Abstract
The survival and reproduction of individual or small groups of modules affords colonial organisms a great regenerative capacity. Consequently, modular loss due to fragmentation or senescence may not necessarily lead to colony mortality. This study (1) examines in situ partial mortality for colonies of the invasive bryozoan Membranipora membranacea in Nova Scotia by quantifying the location, magnitude, and timing of partial mortality for colonies growing on kelp (Saccharina latissima) in the field, and (2) estimates the effects of temperature (5–20 °C), and location and magnitude of modular loss on the recovery capacity of experimentally damaged colonies in the laboratory. In situ zooid mortality was substantial, with 50–100 % of colonies experiencing some level of partial mortality by the end of the growing season. Colonies with damage to older centrally located zooids maintained their capacity for growth and recovery, while colonies where younger peripheral zooids were removed showed no sign of recovery, and often experienced further loss of zooids. The effect of temperature depended on the location of colony damage, with increasing temperature resulting in increased loss of zooids for peripherally damaged colonies, but having no effect on the recovery of colonies with damage to central zooids. Variation in colony recovery may be related to the age distribution and reproductive maturity of zooids within a colony. Alteration of resource allocation between sexual and asexual reproduction may be adaptive in that it maximizes lifetime fitness in response to localized partial mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of colonial organisms to regenerate following damage as a result of injury or prolonged exposure to unfavorable conditions is arguably one of the primary advantages of their modular construction. Regeneration of colonial organisms relies on asexual, vegetative production of new modular units (Henry and Hart 2005), the ability of modular units to survive and reproduce individually or in small groups (Highsmith 1982; Hughes and Jackson 1985), and the sharing and/or reallocation of resources among modules in response to localized demand within a colony (Palumbi and Jackson 1983; Harvell and Helling 1993; Oren et al. 2001). The propensity of colonial organisms to persist and recover following dramatic reductions in colony size makes the accurate measurement of their demographic properties extremely complicated. This is particularly the case for mortality estimates, because modular losses due to fragmentation or senescence may not necessarily lead to colony-wide mortality.
Membranipora membranacea is a colonial cheilostome bryozoan that is native to the Pacific coast of North America and the Atlantic coast of Europe, and was introduced to Nova Scotia in the northwest Atlantic in the early 1990s (Scheibling et al. 1999). It is epiphytic, primarily encrusting laminarian kelps (Yorke and Metaxas 2012), and its colonies grow through the addition of new modules (zooids) to the colony edge. As a result, the age distribution of zooids within a colony is such that the oldest zooids are located at the center of the colony, with peripheral zooids being progressively younger. The age gradient within colonies is reflected in the timing of the onset of reproduction in individual zooids, which varies spatially within a colony. Typically, older more centrally located zooids begin to reproduce before younger more peripheral zooids; however, crowding by conspecifics and simulated predation damage have been shown to influence the timing and pattern of reproduction within experimentally manipulated M. membranacea colonies (Harvell and Helling 1993). Throughout this manuscript, we will refer to interior regions of M. membranacea colonies consisting of older zooids as central and the outer regions consisting of younger zooids as peripheral.
In Nova Scotia, colonies of M. membranacea experience modular loss in one of three ways: (1) degeneration of older central zooids during seasonal colony senescence; (2) removal of younger peripheral zooids due to physical abrasion, as well as erosion and breakage of kelp blades; and (3) incidental rasping of zooids from central and/or peripheral regions of colonies during grazing of kelp by the herbivorous gastropod Lacuna vincta. Although attempts have been made to estimate the mortality of M. membranacea in Nova Scotia (e.g., Saunders et al. 2010), these estimates are based solely on declining numbers of whole colonies; the frequency of occurrence of modular loss and the extent to which colonies are able to recover from this loss remains unknown.
