tested species were about half of that during the warm period and the effect of high ST observed during the fast growing period in A. calycularis was clearly attenuated (Fig. 2b). As discussed earlier, this is most probably related to the positive effect that ST has on growth within the optimum temperature thresholds, as observed in other Mediterranean temperate coral species (e.g., Rodolfo-Metalpa et al. 2008). Thus, detection of an observable effect of the examined environmental variables on coral calcification rate could be better assessed during their maximum growth period in summer. However, it should be taken into account that consideration of summer growth only could overestimate the predicted annual effects of the examined factors and would neglect the potential role that phenotypic plasticity may play in enhancing the resistance of the species to stressful conditions (Chevin and Lande 2010). This would be consistent with the interpretation that the response of corals to OA and global warming may not be as rapid and geographically consistent as expected (Pandolfi et al. 2011), highlighting the necessary but challenging task of long-term experiments to detect with more reliability the likely effects in the field.
Potential to compromise the carbonate skeletal framework
Skeletal porosity and microdensity are factors influencing the ability of corals to withstand breakage caused by natural or anthropogenic disturbance (Brown et al. 1985). However, studies evaluating the potential effect of OA and global warming on the coral structural framework are scarce in the literature. A study of different Mediterranean corals along a latitudinal temperature gradient documented that the skeletal porosity of the zooxanthellate B. europaea correlated positively with ST increase (Caroselli et al. 2011). According to the authors, the attenuation of calcification due to the inhibition of the photosynthetic activity by high ST could have caused the observed increase in the porosity of this coral. The hypothesis was supported by the fact that this effect was not observed in the non-symbiotic species L. pruvoti, where, conversely, high ST correlated with an increase in the microdensity of the skeleton. In contrast, our results did not exhibit any significant difference in skeletal microdensity in A. calycularis or L. pruvoti reared under acidified and warmer conditions with respect to the control treatment (Fig. 3a). However, slight differences between the two studies (including ST regime, exposure time, natural vs. aquarium conditions and technique applied) may explain the divergent results for ST-dependent microdensity effects.
Our results show, however, that the low-pH treatment was conducive to an increase in porosity in the azooxanthellate A. calycularis and L. pruvoti with respect to control conditions (Fig. 3b). Similar results, which in our case contrast with the lack of OA effect in the calcification rate of both tested species at the end of our experiment, have been reported in recent publications. Increasing acidity along a natural pH gradient in a CO2 vent was associated with an increasing skeletal porosity in the symbiotic coral B. europaea (Fantazzini et al. 2015). However, a significant reduction in the calcification rate was detected in this case, leading the authors to suggest that the response could be related to the need to maintain constant linear extension rates. It is therefore possible that the decrease in the skeletal porosity observed in A. calycularis and L. pruvoti under the low-pH treatment, along with the ability to keep constant their calcification rates, may lead to higher linear extension rates in these species under reduced pH. Unfortunately, we could not assess properly this biometric parameter in our studied species. According to Fantazzini et al. (2015), coral efforts to maintain extension rates could be related to a need to achieve a critical size at sexual maturity, at the expense of increasing their susceptibility to mechanical stress under acidified conditions. In the same way, the higher porosity values observed in the tropical coral Stylophora pistillata reared under acidified conditions (pH 7.4 and 7.2) were related to changes in its skeleton phenotype, such as the enlargement of the corallite calyx and thinning of septae and thecae (Tambutté et al. 2015). A similar phenotypic response was detected in the zooxanthellate Mediterranean coral C. caespitosa reared under more similar pH values than the present work (pH 7.8), although porosity of the skeleton was not measured in that study (Movilla et al. 2012). Therefore, the similar response of skeleton porosity between zooxanthellate and azooxanthellate corals suggest that factors other than the inhibition of symbionts by ST must be affecting the calcium carbonate structure of some coral species. Further research on testing the fragility of the coral supporting framework to the long-term exposure to unfavorable conditions, such as those caused by the combination of an acidified and warmed environment, should promote better understanding of the calcification physiology in coral species.
