Abstract
Zooplankton often migrate vertically to deeper dark water during the day to avoid visual predators such as fish, a process which can strengthen benthic–pelagic coupling. In the Gulf of Finland, Baltic Sea, a pronounced hypoxic layer develops when there is an inflow of anoxic bottom water from the Central Baltic Sea, which could be a barrier for vertical migrants. Here, we report an acoustic study of the distributions of crustacean zooplankton (mysid shrimp and the copepod Limnocalanus macrurus), gelatinous zooplankton (Aurelia aurita) and fish. Zooplankton trawl nets were used to ground-truth acoustic data. Vertical profiles of oxygen concentration were taken, and the physiological impact of hypoxia on mysids was investigated using biochemical assays. We hypothesised that the vertical distribution of zooplankton and fish would be significantly affected by vertical heterogeneity of oxygen concentrations because anoxia and hypoxia are known to affect physiology and swimming behaviour. In addition, we hypothesised that mysids present in areas with hypoxia would exhibit a preparatory antioxidant response, protecting them from oxidative damage during migrations. The acoustic data showed peaks of crustacean zooplankton biomass in hypoxic (<2 mL L−1) and low oxygen (2–4 mL L−1) concentrations (depth >75 m), whereas fish shoals and A. aurita medusae were found in normoxic (5–6 mL L−1) upper water layers (<40 m), with individual fish in deeper water excepting that rule. Mysid shrimp from areas with hypoxia had significantly enhanced antioxidant potential compared with conspecifics from areas with no hypoxia and had no significant indications of oxidative damage. We conclude that mysids can protect themselves from oxidative damage, enabling them to inhabit hypoxic water. Our data suggest that hypoxic and low oxygen zones (up to 4 mL L−1) may provide some zooplankton species with a refuge from visual predators such as fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypoxia (<2 mL L−1) in coastal environments around the world is a persistent and growing problem (Vaquer-Sunyer and Duarte 2008). Hypoxia has created large areas of benthic ‘dead zones’ in the Baltic Sea (Modig and Ólafsson 1998; Conley et al. 2009), yet few data exist on the effect of hypoxia on the pelagic community there (cf. Zhang et al. 2009, 2014 for details of impacts in the Gulf of Mexico). Fish are perhaps the best studied pelagic group in terms of their response to and tolerance of hypoxic conditions. Research in the Baltic Sea on species such as cod, herring and sprat found that fish aggregated in normoxic surface waters (Neuenfeldt and Beyer 2003; Ekau et al. 2010). There are fewer data describing the effect of hypoxia on jellyfish in the Baltic Sea, but compared with both fish and crustacean zooplankton, scyphozoans such as Aurelia aurita are more tolerant of low oxygen concentrations (Purcell et al. 2001), and hypoxia can even be advantageous to A. aurita medusae because it enables them to capture larger numbers of larval fish in hypoxic environments where the swimming speed of larval fish is reduced (Shoji et al. 2005). In the Baltic Sea, A. aurita medusae form seasonal blooms in the uppermost water layers (<20 m) during the summer months (Barz and Hirche 2005).
Fish and other visual predators drive the diel vertical migration (DVM) of crustacean zooplankton (Hays 2003; Brierley 2014). Vertically migrating zooplankton can increase the strength of the biological carbon pump through active carbon flux, which is the downward transport of carbon by migrant zooplankton feeding in surface waters and defecating at depth (Angel and Pugh 2000; Hidaka et al. 2001; Burd et al. 2010; Hernandez-Leon et al. 2010). This process plays an important role in biogeochemical cycling, so understanding of DVM and its various controlling factors is needed (Brierley 2014). Crustacean zooplankton fall between fish and non-visual hunters such as the scyphozoans in terms of their tolerance to hypoxia; for example, copepods (Eurytemora affinis) tolerate oxygen concentrations as low as 1 mL L−1 in a North Sea estuary, but their abundance increased when oxygen concentrations increased to 6 mL L−1 (Appletans et al. 2003). In the Gulf of Finland, Limnocalanus macrurus is one of the dominant copepod species by biomass and is an important food source for commercially important fish (Viitasalo 1992; Rajasilta et al. 2014). It is a daily vertical migrant in May and June, ceasing its vertical migrations in August due to high temperatures in the upper water layers (Bityukov 1960). Aside from L. macrurus and A. aurita, mysid shrimp (Mysis spp.) are common macrozooplankton in the northern Baltic Proper (Rudstam and Hansson 1990). DVM by Baltic Sea mysids has been reported; however, it varies seasonally, decreasing during late summer (Rudstam and Hansson 1990). Recently, it has been speculated that worsening hypoxia may partially explain why Mysis mixta has decreased by up to 50 % since the 1980s in an area on the eastern coast of Sweden (Ogonowski et al. 2013). One consequence of hypoxic zones at depth could be a barrier to the DVM of Baltic Sea mysid shrimp, as it is for some migrants attempting to cross oxygen minimum zones in the Benguela Current (Ekau et al. 2010).