The regenerative capacity of colonial marine invertebrates has been linked to colony size (Oren et al. 2001), the number (Oren et al. 2001), and location (Wahle 1983; Harvell 1984; Bone and Keough 2005) of damaged modules; the age distribution of modules throughout a colony (Palumbi and Jackson 1983; Meesters and Bak 1995); and the connectivity of individual modules within a colony (Harvell and Helling 1993; Bone and Keough 2005). In addition, temperature has been shown to affect colony growth rate and consequently colony recovery, for several cheilostome bryozoans (Menon 1972; O’Dea and Okamura 1999; Amui-Vedel et al. 2007; Yorke and Metaxas 2011). For M. membranacea specifically, temperature is known to affect the timing and extent of population outbreaks (Saunders and Metaxas 2007, 2008; Scheibling and Gagnon 2009; Saunders et al. 2010) and has been shown to be positively related to colony growth rates under both field and laboratory conditions (Saunders and Metaxas 2009a). In this study, we (1) quantified the location, magnitude, and timing of zooid mortality for M. membranacea colonies on kelp in the field and (2) estimated the effects of temperature, and location and magnitude of modular loss on the recovery capacity (rate and extent) of damaged M. membranacea colonies in controlled experiments in the laboratory.
Estimates of modular loss and recovery are critical for understanding the population dynamics of colonial organisms such as M. membranacea, for which important demographic characteristics, such as fecundity, are related to the survival and reproduction of individual modules as opposed to colonies as a whole (Tuomi and Vuorisalo 1989).
Materials and methods
Zooid mortality in situ
To measure the location and magnitude of in situ zooid mortality, colonies of Membranipora membranacea growing on Saccharina latissima were collected from 4 to 8 m at two sites on the southwestern shore of Nova Scotia: The Lodge (44°33′3″N, 64°01′9″W) on the western shore of St. Margarets Bay, and Sandy Cove (44°27′6″N, 63°42′4″W) in Terence Bay, 20 km northeast of St. Margarets Bay. These sites were chosen based on previous studies showing consistently high abundance of Membranipora membranacea over multiple years (Saunders and Metaxas 2008, 2009b) and to account for variation in different bays. Blades of S. latissima (n = 15–30) with colonies of M. membranacea were collected haphazardly from each site approximately monthly from July 25 to November 29, 2014. Collected algae were immediately transported to the Aquatron facility at Dalhousie University in coolers without seawater, where they were maintained in aquaria with running ambient seawater until processing was completed, typically within 1–3 days. The location (central or peripheral) and magnitude (% of colony surface area) of zooid mortality was estimated visually for all colonies on the collected algae (n = 34–274). Mortality to central zooids was defined as the loss of colony surface area confined within the colony, leaving the entire circumference of the colony intact (Fig. 1a). Mortality that affected any section of the outer growing edge of the colony was considered to be peripheral (Fig. 1b). If zooid loss extended from the interior of the colony across the growing edge, mortality of both central and peripheral zooids was estimated for each region independently, with peripheral damage incorporating the loss of colony circumference only (Fig. 1c). We could not distinguish visually between living zooids and the remaining exoskeleton of degenerated zooids. However, degenerated zooids tend to be more abundant within colonies over winter and in early spring (D. Denley, unpub data) and typically slough off of the kelp substrate during periods of maximum colony settlement and growth from July to November. As a result, it is unlikely that incorporating degenerated zooids into our categorical estimates of percent damage would change the frequency distribution of colonies among damage categories.
Recovery capacity of Membranipora membranacea colonies in the laboratory
Colonies of M. membranacea on individual blades of Laminaria digitata were collected from 4 to 8 m at Sandy Cove in September 2013 and were transported to the Aquatron facility at Dalhousie University in plastic tubs without seawater. Because growth rate of M. membranacea colonies varies with initial colony size (Saunders and Metaxas 2009a), collected colonies were selected to represent a wide range of sizes (~1–30 cm in length) in order to incorporate any variation in the rate or extent of colony recovery that may result due to differences in colony size. Colonies were randomly assigned to flow-through seawater tables maintained at one of three temperature treatments (5, 12, and 20 °C) and allowed to acclimate for 1 week prior to experimental manipulation. Temperature treatments were chosen to represent seasonal variation in the region (Fig. 2), with 5 °C being typical of early winter (December–January) and early spring (April–May), 12 °C of summer to autumn (July–October), and 20 °C representing the maximum daily averaged temperature at 4 m, typically occurring in August (The Lodge: 20.92 °C, Sandy Cove: 19.64 °C). Following 1 week of acclimation to temperature, colonies were randomly assigned to one of five mortality treatments (n = 13–15 colonies per treatment): a control and four levels of damage that were inflicted by scraping zooids off of the kelp substrate using a scalpel. Damage treatments orthogonally combined two levels of damage location (central