Abstract
Life expectancy of the scyphomedusa Aurelia aurita seems closely linked to seasonal shrinkage, or somatic degrowth, which occurs synchronously with sexual reproduction in temperate regions. In the present study, the mechanisms controlling body mass losses and subsequent disappearance of medusae were examined by following seasonal dynamics in population density, individual size, and sexual reproduction of A. aurita during 2013 and 2014 in the shallow, semi-enclosed Kertinge Nor, and the adjacent Great Belt, Denmark. After sexual maturation in early summer, medusae were characterized by a distinct phase of somatic degrowth, expressed by weight-specific individual growth rates of −0.5 to −1.4 % day−1, which was followed by the absence of A. aurita populations in both ecosystems during winter. The number of planula larvae per female (N L , ind.) was positively correlated with medusa diameter (d, mm), following the exponential relationship N L = 160.8 × e 0.029d. The percentage of body mass made up by planulae ranged from 6 to 11 % in Kertinge Nor and 10 to 33 % in Great Belt. ≤15 % of total size-specific body mass losses were due to the release of planulae in Kertinge Nor, suggesting shortage of zooplankton prey during autumn as main factor causing degrowth in A. aurita. Starving medusae produced significantly fewer oocytes and revealed increased mortality compared to well-fed individuals in supplementary laboratory experiments, indicating that degrowth controls the energetic investment into sexual reproduction. Seasonal variability in food supply, rather than energy allocation to reproduction, appears to shorten life spans of A. aurita medusae in temperate Danish waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The moon jellyfish, Aurelia aurita s. l., has been described as an almost ubiquitous ecological generalist (Dawson and Martin 2001) and belongs to the most widely distributed of all scyphozoans, ranging circumglobally between 70°N and 40°S (Möller 1980; Lucas 1996). A. aurita has a life cycle which includes a pelagic medusa and a benthic polyp stage. Medusae reproduce sexually, and after fertilization of oocytes, females release planula larvae which after settlement metamorphose into polyps. Benthic polyps in turn produce ephyrae that are released into the ambient water to develop into medusae (Hamner and Jenssen 1974; Möller 1980; Hernroth and Gröndahl 1985; Lucas 2001).
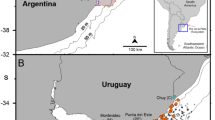
In temperate waters, annual life cycles of A. aurita predominate (Hamner and Jenssen 1974; Lucas 2001), and generally ephyrae are present in spring and grow through summer, become sexually mature medusae and show strong declines in population density after the onset of sexual reproduction (Möller 1980; Gröndahl 1988a; Olesen et al. 1994; Lucas 2001). A. aurita can be very abundant, exerting a considerable predatory impact on zooplankton and fish larvae (Bailey and Batty 1984; Båmstedt 1990; Schneider and Behrends 1994, 1998; Behrends and Schneider 1995; Hansson et al. 2005; Møller and Riisgård 2007a, b). In general, medusa populations of A. aurita are food limited, following seasonal dynamics in zooplankton biomass, as observed, for example, in Kiel Bight (Möller 1980), in a small semi-enclosed Norwegian bay (Ishii and Båmstedt 1998), and in the shallow Danish cove of Kertinge Nor (Fig. 1).
In Kertinge Nor, the maximum diameter of the medusa umbrella is usually only a few centimeters, compared to >20 cm in more open coastal waters (Möller 1980). Extremely high abundance of small jellyfish in Kertinge Nor causes shortage of prey and thus restriction of their own growth (Olesen et al. 1994; Olesen 1995; Riisgård et al. 1995, 1996, 2008, 2010; Frandsen and Riisgård 1997; Nielsen et al. 1997). A survey of data obtained every year in August in the period 1991–2009 in Kertinge Nor indicated that the remarkably high population density of A. aurita in combination with small umbrella diameters had remained similar during the 18-year period, and further, that particularly high population densities of jellyfish in certain years could be correlated with relatively small mean umbrella diameters (Riisgård et al. 2010). Although medusae in Kertinge Nor remain small, they are able to reproduce during summer, and fecundity, i.e., the production of gametes (oocytes or sperm follicles), is typically followed by shrinkage due to body mass losses (designated ‘degrowth’ by Hamner and Jenssen 1974) and the disappearance of the A. aurita population in late autumn (Olesen et al. 1994). The phenomenon of degrowth, in which the animal becomes smaller when deprived of food, regressing until it resembles a diminutive adult (Hamner and Jenssen 1974) has repeatedly been documented for much larger A. aurita medusae around the world (Möller 1980; Uye and Shimauchi 2005; Marques et al. 2015). Besides food as limiting factor, shrinkage of medusae has been contributed to the energetic investment into gamete production and the spawning process, potentially resulting in morphological degradation and death (Marques et al. 2015). It is still unclear to which degree degrowth is controlled by food availability and/or a trade-off between survival and reproduction (Stearns 1976).
In the present study, seasonal dynamics in population density, individual growth, and sexual reproduction of small A. aurita medusae in the semi-enclosed Kertinge Nor and of larger specimen in the adjacent Great Belt, Denmark, were used to examine the hitherto poorly described mechanisms behind somatic degrowth. The importance of food versus sexual reproduction was evaluated by quantifying the actual body weight loss of medusae in the degrowth period compared to estimated (respiratory) maintenance costs and weight losses due to the release of planula larvae. It was further tested whether the yearly cumulative jellyfish population biomass in Kertinge Nor was comparable in 2013 and 2014, outlining to which extent seasonal and inter-annual differences in the maximum umbrella diameter of medusae are related to population density-dependent food availability. Based on these findings, it was possible to unravel controlling factors that determine the life expectancy of A. aurita in Danish and other temperate waters where medusa populations are not persistent throughout the year.