Biochemical balance in the tissue and ecological implications
Elevated ST may stimulate enzyme activity and metabolic rate of organisms within their window of thermal tolerance (Ip et al. 1991), although it could also cause a rapid deterioration of cellular processes when certain ST threshold is exceeded (Pörtner and Farrell 2008; Kroeker et al. 2013). The observed lower values of OM, protein and lipid content of the tissue in L. pruvoti reared under high-ST conditions (Fig. 4) could be related to increased metabolic activity. However, this effect manifested as a species-specific response that was not observed in A. calycularis. In a previous work assessing two Mediterranean cold-water coral species (Movilla et al. 2014), and similar to our results with A. calycularis, no significant effect was detected in the OM amount or the lipid content in the tissue of Dendrophyllia cornigera and Desmophyllum dianthus after more than 300 days of exposure to a 7.8 pH treatment. Similarly, a previous study with the octocoral Corallium rubrum (Bramanti et al. 2013) reported no effect of similar pH levels on the lipid, protein or carbohydrate content of the tissue. However, a significant increase in the OM amount was observed in those specimens of C. rubrum reared under low-pH conditions, at the expense of a reduction in the calcification rate.
The calcification process is still poorly understood and predicting how corals will respond to an increase in CO2 requires a better understanding of the mechanisms involved (Cohen and Holcomb 2009; Cohen et al. 2009; Allemand et al. 2011; Venn et al. 2011; Mass et al. 2012; Holcomb et al. 2014). It has been proposed recently that the physiological mechanisms that drive calcification itself could be crucial in how some coral species are able to resist increasing acidification (Ries et al. 2010; Tambutté et al. 2011; McCulloch et al. 2012). Venn et al. (2013) proposed two models to explain different responses to acidification in reef corals: (1) the energy pool for maintaining internal pH is constant and, therefore, a reduction in the environmental pH would result in a significant reduction in the calcification rate; and (2) the energy input is compensated as the external pH is reduced to maintain constant calcification rate, to the detriment of other vital activities such as locomotion, reproduction, tissue regeneration or to counteract other environmental stresses (Hoegh-Guldberg et al. 2007; Brewer and Peltzer 2009). In this context, our results on the temperate azooxanthellate corals A. calycularis and L. pruvoti conform, respectively, to the energetic scenarios 1 and 2 proposed by Venn et al. (2013). The OM and the lipid and protein content of the tissue of A. calycularis did not become affected by the stress conditions of low pH and high ST, but the species exhibited a decrease in calcification rate of about 25 % under the high-ST conditions during the summer period. Conversely, the absence of effect of low pH and high ST on calcification of L. pruvoti is concomitant with a significant decrease in OM and on the lipid and protein content of the tissue under the high-ST conditions. This could be indicative of the activation of physiological processes to offset the new conditions at the expense of an increase in the energy consumption that leads to a decrease on the global energy resources of the species. Accordingly, the lipid and protein reserves in L. pruvoti under control conditions were twice as high as those in A. calycularis (Fig. 4c), which would allow the former to have a greater energy availability to maintain the energetically costly calcification rate. The species-specific observed response suggests therefore that different internal self-regulation mechanisms may be in place, allowing certain species to cope more successfully with environmental changes.