zooids removed, peripheral zooids removed) and two levels of percent damage (50 % of zooids removed, 75 % of zooids removed). For the central damage location, 50 or 75 % of zooids were removed from the interior of the colony, leaving a ring of younger peripheral zooids the total surface area of which was half or one-quarter the size of the original intact colony, respectively (Fig. 3a, c). Similarly, for the peripheral damage location, 50 or 75 % of zooids were removed from the entire colony perimeter, and the remaining colony was half or one-quarter the size of the original intact colony, respectively, and consisted only of older interior zooids (Fig. 3b, d). Damage percentages were based on pilot studies where recovery of colonies was observed after removal of 50 % of colony surface area. Control colonies were left intact for the duration of the experiment. All colonies were photographed prior to and immediately following experimentally inflicted damage, and initial and post-damage colony surface area was measured using ImageJ photo analysis. Colonies were subsequently photographed at 7 and 14 days after damage, and change in surface area was measured using ImageJ. To further account for any potential variation in growth rate among different sized colonies, change in surface area was standardized by dividing by initial colony size [change in colony surface area = (final surface area − initial surface area)/initial surface area, see “Data Analysis”]. Results of pilot studies confirmed that this method of standardization was appropriate for comparing growth and recovery of colonies within the size range examined. Overall, including both acclimation and experimental periods, colonies were maintained in the laboratory for 21 days. During this time, colonies were fed a combination of live microalgae three times a week at concentrations (~4.5 × 104 cells ml−1) known to be sufficient for unlimited colony growth under laboratory conditions (Saunders and Metaxas 2009a).
Monthly averaged temperature (mean + SD, n = 27–31) at three depths at The Lodge (TL: 4, 8, 12 m) and two depths at Sandy Cove (SC: 4, 8 m) from September 2012 to November 2013. Temperature treatments used in laboratory experiments (5, 12 °C) are indicated by horizontal dashed lines; the highest temperature treatment used in laboratory experiments (20 °C) exceeds the monthly averaged annual maximum temperature in the region
Data analysis
Zooid mortality in situ
To determine whether incidences of damage differed between central and peripheral zooids within a colony, the frequency of percent damage (categories: 0, <25, 25, 50, 75, >75 %) was compared between central and peripheral locations of damaged colonies collected from each site in November 2014, when levels of colony damage were greatest, using Chi-square tests of homogeneity. For colonies that experienced mortality of both central and peripheral zooids, the location of damage was randomly selected for the purpose of analysis to ensure independence. For example, if a colony exhibited 25 % mortality of centrally located zooids and <25 % mortality of peripherally located zooids, either central or peripheral mortality was considered for the analysis. This did not substantially affect the sample size or our results, as comparatively few colonies (n = 0–57) exhibited zooid mortality in both central and peripheral regions during each sampling period. In some instances, >1 colony was sampled per kelp blade leading to potential non-independence of mortality. To account for this, we conducted the analysis on 50 subsamples randomly drawn from the full dataset for each site. Each of the 50 subsamples randomly selected 40 % of the total number of colonies sampled in November 2014 from The Lodge (n total = 100, n subsample = 40) and 90 % of the total number of colonies from Sandy Cove (n total = 34, n subsample = 30). We then calculated a mean χ2 statistic and associated standard error (of the statistics yielded by the 50 random subsamples), which we present along with the associated range of p-values. The proportion of data included in each subsample was determined based on the maximum proportion of colonies that could have been sampled from the same kelp at each site (60 % at The Lodge, 10 % at Sandy Cove).
Growth of control colonies in the laboratory
Relative growth of control colonies was calculated as the percentage change in surface area over time relative to initial colony surface area on day 1 of the experiment (Harvell et al. 1990; Saunders and Metaxas 2009a; Bone and Keough 2010; Marzinelli et al. 2012). The effect of temperature (fixed factor, three levels: 5, 12, 20 °C) on the relative growth of control colonies after 7 and 14 days was examined using two-way ANOVA with repeated measures on day (RM ANOVA). Significant differences between means as detected by RM ANOVA were examined with Tukey’s HSD post hoc tests. For control colonies, measurements of relative growth exhibited heterogeneity of variance as detected by Cochran’s test that could not be alleviated by transformation of the data. To account for this, we adopted a more conservative α (αcritical = 0.01) for the RM ANOVA; however, an α-value of 0.05 was maintained for all post hoc tests, as Tukey’s HSD is already fairly conservative (Crawley 2007). According to the Shapiro–Wilk test (p < 0.05), relative growth of control colonies was not normally distributed, and normal distribution could not be attained through transformation; however, ANOVA is robust to deviations from normality (Zar 1999).