Materials and methods
Study site
The fjord system consisting of Kerteminde Fjord and Kertinge Nor (Fig. 1) covers an area of 8.5 km2 and has a mean water depth of approximately 2 m and a maximum depth of 8 m. The fjord has a sill at its mouth to the open sea (Great Belt). The discharge over the sill is forced by a diurnal tide with average amplitude of approximately 20 cm. The catchment area to Kerteminde Fjord and Kertinge Nor is limited and the fresh water input negligible compared to the water exchange of the fjord system. The salinity in the fjord system varies typically between 15 and 21 over the year (Riisgård et al. 2008). Water exchange of the fjord system is governed by density-driven circulation (Riisgård et al. 1996; Nielsen et al. 1997; Riisgård 1998) which results from salinity variations in Great Belt due to changing flow situations (Jürgensen 1995; Møller 1996). Outflow of water from the Baltic Sea gives salinities down to <10, whereas inflow from Kattegat to the Baltic Sea results in salinities of up to 27 in the upper layer of the Great Belt. Salinity differences between the fjord system Kertinge Nor/Kerteminde Fjord and the adjacent Great Belt can either result in bottom layer inflow of more saline water into the fjord creating a clockwise circulation or surface layer inflow of less saline water creating a counterclockwise circulation pattern (Jürgensen 1995; Nielsen et al. 1997). On an annual timescale, the two circulation directions have equal probability (Riisgård et al. 2010).
Jellyfish in Kertinge Nor in 2013 and 2014
Aurelia aurita ephyrae and medusae were collected during 32 cruises with a small boat at locations 1–3 in Kertinge Nor and at location 6 in Great Belt (Fig. 1) from May 2013 to December 2014. At each sampling site, 3 horizontal hauls (haul length, L haul = 5.4 ± 0.2 m) were performed with a 500-µm-meshed plankton net (mouth area A = 0.25 m2) at a depth of 0.5 m below surface. After each haul, the collected jellyfish were gently rinsed off the plankton net with seawater and subsequently counted and measured in the laboratory. Temperature (3–24 °C) and salinity (13–26) were recorded in 0.5-m steps from the surface to the bottom at all 6 locations (Fig. 1) during each cruise. Population densities (ind. m−3) of A. aurita in Kertinge Nor and Great Belt were estimated from the number of medusae (N M , ind.) in each of 3 replicate hauls on each location per volume of filtered seawater (V f = L haul × A = 1.35 m3) as D = N M /V f . Inter-rhopalia diameters (d, mm) of ephyrae and medusae were determined by measuring all or 30 individuals in each haul to the nearest mm.
The timing of sexual reproduction in A. aurita was determined by tracking the number and sex of sexually mature medusae (N Mm ) in net haul samples, as identified from the presence of oocytes or spermatic follicles in the gonadal tissue of female or male individuals (Schiariti et al. 2012) under a Leica M165 FC stereomicroscope (standard magnification range 7–120-fold). Additional larvae-carrying females were collected in Kertinge Nor during the reproductive season (6–10 individuals per sampling date) to quantify the number of planula larvae per female medusa (N L , ind. female−1) and the daily release of planulae (ρ L ; ind. female−1 d−1). Medusae were individually incubated in small (50 mL) seawater-filled containers at 15–25 °C room temperature exposed to natural light conditions for about 24 h before counting the number of planula larvae on each medusa, as well as the released larvae in the seawater containers, by using a stereomicroscope.
Jellyfish in Great Belt in 2014
Aurelia aurita collected in Great Belt were easily recognized by a bluish coloring and their much larger body size compared to the small red-brownish medusae from Kertinge Nor. To determine the reproductive output in female medusae from Great Belt, larvae-carrying medusae were individually transported to the nearby laboratory in seawater-filled buckets. Subsequently, planulae were removed from each medusa by thoroughly washing the oral arms. Together with already released larvae, they were collected in an 80-µm-meshed plankton filter and augmented in 500 mL seawater. After preservation in Lugol’s solution, 10–20 replicate subsamples (0.1–5 mL) were counted under the stereomicroscope to determine the number of planula larvae per female (N L ). Diameter (d L , µm) and length (L L , µm) of planula larvae (L) from both Kertinge Nor and Great Belt (N L = 50) were measured under the stereomicroscope with integrated camera system (Leica DFC 425 C) using imaging software LAS (Leica Application Suite).
Growth and fecundity experiments
Laboratory experiments were conducted to investigate the influence of food availability on growth and sexual reproduction of A. aurita medusae from Kertinge Nor during the end of the year 2014. After an acclimation period of 24 h under starvation, larvae-carrying females (d ~ 30–60 mm) were individually transferred to 1-L aquaria with filtered (38 µm) seawater (14–20 °C, salinity 20). In a first experimental series (Series #1), medusae collected in September 2014 (d = 32.3 ± 3.8 mm) at sampling location 1 in Kertinge Nor (Fig. 1) were divided into 2 groups (n = 6), one group was starved and the other daily fed with 3-day-old brine shrimp, Artemia salina, nauplii in 5 rations of 2000 ind. medusa−1 within 12-h intervals for 30 days. The seawater in all aquaria was exchanged daily. The inter-rhopalia umbrella diameter (d, mm) of each medusa was measured daily to the nearest mm by gently placing the medusae on laminated millimeter paper. Umbrella diameters were converted to body dry weight to estimate weight-specific growth rates, cf. Eq. (6). A second experimental series (Series #2) with medusae captured at the same location in October 2014 (d = 45.1 ± 7.9 mm) was conducted under similar food conditions for 30 days, additionally including regular counts of the number of oocytes (N O , ind. female−1) observed in gonads and oral arms of each live female medusa under the stereomicroscope.
Equations and biometric conversions
The following equation was used to estimate the individual clearance rate (Cl ind , L day−1) from the mean inter-rhopalia diameter (d, mm) of A. aurita feeding on copepods (Møller and Riisgård 2007c):
When used for investigating the feeding of a jellyfish species on a given kind of zooplankton prey, clearance rates are independent of prey concentration (Purcell 1997; Møller and Riisgård 2007c). The volume-specific population clearance rate (Cl pop , m3; water filtered by the jellyfish population in 1 m3 water per day = m3 m−3 day−1) was estimated as the product of the individual clearance rate (Cl ind , L day−1) and the population density (D, ind. m−3) for each locality:
The time (t 1/2 , day) it takes for a population of jellyfish with known Cl pop to reduce the concentration of prey organisms (copepods) in V = 1 m3 of water by 50 % (i.e., the theoretical half-life of prey) was estimated as (Møller and Riisgård 2007b):
Umbrella diameters (d, mm) of A. aurita ephyrae ( E ) and medusae ( M ) were converted to individual dry weight (DW, mg ind.−1) using the following exponential regression functions.