The Mediterranean is a biodiversity hotspot (Coll et al. 2010; Templado 2014) that is highly vulnerable to global change (e.g., Calvo et al. 2011; Herrmann et al. 2014; Marbà et al. 2015) and where OA and global warming seem to be occurring more rapidly than in the global oceans (Vargas-Yáñez et al. 2010; Touratier and Goyet 2011; Touratier et al. 2012; but see Palmiéri et al. 2014). In this semi-enclosed sea, global warming has been shown to be responsible of a significant lengthening of summer conditions (Coma et al. 2009), being an energetically unfavorable season with high respiratory demand and low food availability due to thermal stratification of the water column. The combination of both factors forces marine benthic invertebrates to a decreased activity pattern described as ‘summer dormancy’ (Coma et al. 2000; Coma and Ribes 2003), and may restrict the accumulation of energy resources that could be devoted to calcification, tissue growth and/or reproduction (Brewer and Peltzer 2009; Hoegh-Guldberg et al. 2007). Nonetheless, although it has been suggested that reduced pH can cause metabolic depression in some marine invertebrates (Edmunds 2012), little information is available about the potential effect of OA, alone or in combination with high ST, on the metabolism and biochemical balance. The decrease in the biochemical variables observed in L. pruvoti in our experiment suggests that, in the near future, the continuing reduced-pH conditions, along with increased ST, may push the metabolism of some of these organisms close to their limits. The potential synergistic effect of the two factors suggests that, in some temperate species, OA could enhance the severity of mass die-off events of benthic organisms associated with long-term warm periods, which are increasing in frequency and intensity in the Mediterranean Sea (Coma et al. 2009). Understanding their biochemical strategies for the reallocation of metabolic energy can contribute to assessing the potential effects and relative resilience under future scenarios of anthropogenic global change and bring new insights into eventual shifts under large-scale stresses. This understanding will be useful for the management and preservation of the Mediterranean infralittoral (Ballesteros 2006), one of the richest and most diverse marine ecosystems on earth.
References
Allemand D, Tambutté É, Zoccola D, Tambutté S (2011) Coral calcification, cells to reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Heidelberg, pp 119–150
Anthony KRN, Connolly SR, Hoegh-Guldberg O (2007) Bleaching, energetics, and coral mortality risk: effects of temperature, light, and sediment regime. Limnol Oceanogr 52:716–726
Anthony KRN, Kline DI, Diaz-Pulido G, Dove SG, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA 105:17442–17446
Ballesteros E (2006) Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr Mar Biol 44:123–195
Bally M, Garrabou J (2007) Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Glob Change Biol 13:2078–2088
Barnes H, Blackstock J (1973) Estimation of lipids in marine animals and tissues: detailed investigation of the sulphophosphovanillin method for total lipids. J Exp Biol 12:103–118
Bianchi CN (2007) Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 580:7–21
Bianchi CN, Morri C (1994) Southern species in the Ligurian Sea (northern Mediterranean): new records and a review. Bollettino dei Musei e degli Istituti biologici dell’Università di Genova (1992–1993) 58–59:181–197
Bramanti L, Movilla J, Guron M, Calvo E, Gori A, Dominguez-Carrió C, Grinyó J, López-Sanz A, Martínez-Quintana A, Pelejero C, Ziveri P, Rossi S (2013) Detrimental effects of Ocean Acidification on the economically important Mediterranean red coral (Corallium rubrum). Glob Change Biol 19:1897–1908
Brewer PG, Peltzer ET (2009) Limits to marine life. Science 324:347
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs 16:S129–S138