Relative recovery of damaged colonies in the laboratory
Relative recovery of damaged colonies through the budding of new zooids was calculated as the percentage change in surface area over time relative to the initial colony surface area following artificially inflicted damage [relative recovery (%) = {[(final surface area − initial surface area post-damage)/initial surface area post-damage] × 100}]. We examined the effects of temperature (fixed factor, three levels: 5, 12, 20 °C), damage percentage (fixed factor, two levels: 50 % of zooids removed, 75 % of zooids removed), and damage location (fixed factor, two levels: central zooids removed, peripheral zooids removed) on the relative recovery of damaged colonies after 7 and 14 days using four-way ANOVA with repeated measures on day (RM ANOVA). Based on the results of RM ANOVA (see “Results”), the effects of temperature, damage percentage, and damage location (all fixed effects) on the relative recovery of damaged colonies were also examined separately for days 7 and 14, using three-way ANOVA. Significant differences between means as detected by ANOVA were further examined using Tukey’s HSD tests. Measurements of relative recovery were arcsine-square root transformed to better approximate the normal distribution and to eliminate heterogeneity of variance as detected by Cochran’s test. As with the control colonies, relative recovery of damaged colonies was not normally distributed (Shapiro–Wilk test, 7 days: p = 0.02, 14 days: p = 0.01), even after transformation.
For damage treatments where the relative recovery of damaged colonies was similar to the relative growth of control colonies, differences in relative growth between control and damaged colonies were examined using one-tailed Student’s t tests (when variances were equal) and Welch’s t tests (for unequal variances).
Results
Zooid mortality in situ
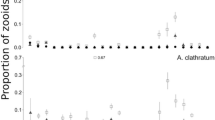
Damage of colonies on kelp increased during the growing season for both sites. In November 2014, when colony damage was greatest, there was no difference in the frequency of percent zooid mortality between central and peripheral locations of colonies (The Lodge: \( \bar{X}_{4}^{2} = 3.30 \pm 0.268, \) p = 0.084–0.967; Sandy Cove: \( \bar{X}_{4}^{2} = 4.70 \pm 0.192, \) p = 0.059–0.641, Figs. 4, 5). Analyses of randomly subsampled data yielded consistent results with those using the complete dataset (The Lodge: X 24 = 1.28, p = 0.865; Sandy Cove: X 24 = 5.70, p = 0.223). Most colonies experienced some level of damage in situ, with >50 % of colonies at The Lodge and >80 % of colonies and Sandy Cove experiencing >50 % mortality at either the central or peripheral location (Figs. 4, 5).
Frequency (%) of all Membranipora membranacea colonies collected on Saccharina latissima at The Lodge from July 2014 to November 2014 and showing different magnitudes of damage (0, <25, 25, 50, 75, >75 %) to central and peripheral zooids. A subsample (n = 40) was randomly drawn from this distribution in November 2014 for analysis (see “Methods”)
Frequency (%) of all Membranipora membranacea colonies collected on Saccharina latissima at Sandy Cove from July 2014 to November 2014 and showing different magnitudes of damage (0, <25, 25, 50, 75, >75 %) to central and peripheral zooids. A subsample (n = 30) was randomly drawn from this distribution in November 2014 for analysis (see “Methods”)
Recovery capacity of Membranipora membranacea colonies in the laboratory
Growth of control colonies
Growth of undamaged control colonies of M. membranacea occurred at 12 and 20 °C in the laboratory, and there was no significant difference in relative growth of colonies between these two temperature treatments after 7 or 14 days (Fig. 6; Table 1). At 5 °C, control colonies appear to decrease in size (Fig. 6); however, growth of these colonies did not differ significantly from zero after 7 or 14 days (Welch’s t test, 7 days: t 14 = −1.57, p = 0.14; 14 days: t 14 = −1.84, p = 0.09). Both the observed growth rates of control colonies and the effect of temperature on these rates are consistent with previous studies in Nova Scotia in both the field and the laboratory, where colony growth rates ranged from 0.01 to 12 mm day−1 (Saunders and Metaxas 2009a).