Aurelia aurita ephyrae (Båmstedt et al. 1999):
Aurelia aurita medusae (Olesen et al. 1994; Fig. 2 therein):
Since dry weight increases with salinity (Hirst and Lucas 1998), Purcell (2009) recommends conversions only from specimens from similar ambient salinity, as has been observed in Kertinge Nor and Great Belt during 2013 and 2014 (Online Resources 1, 2). According to linear regression functions provided by Hirst and Lucas (1998; Fig. 2 and Table 4 therein), converted dry weights may vary by a maximum of 10 % due to salinity differences between jellyfish samplings. Resulting individual dry weights were used to determine weight-specific instantaneous growth rates µ (day−1) by using the following equation (Olesen et al. 1994):
where DW t and DW t+∆t is the mean dry body weight of medusae on day t and day t + ∆t, respectively. The volume-specific dry weight of the jellyfish population (DW pop , g m−3) was calculated as:
where DW E,M (mg ind.−1) expresses the mean individual dry weight of ephyrae and medusa and D (ind. m−3) is the density of the jellyfish population.
The body volume (BV L , µm3 ind.−1) of planula larvae was calculated as a prolate spheroid (Sun and Liu 2003; Table 2 therein):
Body volumes of planulae were converted to biomass (BM L , µg C ind.−1) using a conversion factor of 0.12 pg C µm−3 body volume (Nielsen and Møhlenberg 2004):
The following conversion factors were used: c 1 = 0.02 mg µg−1 C−1 (mg A. aurita medusa dry weight per µg carbon; Schneider 1988); c 2 = 0.02 J µl−1 O −12 (caloric content per µl O2; Eckert 1988); c 3 = 0.45 mg J−1 (mg A. aurita medusa dry weight per J; Schneider 1988).
The total dry weight of planula larvae per female (DW L ; mg) was calculated by the equation:
where N L (ind.) is the number of larvae per female medusa and BM L (µg C ind.−1) the biomass per planula larva.
The standardized daily release of planula larvae (ρ Ls , mg female−1 day−1) in Kertinge Nor was calculated from the slope a (day−1) of linear regressions for measured daily release rates (ρ L , ind. female−1 day−1) versus larval numbers N L (ind. female−1; Fig. 4) by using the equation
The daily respiratory loss in medusa dry weight (DW R , mg day−1) as a function of individual medusa dry weight (DW M , mg ind.−1) was expressed by the equation (Frandsen and Riisgård 1997):
Statistical analyses
Statistical analyses were performed in R (R Core Team 2015) version 3.1.3 using generalized linear models (GLMs) in the stats package or generalized linear mixed-effect models (GLMMs) in R package lme4 (Bates et al. 2013). Seasonal differences in individual medusa size as a function of population density were analyzed as repeated measures over time by means of a GLM with gamma error structure. GLMs with similar error structure were applied to assess inter-annual differences in the cumulative dry weight of the jellyfish population, the number of planula larvae per female medusa relative to umbrella diameter and sampling location in Kertinge Nor and Great Belt, and the change in umbrella diameters of fed or starved medusae over time. A generalized linear mixed-effect model (GLMM) was parameterized with Poisson error structure to test for significant differences in the number of oocytes between fed and starving medusae, taking into account food treatment as fixed effect and time and replicate number as random effects.
Results
Jellyfish in Kertinge Nor in 2013 and 2014
Initially high population densities of A. aurita as observed in Kertinge Nor during spring (Fig. 2a) decreased gradually throughout the years 2013 and 2014 and approached zero during the winter months (Fig. 2a, inset). As expressed by similar cumulative population dry weights during 2013 and 2014 (Fig. 2b; GLM, t = 1.43, P = 0.165), seasonal changes in individual size of medusae (Fig. 3a) were significantly related to changes in population density (GLM, t = 31.9, P < 2 × 10−16). Additional details are given in the Electronic Supplementary Material, showing an inverse relationship between umbrella diameters and jellyfish density in Kertinge Nor during 2013 and 2014 (Online Resource 3). High jellyfish population densities in Kertinge Nor resulted in a theoretical half-life of copepods as short as 0.5–0.6 day during June 2013 and July 2014, indicating a pronounced predatory impact of A. aurita from spring to late autumn during 2013 and 2014 (Online Resource 1). Instantaneous weight-specific growth rates in Kertinge Nor (Online Resource 1) demonstrate a growth phase from early spring to summer and a degrowth phase from late summer to the end of the year. As indicated by exponential regressions for 2013 (t = 4.14, P = 0.006) and 2014 (t = 7.86, P = 5 × 10−5) in Fig. 3b, initial growth rates of medusae reached 2.7 and 3.0 % day−1, respectively, whereas degrowth was observed from September to December, shown by individual growth rates of −0.5 % day−1 (t = −4.13, P = 0.004) and −1.4 % day−1 (t = −6.20, P = 0.002) in 2013 and 2014.
Aurelia aurita. Ephyrae and medusae collected in Kertinge Nor (locations 1–3; Fig. 1) during 2013 and 2014. a Mean (±SD) umbrella diameter, b individual dry weight, c dry weight of planula larvae per female medusa, and d percentage of total medusa body dry weight made up by planula larvae. Data from Online Resource 1
The first planula larvae were seen on female medusae with an umbrella diameter of about 30 mm in early July in both 2013 and 2014 (Fig. 3c), about two months before the onset of degrowth (Fig. 3b). From autumn in both years, the number of planula larvae per female (Fig. 3c) and their percentage of total medusa body mass (Fig. 3d) decreased to become negligible in December. Figure 4 shows the release rates of planula larvae as a function of the total number of larvae per female medusa in Kertinge Nor during 2013 and 2014. The slopes of linear regressions for 2013 (t = 3.87, P = 0.002) and 2014 (t = 9.58, P = 2 × 10−7) indicate production rates of 0.109 and 0.065 day−1, respectively. A mean larva production of about [(0.109 + 0.065)/2 =] 0.087 day−1 has further been used for calculating the standardized daily release of planula larvae during the degrowth periods in 2013 and 2014 (ρ Ls ; Online Resource 4).