In estuarine populations exposed to rapid changes in oxygen conditions, temporary hypoxia and reoxygenation have been linked to many metabolic responses and physiological disorders caused by oxidative stress (Hermes-Lima and Zenteno-Savín 2002). Oxidative stress is manifest as oxyradical-induced damage to major biomolecules (lipids, proteins and DNA) when production of reactive oxygen species (ROS) surpasses background levels and exceeds the antioxidant buffering capacity of the organism. In most vertebrates, both oxygen deprivation and its reintroduction resulting in ROS overproduction cause metabolic injuries. Hypoxia/reoxygenation cycles occurring in nature (e.g. tides, inflows of saline waters replacing hypoxic near-bottom water, vertical migrations) resemble the ischaemic/reoxygenation processes described in vertebrates, including humans (Hermes-Lima et al. 2001). After a reduction in oxygen flow (ischaemia), the return to normoxia causes an increase in the production of ROS, leading to oxidation of cellular components. The electron carriers of the mitochondrial respiratory chain are reduced during ischaemia, whereas immediate reoxygenation of these carriers takes place after the reperfusion, leading to ROS overproduction (Kowaltowski and Vercesi 1999).
It is important for organisms that frequently experience oxygen deprivation to recover quickly and completely when oxygen becomes available. For example, an incident of hypoxia in the Bay of Somme, France, caused the cockle fishery to collapse in 1982, and by 1984, the benthos was dominated by the opportunistic polychaete Pygospio elegans. The cockle (Cerastoderma edule) populations did not recover until 1987, and the fishery did not open again until 1988 (Desprez et al. 1992). A wide variety of invertebrate species can endure extended periods of hypoxia or anoxia (hours to weeks), switch to normoxic conditions and recover from resulting damages (Hermes-Lima et al. 2001). To recover successfully, these animals must have effective mechanisms to minimise oxidative stress during the transition from hypoxic to normoxic conditions. Invertebrates that frequently experience predictable changes in oxygen, as during DVM, are capable of enhancing their antioxidant potential under hypoxia. This enhancement has been suggested to be a preparatory mechanism evolved to deal with a physiological oxidative stress that occurs rapidly during reoxygenation (Hermes-Lima and Zenteno-Savín 2002).
The Gulf of Finland has an average depth of 38 m and a strong riverine freshwater influence. Maximum depths (123 m) are found across its western side, where the Gulf of Finland meets the Baltic Proper (data from www.helcom.fi). Saline water from North Sea intrusions (Kattegat water) resides deep in the Baltic Proper, and tongues of this more saline water enter deeper areas of the Gulf of Finland on its western side. Anoxic and hypoxic deep water from the Baltic Proper enter the Gulf of Finland on its western side, often resulting in oxygen-deficient bottom waters. Our aim here was to investigate the effect of anoxia and hypoxia on the Gulf of Finland’s pelagic community, including fish, gelatinous zooplankton, mysids and copepods. By gaining an understanding of how the spatial distribution of various pelagic species responds to varying oxygen concentrations, we can begin to predict how hypoxia in the Baltic Sea may affect competition and predator–prey interactions. Our objectives were: (1) to investigate the vertical distribution of fish and macrozooplankton using acoustics with additional macrozooplankton sampling using trawl nets to ground-truth acoustic data and (2) to determine the physiological impacts of hypoxia on mysids using biochemical assays. We hypothesised that: (1) of the pelagic community, fish would be the least tolerant of hypoxia due to known physiological constraints and would be found in normoxic upper water layers, (2) A. aurita medusae would be found in the normoxic upper water layers in line with research from the Central Baltic Sea, which reports that 80 % of medusae are found in the upper 20 m (Barz and Hirche 2005), (3) for the most common crustacean macrozooplankton in the Gulf of Finland (L. macrurus and mysids), hypoxia would have a significant, negative effect on biomass, as detected by acoustic backscattering, (4) L. macrurus would have no significant DVM signal because they cease DVM in late summer (Bityukov 1960), whereas mysids would show a significant DVM signal, and (5) mysids undergoing DVM in hypoxic areas would have significantly higher antioxidant levels compared to those from normoxic areas; this would be considered a physiological adaptation as higher antioxidant capacity would reduce risk of oxidative damage (i.e. a preparatory response).