Brown BE, Sya’Rani L, Le Tissier M (1985) Skeletal form and growth in Acropora aspera (Dana) from the Pulau Seribu, Indonesia. J Exp Mar Biol Ecol 86:139–150
Bucher D, Harriott VJ, Roberts LG (1998) Skeletal micro-density, porosity and bulk density of acroporid corals. J Exp Mar Biol Ecol 228:117–136
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol 49:1–42
Calvo E, Simó R, Coma R, Ribes M, Pascual J, Sabatés A, Gili JM, Pelejero C (2011) Effects of climate change on Mediterranean marine ecosystems: the case of the Catalan Sea. Climate Res 50:1–29
Caroselli E, Prada F, Pasquini L, Marzano FN, Zaccanti F, Falini G, Levy O, Dubinsky Z, Goffredo S (2011) Environmental implications of skeletal micro-density and porosity variation in two scleractinian corals. Zoology 114:255–264
Caroselli E, Zaccanti F, Mattioli G, Falini G, Levy O, Dubinsky Z, Goffredo S (2012) Growth and demography of the solitary scleractinian coral Leptopsammia pruvoti along a sea surface temperature gradient in the Mediterranean Sea. PLoS ONE 7:e37848. doi:10.1371/journal.pone.0037848
Casado-Amezúa P, Goffredo S, Templado J, Machordom A (2012) Genetic assessment of population structure and connectivity in the threatened Mediterranean coral Astroides calycularis (Scleractinia, Dendrophylliidae) at different spatial scales. Mol Ecol 21:3671–3685
Casellato S, Masiero L, Sichirollo E, Soresi S (2007) Hidden secrets of the Northern Adriatic: Tegnuè, peculiar reefs. Cent Eur J Biol 2:122–136
Cebrian E, Ballesteros E (2004) Zonation patterns of benthic communities in an upwelling area from the western Mediterranean (La Herradura, Alboran Sea). Sci Mar 68:69–84
Chevin LM, Lande R (2010) When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64:1143–1150
Clayton TD, Byrne RH (1993) Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep Sea Res 40:2115–2129
Cohen AL, Holcomb M (2009) Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22:118–127
Cohen AL, McCorkle DC, De Putron S, Gaetani GA, Rose KA (2009) Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochem Geophys Geosyst. doi:10.1029/2009GC002411
Coll M, Piroddi C, Steenbeek J et al (2010) The biodiversity of the Mediterranean Sea: estimates, patterns and threats. PLoS ONE 5:e11842
Coma R, Ribes M (2003) Seasonal energetic constraints in Mediterranean benthic suspension feeders: effects at different levels of ecological organization. Oikos 101:205–215
Coma R, Ribes M, Gili JM, Zabala M (2000) Seasonality of in situ respiration rate in three temperate benthic suspension feeders. Limnol Oceanogr 47:324–331
Coma R, Ribes M, Gili JM, Zabala M (2002) Seasonality in coastal benthic ecosystems. Trends Ecol Evol 15:448–453
Coma R, Linares C, Ribes M, Diaz D, Garrabou J, Ballesteros J (2006) Consequences of a mass mortality in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean). Mar Ecol Prog Ser 327:51–60
Coma R, Ribes M, Serrano E, Jiménez E, Salat J, Pascual J (2009) Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc Natl Acad Sci USA 106:6176–6181
Cooper TF, De’ath G, Fabricius KE, Lough JM (2008) Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Glob Change Biol 14:529–538
Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biol 101:389–395
De’ath G, Lough JM, Fabricius KE (2009) Declining coral calcification on the Great Barrier Reef. Science 323:116–119
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res Part A Oceanogr Res Pap 34:1733–1743
Dimond J, Carrington E (2007) Temporal variation in the symbiosis and growth of the temperate scleractinian coral Astrangia poculata. Mar Ecol Prog Ser 348:161–172
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1:169–192
Doney SC, Ruckelshaus M, Emmett Duffy J, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Ann Rev Mar Sci 4:11–37
Edmunds PJ (2012) Effect of pCO2 on the growth, respiration, and photophysiology of massive Porites spp. in Moorea, French Polynesia. Mar Biol 159:2149–2160
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1:165–169