The actual and estimated change in body dry weight of medusae during degrowth periods in 2013 and 2014 is shown in Fig. 5. The reductions in weight loss due to food uptake (ΔDW P ; Online Resource 4) are indicated by the difference between ΔDW M and ΔDW R + ΔDW L (i.e., left and right columns in Fig. 5). It is seen that this difference decreased during the last five time intervals in 2014, indicating a gradual reduction in the amount of ingested food (due to increasing starvation during late autumn). In the same time period, weight losses due to the release of planula larvae were gradually reduced to become absent in the last time interval (November 22 to December 06, 2014). From this it is concluded that degrowth is mainly caused by starvation, whereas weight losses due to the production and release of planula larvae play a secondary role.
Aurelia aurita. Actual (left columns) and estimated (right columns) change in body dry weight of medusae in Kertinge Nor during time intervals in the degrowth periods in 2013 and 2014. Shown are actual measured changes in medusa dry weight (∆DW M = DW M(t+Δt) − DW M(t)) and estimated dry weight losses due to respiration (∆DW R = DW R × ∆t) or release of planula larvae (∆DW L = ρ Ls × ∆t) during each time interval. Data from Fig. 4 and Online Resource 4
Jellyfish in Great Belt in 2014
Aurelia aurita collected in Great Belt during 2014 reached maximum umbrella diameters of approximately 250 mm (Fig. 6a; Online Resource 2). The exponential regressions for the dry weight of medusae during the year (Fig. 6b) indicate an initial weight-specific growth rate of 7.5 % day−1 (t = 10.7, P = 4 × 10−4) and a much less defined degrowth rate of about −0.5 % day−1 (t = −0.78, P = 0.477). Planula larvae were first seen on medusae from Great Belt in early July, whereas their number decreased to become zero by early October 2014 (Fig. 6c). During the reproductive season, produced planula larvae made up 9.7–33.0 % of the total medusa dry weight (Fig. 6d; Online Resource 2). Medusae from Great Belt produced significantly more offspring compared to small medusae from Kertinge Nor (GLM, t = −8.10, P = 4 × 10−9), and the number of planula larvae increased with increasing medusa size (GLM, t = −3.35, P = 0.001), revealing an exponential relationship that indicates 2.9 % increase in reproductive output per mm increase in umbrella diameter (Fig. 7).
Aurelia aurita. Medusae collected in Great Belt (location 6; Fig. 1) during 2014. a Mean (±SD) umbrella diameter, b individual dry weight, c dry weight of planula larvae per female medusa, d percentage of total medusa body dry weight made up by planula larvae. Data from Online Resource 2
Growth and fecundity of Aurelia aurita medusae
Figure 8a, b shows umbrella diameters and estimated individual dry weights of A. aurita medusae when kept under different food conditions in the laboratory. In both experimental series, umbrella diameters increased significantly over time in fed medusae (GLM, t = 48.4, P < 2 × 10−16), while they decreased in starved medusae (GLM, t = 42.4, P < 2 × 10−16; Fig. 8a). Exponential regression lines in Fig. 8b indicate initial weight-specific growth rates of 5.9 % day−1 (t = 30.0, P < 2 × 10−16) and 3.6 % day−1 (t = 14.9, P = 1 × 10−11) for fed medusae and degrowth rates of −5.4 % day−1 (t = −35.8, P < 2 × 10−16) and −5.3 % day−1 (t = −29.9, P < 2 × 10−16) for starved medusae from Series #1 and #2, respectively. A mean initial number of 817 ± 483 oocytes per female medusa was counted at the start of Series #2. In fed medusae, this number had increased to 1056 ± 534 after 30 days, while it decreased significantly to 8 ± 20 oocytes per female in starved medusae (Fig. 8c; GLMM, z = 111.8, P < 2 × 10−16). In both Series #1 and #2, all individuals survived the experimental period of 30 days. After further 115 days, all medusae had died in the starvation treatment.
Discussion
Seasonal growth patterns of Aurelia aurita medusae
The actual measured degrowth of A. aurita in Kertinge Nor compared to estimated respiratory losses in body mass suggests insufficient compensation of weight losses by food intake with respect to the energetic demands of medusae during autumn 2013 and 2014. As demonstrated in 1991, 1992 (Olesen et al. 1994), 1995 (Nielsen et al. 1997), and 2009 (Riisgård et al. 2010), when the dense jellyfish population in Kertinge Nor caused strong decreases in zooplankton biomass in summer and autumn, short theoretical copepod half-lives of 0.5–13 days suggest predatory control of the zooplankton community (cf., e.g., Hansson et al. 2005; Møller and Riisgård 2007b; Riisgård et al. 2012) from April to November 2013 and 2014. Despite the lack of zooplankton data in the present study, similar seasonal dynamics as observed in previous years suggest food limitation due to the high estimated predation impact of A. aurita (cf. Riisgård et al. 2010). After the release of ephyrae during early spring, Kertinge Nor was characterized by extremely high population densities of small A. aurita medusae, as has been shown by Olesen et al. (1994), who observed maximum umbrella diameters of 55 and 40 mm in 1991 and 1992, before degrowth in September and July, respectively, followed declines in zooplankton biomass.