Materials and methods
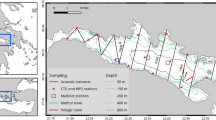
Sampling was conducted in the Gulf of Finland (Fig. 1) from the R/V Aranda. In August 2012, we examined the day- and night-time vertical distribution of macrozooplankton, and in August 2007, M. relicta and M. mixta were collected for analyses of physiological effects of hypoxia. Sampling took place in coastal areas in northern Finnish waters (>30 m) and west across the deeper opening of the Gulf (>80 m; Fig. 1). Physico-chemical data (salinity, temperature, depth, oxygen concentration) were collected using a SBE 911plus CTD system (12 deployments). Oxygen concentration, pH, chlorophyll and nutrient (nitrogen, phosphorus, silicate) concentrations were measured in the laboratory onboard using standard methods as set out in the HELCOM monitoring guidelines (HELCOM 1988; also available online at www.helcom.fi). Percentage of photosynthetically available radiation (%PAR) reaching each depth (every 0.5 m) was calculated from Secchi depth measured during the cruise (Tyler 1968).
Acoustics
Split beam 70 and 120 kHz transducers, and a single beam 200 kHz transducer driven by Simrad EK60 echosounders were installed onto a towed body. All three transducers were circular with 7° beam widths. Calibrations were performed with standard copper spheres, as recommended by the manufacturer. A ping rate of 2 s−1 and a pulse duration of 512 µs were used. Depth of towed body was kept constant (2 m), and depth was recorded using a Sensus Ultra (ReefNet Inc.) depth sensor. Ship speed was 3.5 knots during acoustic data collections. Post-processing of acoustic data was carried out using the software Echoview v4.9. The nearfield was calculated for 70 kHz as 4 m, 120 kHz as 3 m, 200 kHz as 5 m and was excluded for all frequencies from further analysis. To compensate for signal loss with beam spreading and absorption, time varied gain (TVG) was applied (function 20logR + 2αR), and amplified background noise from regions of the echogram that were essentially blank was removed (Watkins and Brierley 1996). Acoustic backscattering, expressed as mean volume backscattering strength S v (dB re 1 m−1), was calculated separately for each frequency over a grid of 60 pings (30 s) horizontally × 0.5 m vertically.
Echoes arising from crustacean macrozooplankton (L. macrurus, Mysis spp.), A. aurita and fish were discriminated and classified using the relative frequency response classification procedure (Korneliussen and Ona 2003). S v values from each frequency for each cell were used to produce frequency responses for each cell (De Robertis et al. 2010). Fish with swimbladders are expected to yield a stronger echo at 70 than at 120 or 200 kHz (Kloser et al. 2002; Knudsen 2009). After a threshold of −60 dB re 1 m−1, appropriate to Baltic fish surveys (Peltonen and Balk 2005), was applied to 70 kHz data, cells for which S v 70 > 120 > 200 kHz were classified as ‘fish’. Grid cells with this characteristic were excluded from analyses of macrozooplankton distributions. Organisms such as A. aurita medusae, mysids and copepods can be considered fluid-like scatterers (Simmonds and MacLennan 2005) because their bodies have a sound speed and density similar to sea water. Various empirical observations and models (Greenlaw 1979; Holliday and Pieper 1980; Stanton et al. 1993) have shown that zooplankton, such as mysids and large copepods, produce higher backscatter at 200 than 120 kHz (Stanton et al. 1994). Cells for which S v was 70 < 120 < 200 kHz were ascribed crustacean zooplankton. Scyphozoan medusae are also fluid-like zooplankton, and although there are fewer data on gelatinous plankton than crustaceans, it has been observed that ‘jellyfish’ have higher target strengths at lower frequencies than crustacean zooplankton of similar mass. (Mutlu 1996; Colombo et al. 2003; Warren and Smith 2007, Brierley et al. 2005). We classified cells for which S v was 70 < 120 > 200 kHz as large fluid-like zooplankton, using trawl data (see below) to confirm the presence of A. aurita. All frequency differences included a ±2 dB margin of error (Bertrand et al. 2010), after which a Boolean mask (true/false) was applied to each frequency, and the three scattering types were classified by colour for display as a synthesised echogram (Korneliussen and Ona 2003).