Fantazzini P, Mengoli S, Pasquini L, Bortolotti V, Brizi L, Mariani M, Di Giosia M, Fermani S, Capaccioni B, Caroselli E, Prada F, Zaccanti F, Levy O, Dubinsky Z, Kaandorp JA, Konglerd P, Hammel JU, Dauphin Y, Cuif JP, Weaver JC, Fabricius KE, Wagermaier W, Fratzl P, Falini G, Goffredo S (2015) Gains and losses of coral skeletal porosity changes with ocean acidification acclimation. Nat Commun 6:7785. doi:10.1038/ncomms8785
Fine M, Tchernov D (2007) Scleractinian coral species survive and recover from decalcification. Science 315:1811
Gambi MC, Barbieri F, Signorelli S, Saggiomo V (2010) Mortality events along the Campania coast (Tyrrhenian Sea) in summers 2008 and 2009 and relation to thermal conditions. Biol Mar Mediterr 17:126–127
Garrabou J, Coma R, Bensoussan N, Bally M, Chevaldonné P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, Ledoux JB, Lejeusne C, Linares C, Marschal C, Pérez T, Ribes M, Romano JC, Serrano E, Teixido N, Torrents O, Zabala M, Zuberer F, Cerrano C (2009) Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Change Biol 15:1090–1103
Gattuso JP, Hansson L (2011) Ocean acidification: background and history. In: Gattuso JP, Hansson L (eds) Ocean acidification. Oxford University Press, Oxford, pp 1–20
Gattuso JP, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Integr Comp Biol 39:160–183
Goffredo S, Airi V, Radetic J, Zaccanti F (2006) Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean. 2. Quantitative aspects of the annual reproductive cycle. Mar Biol 148:923–932
Goffredo S, Caroselli E, Pignotti E, Mattioli G, Zaccanti F (2007) Variation in biometry and population density of solitary corals with solar radiation and sea surface temperature in the Mediterranean Sea. Mar Biol 152:351–361
Grubelić I, Antolić B, Despalatović M, Grbec B, Paklar GB (2004) Effect of climatic fluctuations on the distribution of warm-water coral Astroides calycularis in the Adriatic Sea: new records and review. J Mar Biol Ass UK 84:599–602
Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Ann NY Acad Sci 1134:320–342
Herrmann M, Estournel C, Adloff F, Diaz F (2014) Impact of climate change on the northwestern Mediterranean Sea pelagic planktonic ecosystem and associated carbon cycle. J Geophys Res Oceans 119:5815–5836
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Holcomb M, McCorkle DC, Cohen AL (2010) Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander 1786). J Exp Mar Bio Ecol 386:27–33
Holcomb M, Cohen AL, McCorkle DC (2012) An investigation of the calcification response of the scleractinian coral Astrangia poculata to elevated pCO2 and the effects of nutrients, zooxanthellae and gender. Biogeosciences 9:29–39
Holcomb M, Venn AA, Tambutté E, Tambutté S, Allemand D, Trotter J, McCulloch MT (2014) Coral calcifying fluid pH dictates response to ocean acidification. Sci Rep 4:5207
Ip YK, Lim ALL, Lim RWL (1991) Some properties of calcium-activated adenosine triphosphatase from the hermatypic coral Galaxea fascicularis. Mar Biol 1991:191–197
IPCC (2013) Summary for policymakers. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York
Jokiel PL, Maragos JE, Franzisket L (1978) Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE (eds) Coral reefs: monographs on oceanographic methodology. UNESCO, Paris, pp 529–541
Khatiwala S, Tanhua T, Mikaloff-Fletcher S, Gerber M, Doney SC, Graven HD, Gruber N, McKinley GA, Murata A, Ríos AF, Sabine CL, Sarmiento JL (2013) Global ocean storage of anthropogenic carbon. Biogeosciences 10:2169–2219
Kroeker KJ, Kordas RL, Crim R, Hendriks IE (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896
Kružić P, Zibrowius H, Pozar-Domac A (2002) Actiniaria and Scleractinia (Cnidaria, Anthozoa) from the Adriatic Sea: first records, confirmed occurrences and significant range extensions of certain species. Italian J Zool 69:345–353
Kushmaro A, Rosenberg E, Fine M, Ben Haim Y, Loya Y (1998) Effect of temperature on bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar Ecol Prog Ser 171:131–137