As shown for Kertinge Nor, food scarcity could be the main driver of seasonal degrowth in A. aurita medusae in other temperate regions (Möller 1980; Lucas 1996; Ishii and Båmstedt 1998). Compared to the dense population of small medusae in Kertinge Nor, A. aurita collected in Great Belt during 2014 showed approximately fivefold maximum umbrella diameters, reflecting higher growth potential due to their generally low abundance (≤4 ind. m−3), as typical for larger A. aurita medusae in Kiel Bight (Möller 1980; Schneider 1989), where growth rates of up to 20 % day−1 in medusa diameter [corresponding to approximately 16.2 % day−1 in dry weight; cf. Eq. (5)] have been observed (Möller 1980). The theoretical half-lives of copepods in Great Belt were ≥21 days throughout the year 2014, demonstrating a much less pronounced predatory impact than exerted by the food-limited medusae in Kertinge Nor despite similar body mass losses (degrowth) in late summer. Seasonality in growth and degrowth patterns has also been documented in a population of abundant, small A. aurita medusae in Horsea Lake, UK (Lucas 1996). From corresponding data on umbrella diameters and jellyfish population density (Lucas 1996, Fig. 4a, b therein), the theoretical copepod half-life in Horsea Lake may be estimated to 3–11 days in the period April to November 1996 using present Eqs. (1–3), suggesting food limitation during the winter months.
Population density-driven food availability
Observations support a suggested population density-dependent mechanism controlling adult medusa size with respect to available food resources (Schneider and Behrends 1994; Lucas 2001; Riisgård et al. 2010). Previous data collected in Kertinge Nor over the last 24 years suggest unchanged seasonal patterns in the abundance and individual size of the local jellyfish population (Riisgård et al. 2010, Fig. 7 therein). This was also supported by near constant yearly cumulative population dry weights observed during 2013 and 2014, reflecting that smaller umbrella diameters coincide with higher population densities and vice versa. In contrast to the less abundant, larger medusae in Great Belt, which may compose less discrete populations considering advection from both the Baltic Sea and the North Sea (Barz et al. 2006), the annual A. aurita population in Kertinge Nor may be described as stable and homogenous, with medusae being most likely restocked by local polyps each spring (Olesen et al. 1994).
The distribution of medusae in Kertinge Nor has been shown to be influenced by density-driven currents created by salinity changes in the adjacent Great Belt (Nielsen et al. 1997). The estimated residence time of water in Kerteminde Fjord/Kertinge Nor lays between 1 week and a few months (Riisgård et al. 2008), while there seems to be no significant seasonal variation of the amount of water exchange discharge (Jürgensen 1995). Present data on the jellyfish abundance in Kertinge Nor and Great Belt are based on near-surface sampling and hence neglect stratification due to water exchange between the highly saline North Sea and the brackish Baltic. While the shallow Kertinge Nor is subject to frequent vertical mixing by wind-, wave-, and current action (Riisgård 1998), the possibility of medusae over-wintering in deep water layers of Great Belt cannot be excluded (cf. Hernroth and Gröndahl 1985). Observed declines in the jellyfish population density in Kertinge Nor throughout the year and differences in the cumulative population dry weight of A. aurita suggest that a certain fraction of medusae may have gradually been washed out from the fjord system during 2013 and 2014. Stronger washout in 2014 and consequently decreased jellyfish density hence may have resulted in increased food supply for the remaining medusae, as reflected by larger maximum umbrella diameters than observed in 2013.
As mortality of A. aurita medusae has been suggested to be low before sexual maturation (Möller 1980), the population density-driven availability of food resources as a function of predominating water exchange processes in Kertinge Nor can be considered as major factor driving individual development of ephyrae after their release in early spring. The complete absence of medusae in Kertinge Nor and Great Belt during winter, however, suggests that increased mortality due to insufficient coverage of energetic demands during reproductive and degrowth periods contributes to seasonal declines in population density. Gradual declines in population density of A. aurita throughout the year have also been observed in the brackish Horsea Lake (Lucas 1996, Fig. 4a therein), where dilution of the jellyfish population due to washout is rather unlikely due to its semi-enclosed nature (Cathy H. Lucas, pers comm), thus suggesting mortality of medusae as important influencing variable.
Results however demonstrate that degrowth is a reversible process which is not inevitably accompanied by mortality, since medusae collected in the field during the degrowth period survived and regrew when sufficiently fed in the laboratory. Although cultivation of medusae in the laboratory involved restricted feeding due to small containers and regular disturbance, as reflected by comparatively low growth rates of 5.9 and 3.6 % day−1 in our well-fed individuals (cf. Frandsen and Riisgård 1997), medusae in both experimental series showed positive growth synchronously with continuous degrowth of their conspecifics in Kertinge Nor. Medusae kept under starvation in the laboratory revealed growth rates of −5.3 to −5.4 % day−1, in agreement with earlier observations by Hamner and Jenssen (1974, Fig. 3 therein) or Frandsen and Riisgård (1997, Table 2 therein). Compared to the present degrowth rates of −0.5 to −1.4 % day−1 observed in Kertinge Nor, our findings underline that degrowth of medusae in the cove was not caused by complete starvation, yet available food resources could not compensate for observed weight losses on the long term. Further, data of the present study emphasize that growth patterns of A. aurita are not constrained by the initial developmental stage (Hamner and Jenssen 1974), suggesting that degrowth and subsequent mortality of starving medusae are rather caused by food limitation than by energy allocation to sexual reproduction.
Energetic investment into sexual reproduction
Body mass losses due to the production of planula larvae, relative to overall body maintenance costs, were low in A. aurita medusae from Kertinge Nor, accounting for minor fractions observed during degrowth periods. An estimated percentage of 6–11 and 10–33 % medusa dry weight was made up by planula larvae in Kertinge Nor and Great Belt, respectively. Data of the present study further support a strong positive correlation between fecundity and body size (Schneider 1988; Lucas 1996), confirming that reduced amounts of energy are invested into sexual reproduction under food limitation, as has been shown by means of gonad indices (GI) in a study by Lucas and Lawes (1998, Fig. 9 therein). For a ‘small’ 40-mm bell-diameter medusa, the number of planula larvae can thus be estimated to 513 ind. female−1 using the exponential regression equation N L = 160.8 × e 0.029d (Fig. 7). This is in comparable order of magnitude with previous estimates of 184 ind. female−1 for small medusae from Horsea Lake, as can be derived from the linear regression function N L = 99.298 × WW − 80.090 (Lucas 1996; Fig. 8 therein) after calculating wet weight (WW, g) as a function of umbrella diameter (d, mm) using the equation WW = 0.0902 d 2.79 (Olesen et al. 1994; Fig. 2 therein). The number of planulae per ‘standard’ 150 mm medusa can comparatively be calculated using the exponential regression function from the present study, hence accounting for 1.3 × 104 ind. female−1.