Relative growth (mean + SE, n = 13–15) of undamaged Membranipora membranacea colonies under three temperatures after 7 and 14 days in the laboratory. Relative growth was calculated as a percentage of the initial colony size [relative growth (%) = ((final surface area − initial surface area)/initial surface area) × 100]. Negative values indicate partial mortality. Letters above bars indicate homogeneous subsets among temperature treatments, identified using Tukey’s HSD test, α = 0.05
Relative recovery of damaged colonies
Relative recovery of damaged colonies differed between days, but only in magnitude and not in direction, and these differences varied with temperature (Fig. 7; results of RM ANOVA are presented in Online Resource 1). The effects of temperature, damage percentage, and damage location on the relative recovery of damaged colonies were then examined separately for days 7 and 14, using three-way ANOVA. Colonies showed greater recovery after 7 days and lower further loss of zooids after 14 days, when central zooids were removed than when peripheral zooids were removed for all temperature treatments (Fig. 7; Table 2). Recovery of colonies from which central zooids were removed occurred by the addition of new zooids via asexual budding around the intact colony periphery and not through the regeneration of damaged central zooids. Magnitude of damage (damage percentage) had no significant effect on the relative recovery of colonies after 7 and 14 days. Recovery of colonies did not vary among temperatures when central zooids were removed; however, when peripheral zooids were removed, further loss of zooids increased significantly with increasing temperature (Fig. 7; Table 2).
Relative recovery (mean + SE, n = 14) of Membranipora membranacea colonies after 7 and 14 days of four types of experimentally inflicted damage (50 % of central zooids removed, 50 % of peripheral zooids removed, 75 % of central zooids removed, 75 % of peripheral zooids removed) under each of three temperatures (5, 12, and 20 °C). Relative recovery was calculated as a percentage of the initial colony size following artificially inflicted damage [relative recovery (%) = ((final surface area − initial surface area post-damage)/initial surface area post-damage) × 100]. Negative values indicate negative growth or increased loss of zooids following damage. Bars with different letters are significantly different within each day at α = 0.05 (Tukey’s HSD test)
Relative growth of control colonies was equal to or exceeded relative recovery of damaged colonies after 7 and 14 days at 12 and 20 °C. However, this pattern was not consistent at 5 °C when 50 % of central zooids were removed after 7 and 14 days, and 75 % of central zooids were removed after 7 days (Figs. 6, 7; Table 3).
It should be noted that for colonies where central zooids were damaged, negligible relative growth after 14 days is the result of continued loss of central zooids and does not reflect lack of recovery at the growing edge. While the addition of new zooids through peripheral budding was observed for these colonies, simultaneous loss of central zooids resulted in net colony recovery that did not differ significantly from zero. No addition of new zooids was observed for colonies where peripheral zooids were removed.
Discussion
Levels of partial mortality for Membranipora membranacea colonies in the field did not differ between central and peripheral locations within colonies; however, in situ zooid mortality was substantial, with >50 % of colonies experiencing >30 % partial mortality by the end of the growing season. In Nova Scotia, seasonal senescence of M. membranacea typically begins in late summer to early autumn (D. Denley, unpubl data), a pattern consistent with the observed increase in partial mortality of centrally located zooids later in the season. Similarly, increased mortality of peripheral zooids in autumn may be related to seasonal increases in temperature and wave action that occur during this time (Fig. 2; D’Amours and Scheibling 2007). Erosion rates for the two most abundant kelp species in Nova Scotia, S. latissima and L. digitata, are positively related to water temperature and site exposure, respectively (Krumhansl and Scheibling 2011). Although levels of partial mortality did not differ between central and peripheral locations within M. membranacea colonies in the field, the location of modular loss significantly affected the recovery capacity of damaged M. membranacea colonies in the laboratory, irrespective of the level of damage inflicted. Lack of colony recovery following damage to peripheral zooids suggests that spatial and temporal variations in temperature and wave intensity, as they relate to increased breakage and erosion of kelp blades, will greatly influence recovery capacity and consequently partial mortality of M. membranacea colonies, both seasonally and interannually.