Maier C, Schubert A, Berzunza Sánchez MM, Weinbauer MG, Watremez P, Gattuso J-P (2013) End of the century pCO2 levels do not impact calcification in Mediterranean Cold-water corals. PLoS ONE. doi:10.1371/journal.pone.0062655
Marbà N, Jorda G, Agusti S, Girard C, Duarte CM (2015) Footprints of climate change on Mediterranean Sea biota. Front Mar Sci 2:56. doi:10.3389/fmars.2015.00056
Mass T, Drake JL, Haramaty L, Rosenthal Y, Schofield OME, Sherrell RM, Falkowski PG (2012) Aragonite precipitation by “proto-polyps” in coral cell cultures. PLoS ONE 7:4. doi:10.1371/journal.pone.0035049
McCulloch MT, Falter J, Trotter J, Montagna P (2012) Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Change 2:623–627
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RN (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Movilla J, Calvo E, Pelejero C, Coma R, Serrano E, Fernández-Vallejo P, Ribes M (2012) Calcification reduction and recovery in native and non-native Mediterranean corals in response to ocean acidification. J Exp Mar Biol Ecol 438:144–153
Movilla J, Orejas C, Calvo E, Gori A, López-Sanz À, Grinyó J, Domínguez-Carrió C, Pelejero C (2014) Differential response of two Mediterranean cold-water coral species to ocean acidification. Coral Reefs 33:675–686
Muehllehner N, Edmunds PJ (2008) Effects of ocean acidification and increased temperature on skeletal growth of two scleractinian corals, Pocillopora meandrina and Porites rus. In: Proceedings of 11th international coral reef symposium, pp 7–11
Palmiéri J, Orr JC, Dutay J-C, Béranger K, Schneider A, Beuvier J, Somot S (2014) Simulated anthropogenic CO2 uptake and acidification of the Mediterranean Sea. Biogeosci Discuss 11:6461–6517
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333:418–422
Parker L, Ross P, Connor W, Pörtner H, Scanes E, Wright J (2013) Predicting the response of molluscs to the impact of ocean acidification. Biology 2:651–692
Pelejero C, Calvo E, Hoegh-Guldberg O (2010) Paleo-perspectives on ocean acidification. Trends Ecol Evol 25:332–344
Pelletier F, Clutton-Brock T, Pemberton J, Tuljapurkar S, Coulson T (2007) The evolutionary demography of ecological change: linking trait variation and population growth. Science 315:1571–1574
Perez FF, Fraga F (1987) A precise and rapid analytical procedure for alkalinity determination. Mar Chem 21:169–182
Perez FF, Rios AF, Rellán T, Alvarez M (2000) Improvements in a fast potentiometric seawater alkalinity determination. Cienc Mar 26:463–478
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pagès C, Jaubert J, Gattuso J-P (2003) Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob Change Biol 9:1660–1668
Reynaud S, Ferrier-Pagès C, Meibom A, Mostefaoui S, Mortlock R, Fairbanks R, Allemand D (2007) Light and temperature effects on Sr/Ca and Mg/Ca ratios in the scleractinian coral Acropora sp. Geochim Cosmochim Acta 71:354–362
Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Ries JB, Cohen AL, McCorkle DC (2010) A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 29:661–674
Rodolfo-Metalpa R, Richard C, Allemand D, Ferrier-Pagès C (2006) Growth and photosynthesis of two Mediterranean corals, Cladocora caespitosa and Oculina patagonica, under normal and elevated temperatures. J Exp Biol 209:4546–4556
Rodolfo-Metalpa R, Reynaud C, Allemand D, Ferrier-Pagès C (2008) Temporal and depth responses of two temperate corals, Cladocora caespitosa and Oculina patagonica, from the North Mediterranean Sea. Mar Ecol Prog Ser 369:103–114
Rodolfo-Metalpa R, Martin S, Ferrier-Pagès C, Gattuso J-P (2010) Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 ad. Biogeosciences 7:289–300
Rodolfo-Metalpa R, Houlbrèque F, Tambutté É, Boisson F, Baggini C, Patti FP, Jeffree R, Fine M, Foggo A, Gattuso J-P, Hall-Spencer JM (2011) Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat Clim Change 1:308–312
Rodolfo-Metalpa R, Hoogenboom MO, Rottier C, Ramos-Esplá A, Baker AC, Fine M, Ferrier-Pagès C (2014) Thermally tolerant corals have limited capacity to acclimatize to future warming. Glob Change Biol 20:3036–3049