Sexually mature medusae were first observed in Kertinge Nor during early summer, with oocyte development beginning in June and the smallest larvae-carrying female with an umbrella diameter of 27 mm being observed in July 2013. A. aurita medusae in Kertinge Nor revealed a relatively long reproductive period of 6 months from July to December in both 2013 and 2014, potentially compensating for reduced reproductive output due to their small body size, similarly as has been described for Horsea Lake (Lucas 2001, Fig. 2 therein). Larger medusae in Great Belt presumably started reproducing earlier than in Kertinge Nor in 2014, with an estimated reproductive period of 4 months from June to early October, coinciding with the seasonal occurrence of larvae-carrying A. aurita medusae observed in Gullmar Fjord (Gröndahl 1988a). The smallest larvae-carrying females caught in Kertinge Nor and Great Belt during the reproductive season had an umbrella diameter of 14 and 70 mm, respectively (Fig. 7, inset), both observed during degrowth phases in 2013. These observations are comparable to minimum umbrella diameters of 19–20 mm in small sexually mature medusae (d = 54 ± 20 mm) from Horsea Lake (Lucas 1996) and 64 mm in intermediate-sized medusae (d = 78–100 mm) from Southampton Water (Lucas and Williams 1994). All individuals reached sexual maturity shortly after or before the annual temperature maxima of 24.1 and 20.6 °C were measured in Kertinge Nor and Great Belt in July 2014. While the present study emphasizes control of medusa size and reproductive output by food availability, variable size at sexual maturity may be a consequence of additional synchronization of reproductive periods by environmental factors such as temperature, salinity, or photoperiod (Lucas and Lawes 1998).
The present study confirms previous observations by Ishii and Båmsted (1998), demonstrating that food scarcity not only reduces growth rates but also changes the energy allocation toward sexual reproduction. Lucas et al. (2012) pointed out the capability of A. aurita living in food-rich environments to produce large numbers of small planula larvae with low organic content (0.28 µg C), while medusae in food-limited environments can produce fewer numbers of large, carbon-rich (0.68 µg C) larvae. Planulae of A. aurita from Kertinge Nor and Great Belt were of generally low carbon content (0.2 µg C ind.−1) and similarly small size (Table 1), comparable to observations by Gröndahl (1988b) at the Swedish west coast. However, adjustment of the reproductive output as a function of medusa size was supported by significantly reduced numbers of oocytes in starving (degrowing) females, whereas fed medusae continuously produced oocytes in laboratory experiments. Results emphasize that reproductive and somatic tissues do not necessarily degenerate after spawning, as has also been demonstrated in controlled feeding experiments with male A. aurita (Hamner and Jenssen 1974). In contrast with previous assumptions by Marques et al. (2015), observations of the present study indicate that sexual reproduction is not essentially accompanied by increased mortality.
Life history of Aurelia aurita in temperate regions
The present study emphasizes the importance of seasonal dynamics in food availability for the population density and individual growth of A. aurita medusae in Danish coastal waters and suggests a weak trade-off between survival and sexual reproduction. Degrowth and eventual death of adults have been observed to follow the release of planula larvae (Hamner and Jenssen 1974; van der Veer and Oorthuysen 1985; Lucas 1996), which implies increased mortality of medusae due to energy allocation from survival to reproduction, i.e., senescence. The present study emphasizes that body mass losses expressed by degrowth of A. aurita as observed in Kertinge Nor and Great Belt, are mainly caused by food limitation during autumn which is likely accompanied by increased mortality. Likewise, the amount of energy invested into sexual reproduction seems to strictly depend on food availability, hence following medusa size instead of controlling growth rates. Their capability of degrowth and regrowth therefore allows medusae to survive considerable periods of starvation (Hamner and Jenssen 1974; Lucas 2001).
The production of gametes, however, may involve enhanced parasitic invasion, higher susceptibility to morphological degradation (Möller 1980; Lucas 2001), and weakened regrowth potentials of medusae (as indicated in Fig. 8b). The parasitic amphipod Hyperia galba was frequently observed in sexually mature A. aurita medusae from Kertinge Nor during late autumn 2013 and 2014. Along with parasitic infection, degraded medusae from Great Belt (i.e., significantly reduced in size, often with several body parts damaged or missing) were frequently found stranded on the beaches and in Kerteminde harbor, and no more jellyfish were observed in Great Belt at the end of October 2014. As suggested for hydromedusae from the northeast Pacific (Mills 1993), starvation combined with an accumulation of damage due to parasitic grazing should hence be taken into consideration as primary cause of death in A. aurita medusae during autumn, potentially promoting age-related mortality on the long term.
Longevity and sexual maturation of medusae generally seem to be neither size nor age-dependent (Lucas et al. 2012), with estimated life spans of A. aurita ranging from 3 to 10 months in most temperate waters (reviewed by Lucas 2001). Jellyfish of constantly low size variability were present in Kertinge Nor for ~10 months (Fig. 2a, Online Resource 1) and in Great Belt for ~7 months (Online Resource 2), suggesting shortened life spans compared to year-round populations in Japanese waters (Yasuda 1971; Omori et al. 1995; Miyake et al. 1997; Makabe et al. 2012) or the tropical Jellyfish Lake (Hamner et al. 1982), where longevity of A. aurita medusae may exceed 1 year. Seasonal declines in jellyfish abundance as typically observed in temperate regions (Möller 1980; Gröndahl 1988a; Lucas 1996) may thus be primarily caused by a combination of environmental properties including population density-dependent food availability, whereas age-related mortality triggered by the release of gametes could play a decisive role in longer-lived medusae (Omori et al. 1995).