Growth of M. membranacea colonies through the budding of new zooids at the colony edge generates an age gradient within the colony, with central zooids being the oldest and successively younger zooids being found more distally toward the colony periphery. Thus, it is possible that the inability of colonies to regenerate following removal of the peripheral growing edge reflects intra-colonial differences in zooid function associated with zooid age. Similarly, only young zooids of M. membranacea were competent to produce defensive spines in response to exposure to a predatory nudibranch extract regardless of their location within the colony, which had been experimentally manipulated (Harvell 1991). Regenerative capacity was also greater in younger peripheral regions compared to older more central regions of the cheilostome bryozoan Dendrobeania lichenoides (Harvell 1984). Regeneration rate has been negatively correlated with both the number of brown bodies per zooid in the damaged region, a proxy for zooid age, and the distance of the damaged region from the colony edge in Steginoporella (Palumbi and Jackson 1983). In contrast, central fragments of the encrusting bryozoan Parasmittina delicatula were capable of rapid regrowth and exhibited high survivorship following experimentally inflicted damage in the field, suggesting this species may not be as susceptible to colony-wide mortality following partial zooid mortality (Bone and Keough 2010).
The difference in the relative regenerative capacity of central versus peripheral zooids among different species of bryozoans may be related to intracolonial transfer of resources among zooids. In cheilostome bryozoans, individual zooids are physiologically linked by perforated communication plates (Bobin 1977). The special cells in the pore plates are morphologically polar, orienting transport of lipids and other nutrients from central to peripheral zooids within the colony (Bobin 1977). In M. membranacea, metabolites and carbon are translocated in a distal direction only (Best and Thorpe 1985; Miles et al. 1995). In our study, the lack of recovery after 14 days when central zooids were damaged may be the result of the transfer of nutrients only from central to peripheral zooids. For these colonies, negligible relative growth does not reflect lack of recovery at the growing edge. Rather, the continued loss of central zooids coincided with the addition of new zooids through peripheral budding, resulting in net colony recovery that, however, did not differ significantly from zero.
Reallocation of resources in response to injury has been observed in M. membranacea (Harvell and Helling 1993). When growth of colonies was disrupted by removal of the growing edge from one half of the colony perimeter, the intact side of damaged colonies exhibited elevated rates of edge extension exceeding those of undamaged control colonies (Harvell and Helling 1993). This suggests colony-wide transfer of resources from damaged to undamaged regions of injured colonies. In our study, although the relative recovery of damaged colonies where central zooids had been removed exceeded growth of control colonies at 5 °C after 7 days, this pattern was not consistent after 14 days, and growth of control colonies often exceeded recovery of damaged colonies at higher temperature treatments. Further, the level of damage, or alternatively the proportion of zooids remaining following damage, did not significantly affect the recovery rate of colonies for either damage location. Similarly, colonies of the aborescent bryozoan Bugula neritina with an entire branch removed maintained an average growth rate similar to that of undamaged colonies and significantly greater than that of colonies from which half or all of the branching tips were removed (Bone and Keough 2005). In the encrusting Watersipora subtorquata, regeneration following experimental removal of zooids was directional, occurring only along the remaining colony margin, and growth rate depended exclusively on the length of the remaining growing edge and not on the size of the colony (Hart and Keough 2009).
In our study, when peripheral zooids were damaged, further loss of zooids increased with increasing temperature. This result suggests that the effect of temperature on recovery of damaged M. membranacea colonies may be more complex than the positive relationship observed between temperature and growth of control colonies. Similar decoupling of growth and regeneration in response to temperature was observed in the coral Montastrea annularis, for which daily influxes of colder deep water inhibited regeneration of tissue lesions but not the linear growth of colonies (Lester and Bak 1985). Increased loss of zooids in peripherally damaged colonies at 20 °C may have been a stress response resulting from prolonged exposure to a temperature that approximates the daily averaged annual maximum. An increase of 3 °C above ambient temperature significantly reduced the growth of the bryozoan Celleporaria nodulosa in the laboratory during summer when ambient temperatures were already at their seasonal maximum, but had no significant effect on growth in the winter when ambient temperature was lower (Durrant et al. 2013). However, thermal stress cannot explain the significant decrease in recovery after 14 days between 5 and 12 °C when 75 % of peripheral zooids were removed, since 12 °C represents an intermediate temperature in our region, to which M. membranacea is well adapted. More commonly, recovery of benthic marine invertebrates is associated with comparatively warmer temperatures (Menon 1972; Lester and Bak 1985; Kramarsky-Winter and Loya 2000), resulting in faster growth and regeneration of lost tissue through increased metabolic rate (e.g., O’Dea and Okamura 1999; Kramarsky-Winter and Loya 2000). Conversely, it has been suggested that increased metabolic rate, while enhancing growth rate, may also lead to higher energy demands required for maintenance (Denis et al. 2011).