Trawl sampling
To sample macrozooplankton, and ground-truth acoustic data, a three-bag opening and closing Tucker trawl (500 µm mesh size, opening 0.25 m2) was deployed obliquely and towed at a speed of 1.5 knots (three deployments). The mode of operation for the net is that as the net descends, the first net is open. As the net one closes, the second net opens. As that closes, the third net opens and remains open until recovery. This means that both the first and third nets sample whilst descending and ascending as well as during the oblique tow at the target depth. The trawl was equipped with a Simrad PI32 net monitoring system with depth sensor which enabled real-time control of trawling depth. A recording Sensus Ultra depth sensor was attached to the upper bar of each of the three trawl bags. When a bag was closed, it caused an immediate 0.5 m depth increase, and this depth change was used to check that the bag opening/closing took place as intended. Any gelatinous zooplankton in the sample were immediately counted, measured and identified to the lowest possible taxonomic level on board. Remaining catch material was preserved in 70 % ethanol and counted, measured and identified later in the laboratory.
Biochemical assays
We used: (1) total oxygen radical absorbance capacity (ORAC; represents level of antioxidant defences) and (2) lipid peroxidation (TBARS; thiobarbituric acid reactive substances represent level of oxidative damage) as proxies for antioxidant capacity and oxidative damage, respectively. These biomarkers have been found to be informative for detecting and interpreting oxidative responses in amphipods exposed to a combination of hypoxia and contaminated sediments (Gorokhova et al. 2013). Both biomarkers were expressed here as measures normalised to protein content, which was measured in concert.
CTD data and specimens were obtained in August 2007 from the same sampling stations as for the acoustic studies described above. Mysid shrimp were identified to species level and length-measured (body length, BL, from the tip of the rostrum to the base of the telson), and individuals were snap-frozen at −80 °C. Only juveniles or females without embryos in brood pouches were used for the analyses, which were conducted within a week of collection. Two species that were present throughout the study area in both normoxic and hypoxic areas were analysed: M. mixta (91 individuals, BL range 10.2–21.2 mm) and M. relicta (28 individuals, BL range 4.6–18.9 mm). For extraction, whole bodies (i.e. a single individual per sample) were homogenised (20 % w/v) in a cold (4 °C) buffer (pH 7.2, containing 50 mmol L−1 TRIS, 0.15 mol L−1 NaCl, 1 mmol L−1 EDTA, 0.02 mol L−1 NaH2PO4, and 0.1 % Triton X−100) for 2 min using a FastPrep homogenizer with cooling function. The homogenate was centrifuged at 15,000×g for 15 min at 4 °C, and two 40 µL aliquots of the supernatant were taken, one for direct ORAC measurements and one for total protein content. The remaining supernatant was used for TBARS assays. All biochemical analyses were performed using microplate reader FLUOstar Optima (BMG Lab Technologies, Germany) with absorbance and fluorescence configurations, depending on a particular assay. All measurements were performed in duplicates.
Protein assay
Protein concentration (mg mL−1) was measured using the bicinchoninic acid assay (BCA, Pierce Ltd.) with bovine serum albumin (BSA) as the standard according to the manufacturer’s instructions. For each assay, 20 µL of the homogenate were used; the absorbance was measured at 540 nm, with integration time of 3 s; 10 measurements were made per well.
ORAC assay
We used the modified ORACFL method (Prior et al. 2003) with fluorescein (Fluka; 79.6 nM well−1) as a fluorescent probe, 2,29-azobis (2-amidinopropane) dihydrochloride (AAPH; Sigma-Aldrich; 23 mM well−1) as a peroxyl radical source and Trolox (Sigma-Aldrich; 21.7 mM well−1) as a standard. For each assay, 20 µL of the homogenate were used to measure ORAC during 120-min assay; the remaining fluorescence never exceeded 5 % of the initial value. The ORAC values were expressed in Trolox equivalents in mg protein−1.