References
Bailey KM, Batty RS (1984) Laboratory study of predation by Aurelia aurita on larvae of cod, flounder, plaice and herring: development and vulnerability to capture. Mar Biol 83:287–291
Båmstedt U (1990) Trophodynamics of scyphomedusae Aurelia aurita. Predation rate in relation to abundance, size and type of prey organism. J Plankton Res 12:215–229
Båmstedt U, Lane J, Martinussen MB (1999) Bioenergetics of ephyra larvae of the scyphozoan jellyfish Aurelia aurita in relation to temperature and salinity. Mar Biol 135:89–98
Barz K, Hinrichsen HH, Hirche HJ (2006) Scyphozoa in the Bornholm Basin (central Baltic Sea)—the role of advection. J Mar Syst 60:167–176
Bates D, Maechler M, Bolker B (2013) lme4: linear mixed-effects models using S4 classes. R package version 0.999999-0. 2012. http://CRAN.R-project.org/package=lme4/
Behrends G, Schneider G (1995) Impact of Aurelia aurita medusae (Cnidaria, Schyphozoa) on the standing stock and community composition of mezozooplankton in the Kiel Bight (western Baltic Sea). Mar Ecol Prog Ser 127:39–45
Dawson MN, Martin LE (2001) Geographic variation and ecological adaptation in Aurelia (Scyphozoa, Semaeostomeae): some implications from molecular phylogenetics. Hydrobiologia 451:259–273
Eckert R (1988) Animal physiology, 3rd edn. WH Freeman & Company, New York
Frandsen K, Riisgård HU (1997) Size dependent respiration and growth of jellyfish (Aurelia aurita). Sarsia 82:307–312
Gröndahl F (1988a) A comparative ecological study on scyphozoans Aurelia aurita, Cyanea capillata and C. lamarckii in the Gullmar Fjord, western Sweden, 1982 to 1986. Mar Biol 97:541–550
Gröndahl F (1988b) Interactions between polyps of Aurelia aurita and planktonic larvae of scyphozoans: an experimental study. Mar Ecol Prog Ser 45:87–93
Hamner WM, Jenssen RM (1974) Growth, degrowth, and irreversible cell differentiation in Aurelia aurita. Am Zool 14:833–849
Hamner WM, Gilmer RW, Hamner PP (1982) The physical, chemical, and biological characteristics of a stratified, saline, sulfide lake in Palau. Limnol Oceanogr 27:896–909
Hansson LJ, Moeslund O, Kiørboe T, Riisgård HU (2005) Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark). Mar Ecol Prog Ser 304:117–131
Hernroth L, Gröndahl F (1985) On the biology of Aurelia aurita (L.): 2. Major factors regulating the occurrence of ephyrae and young medusae in the Gullmar Fjord, western Sweden. Bull Mar Sci 37:567–576
Hirst AG, Lucas CH (1998) Salinity influences body weight quantification in the scyphomedusa Aurelia aurita: important implications for body weight determination in gelatinous zooplankton. Mar Ecol Prog Ser 165:259–269
Ishii H, Båmstedt U (1998) Food regulation of growth and maturation in a natural population of Aurelia aurita (L.). J Plankton Res 20:805–816
Jürgensen C (1995) Modelling of nutrient release from sediment in a tidal inlet, Kertinge Nor, Funen, Denmark. Ophelia 42:163–178
Lucas CH (1996) Population dynamics of Aurelia aurita (Scyphozoa) from an isolated brackish lake, with particular reference to sexual reproduction. J Plankton Res 18:987–1007
Lucas CH (2001) Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 451:229–246
Lucas CH, Lawes S (1998) Sexual reproduction of the scyphomedusa Aurelia aurita in relation to temperature and variable food supply. Mar Biol 131:629–638
Lucas CH, Williams JA (1994) Population dynamics of the scyphomedusa Aurelia aurita in Southampton Water. J Plankton Res 16:879–895
Lucas CH, Graham WM, Widmer C (2012) Jellyfish life histories: role of polyps in forming and maintaining scyphomedusa populations. Adv Mar Biol 63:133–196
Makabe R, Kurihara T, Uye S-I (2012) Spatio-temporal distribution and seasonal population dynamics of the jellyfish Aurelia aurita sl. Studied with Dual-frequency IDentification SONar (DIDSON). J Plankton Res 34:936–950
Marques R, Albouy-Boyer S, Delpy F, Carré C, Le Floc’H É, Roques C, Molinero J-C, Bonnet D (2015) Pelagic population dynamics of Aurelia sp. in French Mediterranean lagoons. J Plankton Res 37:1019–1035
Mills CE (1993) Natural mortality in NE Pacific coastal hydromedusae: grazing predation, wound healing and senescence. Bull Mar Sci 53:194–203
Miyake H, Iwao K, Kakinuma Y (1997) Life history and environment of Aurelia aurita. S Pac Study 17:273–285
Möller H (1980) Population dynamics of Aurelia aurita medusae in Kiel Bight, Germany (FRG). Mar Biol 60:123–128
Møller JS (1996) Water masses, stratification and circulation. In: Jørgensen BB, Richardson K (eds) Eutrophication in a coastal marine ecosystem. American Geophysical Union, Coast Estuar Stud, vol 52, pp 51–66
Møller LF, Riisgård HU (2007a) Impact of jellyfish and mussels on algal blooms caused by seasonal oxygen depletion and nutrient release from the sediment in a Danish fjord. J Exp Mar Biol Ecol 351:92–105
Møller LF, Riisgård HU (2007b) Population dynamics, growth and predation impact of the common jellyfish Aurelia aurita and two hydromedusae, Sarsia tubulosa and Aequorea vitrina in Limfjorden (Denmark). Mar Ecol Prog Ser 346:153–165
Møller LF, Riisgård HU (2007c) Feeding, bioenergetics and growth in the common jellyfish Aurelia aurita and two hydromedusae, Sarsia tubulosa and Aequorea vitrina. Mar Ecol Prog Ser 346:167–177