For modular individuals, reproductive output is a function of the total number of modules and the average number of offspring produced per module (Tuomi and Vuorisalo 1989). This makes colony growth through the asexual budding of new modules an important component of lifetime fitness in colonial organisms. Since for M. membranacea, older centrally located zooids typically become reproductive before younger more peripheral zooids (Harvell and Helling 1993), investing in peripheral growth following damage to central zooids may reflect a shift in energy investment from sexual to asexual reproduction following the loss of reproductive zooids. Similarly, fitness consequences associated with lack of recovery following damage to younger peripheral zooids may be partially offset by increased reproductive output of the remaining older zooids. Although not explicitly examined here, previous studies involving M. membranacea have shown that damage to peripheral zooids can trigger reproduction in adjacent more centrally located zooids (Harvell and Grosberg 1988; Harvell and Helling 1993). This shift in allocation of resources between sexual and asexual reproduction may be adaptive in that it maximizes lifetime fitness in response to localized partial mortality.
References
Amui-Vedel A-M, Hayward PJ, Porter JS (2007) Zooid size and growth rate of the bryozoan Cryptosula pallasiana Moll in relation to temperature, in culture and in its natural environment. J Exp Mar Biol Ecol 353:1–12
Best MA, Thorpe JP (1985) Autoradiographic study of feeding and the colonial transport of metabolites in the marine bryozoan Membranipora membranacea. Mar Biol 84:295–300
Bobin G (1977) Interzooecial communication and the funicular system. In: Woollacott RM, Zimmer RL (eds) Biology of Bryozoans. Academic Press, New York, pp 307–333
Bone EK, Keough MJ (2005) Responses to damage in an arborescent bryozoan: effects of injury location. J Exp Mar Biol Ecol 324:127–140
Bone EK, Keough MJ (2010) Competition may mediate recovery from damage in an encrusting bryozoan. Mar Ecol 31:439–446
Crawley MJ (2007) The R Book. England, Chichester
D’Amours O, Scheibling RE (2007) Effect of wave exposure on morphology, attachment strength and survival of the invasive green alga Codium fragile ssp. tomentosoides. J Exp Mar Bio Ecol 351:129–142
Denis V, Debreuil J, De Palmas S, Richard J, Guillaume MMM, Bruggemann JH (2011) Lesion regeneration capacities in populations of the massive coral Porites lutea at Réunion Island: environmental correlates. Mar Ecol Prog Ser 428:105–117
Durrant HMS, Clark GF, Dworjanyn SA, Byrne M, Johnston EL (2013) Seasonal variation in the effects of ocean warming and acidification on a native bryozoan, Celleporaria nodulosa. Mar Biol 160:1903–1911
Hart SP, Keough MJ (2009) Does size predict demographic fate? Modular demography and constraints on growth determine response to decreases in size. Ecology 90:1670–1678
Harvell D (1984) Why nudibranchs are partial predators: intracolonial variation in bryozoan palatability. Ecology 65:716–724
Harvell DC (1991) Coloniality and inducible polymorphism. Am Nat 138:1–14
Harvell DC, Grosberg RK (1988) The timing of sexual maturity in clonal animals. Ecology 69:1855–1864
Harvell D, Helling R (1993) Experimental induction of localized reproduction in a marine bryozoan. Biol Bull 184:286–295
Harvell CD, Caswell H, Simpson P (1990) Density effects in a colonial monoculture: experimental studies with a marine bryozoan (Membranipora membranacea L.). Oecologia 82:227–237
Henry L-A, Hart M (2005) Regeneration from injury and resource allocation in sponges and corals: a review. Internat Rev Hydrobiol 90:125–158
Highsmith R (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226
Hughes TP, Jackson JBC (1985) Population dynamics and life histories of foliaceous corals. Ecol Monogr 55:141–166
Kramarsky-Winter E, Loya Y (2000) Tissue regeneration on the coral Fungia granulosa: the effect of extrinsic and intrinsic factors. Mar Biol 137:867–873
Krumhansl KA, Scheibling RE (2011) Detrital production in Nova Scotian kelp beds: patterns and processes. Mar Ecol Prog Ser 421:67–82