Rossi L (1971) Cnidari e Ctenofori d’Italia. Quaderni della Civica Stazione Idrobiologica di Milano 2:77–86
Slattery M, McClintock JB, Heine J (1995) Chemical defenses in Antarctic soft corals: evidence for antifouling compounds. J Exp Mar Biol Ecol 190:61–77
Sokolov AP, Stone PH, Forest CE, Prinn RG, Sarofim MC, Webster M, Paltsev S, Schlosser CA, Kicklighter D, Dutkiewicz S, Reilly J, Wang C, Felzer B, Melillo J, Jacoby HD (2009) Probabilistic forecast for 21st century climate based on uncertainties in emissions (without policy) and climate parameters. J Clim 22:5175–5204
Solomon S, Qin D, Manning MR, Marquis M, Averyt K, Tignor MMB, Miller HLJ, Zhenlin C (2007) Climate change 2007: the physical science basis: Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge Univ Press, Cambridge
Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud S, Tambutté E, Zoccola D, Allemand D (2011) Coral biomineralization: from the gene to the environment. J Exp Mar Bio Ecol 408:58–78
Tambutté E, Venn AA, Holcomb M, Segonds N, Techer N, Zoccola D, Allemand D, Tambutté S (2015) Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat Commun. doi:10.1038/ncomms8368
Templado J (2014) Future trends of Mediterranean biodiversity. In: Goffredo S, Dubinsky Z (eds) The Mediterranean Sea: its history and present challenges. Springer, London, pp 479–498
Touratier F, Goyet C (2011) Impact of the Eastern Mediterranean transient on the distribution of anthropogenic CO2 and first estimate of acidification for the Mediterranean Sea. Deep Sea Res I: Oceanogr Res Pap 58:1–15
Touratier F, Guglielmi V, Goyet C, Prieur L, Pujo-Pay M, Conan P, Falco C (2012) Distributions of the carbonate system properties, anthropogenic CO2, and acidification during the 2008 BOUM cruise (Mediterranean Sea). Biogeosci Discuss 9:2709–2753
Trenberth K (2012) Framing the way to relate climate extremes to climate change. Clim Change 115:283–290
Vargas-Yáñez M, Moya F, García-Martínez MC, Tel E, Zunino P, Plaza F, Salat J, Pascual J, López-Jurado JL, Serra M (2010) Climate change in the Western Mediterranean Sea 1900–2008. J Mar Syst 82:171–176
Venn AA, Tambutté E, Holcomb M, Allemand D, Tambutté S (2011) Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 6:e20013
Venn AA, Tambutté E, Holcomb M, Laurent J, Allemand D, Tambutté S (2013) Impact of seawater acidification on pH at the tissue—skeleton interface and calcification in reef corals. Proc Natl Acad Sci USA 110:1634–1639
Weinberg S (1979) Autecology of shallow-water Octocorallia from Mediterranean rocky substrata, I. The Banyuls area. Bijdragen tot de Dierkunde 49:1–15
Wicks L, Roberts JM (2012) Benthic invertebrates in a high-CO2 world. Oceanogr Mar Biol Annu Rev 50:127–188
Zibrowius H (1980) Les scléractiniaires de la Méditerranée et de l’Atlantique nord-oriental. Mem Inst Oceanogr 11:1–284
Zibrowius H (1995) The ‘‘southern’’ Astroides calycularis in the Pleistocene of the northern Mediterranean—an indicator of climatic changes (Cnidaria, Scleractinia). Geobios 28:9–16
Acknowledgments
Thanks are due to A. Olariaga (ICM) for technical help in the aquaria design and to M. Delgado (ZAE staff at the ICM) for technical assistance. The authors are also grateful to R. Sherrell and A. Williams for their insightful comments and help with the English. This work was supported by projects CTM2012-32017, CGL2013-43106-R and a FPI fellowship (BES-2007-16537) to JM from the Spanish Government. This is a contribution from the Marine Biogeochemistry and Global Change research group (Grant 2014SGR1029, Generalitat de Catalunya).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: D. Gochfeld.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Movilla, J., Calvo, E., Coma, R. et al. Annual response of two Mediterranean azooxanthellate temperate corals to low-pH and high-temperature conditions. Mar Biol 163, 135 (2016). https://doi.org/10.1007/s00227-016-2908-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2908-9