Nielsen TG, Møhlenberg F (2004) NOVANA. Teknisk anvisning for marin overvågning. 2.7 Mesozooplankton. Miljøministeriet. Danmarks Miljøundersøgelser (report in Danish). http://www2.dmu.dk/1_Om_DMU/2_tvaerfunk/3_fdc_mar/programgrundlag/TekAnv2004_2009/Del2/TA04_2_7_Mesozooplankton.pdf
Nielsen AS, Pedersen AW, Riisgård HU (1997) Implications of density driven currents for interaction between jellyfish (Aurelia aurita) and zooplankton in a Danish fjord. Sarsia 82:297–305
Olesen NJ (1995) Clearance potential of jellyfish Aurelia aurita and predation impact on zooplankton in a shallow cove. Mar Ecol Prog Ser 124:63–72
Olesen NJ, Frandsen K, Riisgård HU (1994) Population dynamics, growth and energetics of jellyfish Aurelia aurita in a shallow fjord. Mar Ecol Prog Ser 105:9–18
Omori M, Ishii H, Fujinaga A (1995) Life history strategy of Aurelia aurita (Cnidaria, Scyphomedusae) and its impact on the zooplankton community of Tokyo Bay. ICES J Mar Sci J du Conseil 52:597–603
Purcell JE (1997) Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates, and effects on prey populations. Ann Inst Oceanogr 73:125–137
Purcell JE (2009) Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia 616:23–50
Riisgård HU (1998) Filter feeding and plankton dynamics in a Danish fjord: a review of the importance of flow, mixing and density-driven circulation. J Environ Manag 53:195–207
Riisgård HU, Christensen PB, Olesen NJ, Petersen JK, Møller MM, Andersen P (1995) Biological structure in a shallow cove (Kertinge Nor, Denmark): control by benthic nutrient fluxes and suspension-feeding ascidians and jellyfish. Ophelia 41:329–344
Riisgård HU, Jürgensen C, Andersen F (1996). Case study: Kertinge Nor. In: Barker Jørgensen B, Richardson K (eds) Eutrophication in coastal marine ecosystems. Coast Estuar Stud, vol 52, pp 205–221
Riisgård HU, Jensen MH, Rask N (2008) Odense Fjord and Kerteminde Fjord/Kertinge Nor. In: Schiewer U (ed) Ecology of Baltic coastal waters, ecological studies, vol 197. Springer, Berlin, pp 361–394
Riisgård HU, Barth-Jensen C, Madsen CV (2010) High abundance of the jellyfish Aurelia aurita excludes the invasive ctenophore Mnemiopsis leidyi to establish in a shallow cove (Kertinge Nor, Denmark). Aquat Invasions 5:347–356
Riisgård HU, Madsen CV, Barth-Jensen C, Purcell JE (2012) Population dynamics and zooplankton-predation impact of the indigenous scyphozoan Aurelia aurita and the invasive ctenophore Mnemiopsis leidyi in Limfjorden (Denmark). Aquat Invasions 7:147–162
Schiariti A, Christiansen E, Morandini AC, da Silveira FL, Giberto DA, Mianzan HW (2012) Reproductive biology of Lychnorhiza lucerna (Cnidaria: Scyphozoa: Rhizostomeae): individual traits related to sexual reproduction. Mar Biol Res 8:255–264
Schneider G (1988) Larvae production of the common jellyfish Aurelia aurita in the Western Baltic 1982–1984. Kiel Meeresforsch 6:295–300
Schneider G (1989) The common jellyfish Aurelia aurita: standing stock, excretion and nutrient regeneration in the Kiel Bight, Western Baltic. Mar Biol 100:507–514
Schneider G, Behrends G (1994) Population dynamics and the trophic role of Aurelia aurita medusae in the Kiel Bight and western Baltic. ICES J Mar Sci 51:359–367
Schneider G, Behrends G (1998) Top-down control in a neritic plankton system by Aurelia aurita medusae - a summary. Ophelia 48:71–82
Stearns SC (1976) Life-history tactics: a review of the ideas. Q Rev Biol 51:3–47
Sun J, Liu D (2003) Geometric models for calculating cell biovolume and surface area for phytoplankton. J Plankton Res 25:1331–1346
R Core Team (2015) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Uye S-I, Shimauchi H (2005) Population biomass, feeding, respiration and growth rates, and carbon budget of the scyphomedusa Aurelia aurita in the Inland Sea of Japan. J Plankton Res 27:237–248
Van Der Veer HW, Oorthuysen W (1985) Abundance, growth and food demand of the scyphomedusa Aurelia aurita in the western Wadden Sea. Neth J Sea Res 19:38–44
Yasuda T (1971) Ecological studies on the jellyfish Aurelia aurita in Urazoko Bay, Fukui Prefecture IV Monthly change in bell-length composition and breeding season. Bull Jpn Soc Sci Fish 37:364–370 (in Japanese; English abstract)
Acknowledgments
We thank Florian Lüskow, Jan Brandt Wiersma, Mie Thorborg Pedersen, David Zalacáin Domench, Ruben van Beek, and Ida Krogsgaard Svendsen who took part in the boat trips and assisted in counting and measurement of collected jellyfish. We are grateful to Kim Lundgreen for technical assistance and to Mikkel Larris for help with the map. Further, we would like to thank Cathy H. Lucas, Ulrich Steiner, Jennifer E. Purcell, and two reviewers for providing constructive feedback that improved the manuscript. This study was financially supported by the Max-Planck Society (Max-Planck Odense Center on the Biodemography of Aging, Denmark) and by a grant (HUR) from Det Frie Forskningsråd (DFF-4002-00494).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. Purcell.
Reviewed by A. Schiariti, S.-I. Uye.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goldstein, J., Riisgård, H.U. Population dynamics and factors controlling somatic degrowth of the common jellyfish, Aurelia aurita, in a temperate semi-enclosed cove (Kertinge Nor, Denmark). Mar Biol 163, 33 (2016). https://doi.org/10.1007/s00227-015-2802-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-015-2802-x