Lester RT, Bak RPM (1985) Effects of environment on regeneration rate of tissue lesions in the reef coral Montastrea annularis (Scleractinia). Mar Ecol Prog Ser 24:183–185
Marzinelli EM, Underwood AJ, Coleman RA (2012) Modified habitats change ecological processes affecting a non-indigenous epibiont. Mar Ecol Prog Ser 446:119–129
Meesters EH, Bak RPM (1995) Age-related deterioration of a physiological function in the branching coral Acropora palmata. Mar Ecol Prog Ser 121:203–209
Menon NR (1972) Heat tolerance, growth and regeneration in three North Sea bryozoans exposed to different constant temperatures. Mar Biol 15:1–11
Miles JS, Harvell CD, Griggs CM, Eisner S (1995) Resource translocation in a marine bryozoan: quantification and visualization of 14C and 35S. Mar Biol 122:439–445
O’Dea A, Okamura B (1999) Influence of seasonal variation in temperature, salinity and food availability on module size and colony growth of the estuarine bryozoan Conopeum seurati. Mar Biol 135:581–588
Oren U, Benayahu Y, Lubinevsky H, Loya Y (2001) Colony integration during regeneration in the stony coral Favia favus. Ecology 82:802–813
Palumbi SR, Jackson JB (1983) Aging in modular organisms: ecology of zooid senescence in Steginoporella sp. (bryozoan; cheilostomata). Biol Bull 164:267–278
Saunders M, Metaxas A (2007) Temperature explains settlement patterns of the introduced marine bryozoan Membranipora membranacea in Nova Scotia, Canada. Mar Ecol Prog Ser 344:95–106
Saunders M, Metaxas A (2008) High recruitment of the introduced bryozoan Membranipora membranacea is associated with kelp bed defoliation in Nova Scotia, Canada. Mar Ecol Prog Ser 369:139–151
Saunders M, Metaxas A (2009a) Effects of temperature, size, and food on the growth of Membranipora membranacea in laboratory and field studies. Mar Biol 156:2267–2276
Saunders M, Metaxas A (2009b) Population dynamics of a nonindigenous epiphytic bryozoan Membranipora membranacea in the western North Atlantic: effects of kelp substrate. Aquat Biol 8:83–94
Saunders M, Metaxas A, Filgueira R (2010) Implications of warming temperatures for population outbreaks of a nonindigenous species (Membranipora membranacea) in rocky subtidal ecosystems. Limnol Oceanogr 55:1627–1642
Scheibling RE, Gagnon P (2009) Temperature-mediated outbreak dynamic of the invasive bryozoan Membranipora membranacea in Nova Scotian kelp beds. Mar Ecol Prog Ser 390:1–13
Scheibling RE, Hennigar AW, Balch T (1999) Destructive grazing, epiphytism, and disease: the dynamics of sea urchin: kelp interactions in Nova Scotia. Can J Fish Aquat Sci 56:2300–2314
Tuomi J, Vuorisalo T (1989) What are the units of selection in modular organisms? Oikos 54:227–233
Wahle CM (1983) Regeneration of injuries among Jamaican gorgonians: the roles of colony physiology and environment. Biol Bull 165:778–790
Yorke AF, Metaxas A (2011) Interactions between an invasive and native bryozoan (Membranipora membranacea and Electra pilosa) species on kelp and Fucus substrates in Nova Scotia, Canada. Mar Biol 158:2299–2311
Yorke AF, Metaxas A (2012) Relative importance of kelps and fucoids as substrata of the invasive epiphytic bryozoan Membranipora membranacea in Nova Scotia, Canada. Aquat Biol 16:17–30
Zar JH (1999) Biostatistical analysis. Prentice-Hall, NJ
Acknowledgments
John Lindley, Robert Scheibling, Colette Feehan, Karen Filbee-Dexter, John O’Brien, Erika Simonson, and Kevin Sorochan provided field assistance. Andrea Moore, Olivia Pisano, and Delphine Durette-Morin assisted with laboratory experiments. Robert Scheibling and three anonymous reviewers provided comments on earlier versions of the manuscript. This research was supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery grant to AM and a Fellowship from the Dalhousie Faculty of Graduate Studies to DD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Denley, D., Metaxas, A. Recovery capacity of the invasive colonial bryozoan Membranipora membranacea from damage: effects of temperature, location, and magnitude of damage. Mar Biol 162, 1769–1778 (2015). https://doi.org/10.1007/s00227-015-2707-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2707-8