TBARS assay
Quantification of a specific end product of the oxidative degradation process of lipids, malondialdehyde (MDA), was conducted according to Mihara and Uchiyama (1978). Briefly, lipid peroxidation was measured in 200 µL homogenate mixed 1:1 ice-cold trichloroacetic acid. The mixture was incubated on ice for 5 min and centrifuged at 10,000×g for 5 min. Reaction solution consisting of 200 µL of 83 mM thiobarbituric acid (TBA) mixed with 12.5 μL of sodium dodecyl sulphate (8.1 %) and glacial acetic acid (20 %, pH 3.5) was added to 200 µL of supernatant and incubated for 60 min at 100 °C. After cooling, 220 µL of 1-butanol/pyridin (15:1, v/v; Sigma-Aldrich, Germany) mixture was added to all samples and standards and mixed for 2 × 10 s. Concentrations were derived from a standard curve of 1,1,3,3-tetramethoxypropane measured as fluorescence in the organic phase at excitation/emission wavelengths of 540/590 nm, and 50 mM monobasic sodium phosphate buffer was used as a blank. The TBARS values were expressed in nmol MDA mg protein−1.
Statistical modelling
To investigate our null hypothesis that the distribution of crustacean zooplankton would not be significantly affected by hypoxia, we explored the distributions of zooplankton in depth/time using generalised additive models (GAMs; Hastie and Tibshirani 1990). GAMs are a regression method that fits smoothing functions between explanatory variables and the response variable and allow for nonlinear relationships (Hastie and Tibshirani 1990). In GAMs, cross-validated cubic spline smoothers (Hamming 1973) replace the least squares estimate of the multiple linear regression. Acoustic grid cell data (original resolutions) were further binned into 30 s × 0.5 m intervals before fitting GAMS to facilitate computational model fitting. GAMs were used because we did not expect a linear response between zooplankton biomass as detected by acoustic backscattering and explanatory variables. A gamma error distribution and a log-link function were found to be the most appropriate because they did not violate model assumptions (Wood 2006). To address any geographic non-independence of data, we incorporated latitude/longitude and depth smooths into the models. It was not possible with present computing constraints to incorporate a temporal autocorrelation structure into the models so we limited the degrees of freedom to six and used penalised splines to reduce the complexity of the smooths and avoid overfitting of the model (Zuur et al. 2009). To begin with, we had 16 environmental parameters that zooplankton biomass could be related to laboratory-derived variables (pH of sea water, concentration of chlorophyll, silicate, nitrogen and phosphorus), CTD-derived variables (depth, temperature, salinity, oxygen concentration), plus day/night, station number, average and maximum depth, and %PAR. Akaike information criteria (AIC) were used to assess the candidate GAMs (Akaike 1974). Statistical modelling and analyses were conducted within the R software environment v2.13.2 using lattice (Sarkar 2008), glme (Hastie and Tibshirani 1990) and mgcv packages (Wood 2006).
We used generalised linear models (GLMs) to investigate our final null hypothesis, which was the expectation of no preparatory antioxidant response in mysid shrimp, to evaluate whether bottom oxygenation status (oxygen status; normoxic vs. hypoxic) could explain the variation in ORAC and TBARS in two co-occurring Mysis species (M. mixta and M. relicta). Analyses were conducted in (GLZ module) in STATISTICA 8.0 (StatSoft 2001, Tulsa, USA) with normal error function and log-link function. As oxidative status biomarkers are known to vary ontogenetically in similar crustaceans such as the grass shrimp and the freshwater prawn (Dandapat et al. 2003; Hoguet and Key 2007), body length (BL; a proxy for age) was used in all GLMs as a covariate. Since BL range varied among the collections and species, data centring was applied to facilitate interpretation of main effects (Schielzeth 2010). The measured values are shown as mean and standard deviation.
Results
Acoustic sampling and net trawls
The final synthesised echogram for the entire cruise track covers areas which were normoxic at all depths and areas which were hypoxic (Fig. 2). Hypoxic conditions (oxygen concentration <2 mL L−1) were only ever encountered below 75 m and encompassed about 18 % of the total water column. At hypoxic locations, normal oxygen concentrations (5–6 mL L−1) were present from the surface to c. 40 m, followed by a gradient of decreasing oxygen concentration to hypoxic conditions at 75 m, reaching anoxia (<0.2 mL L−1) between 85 m and the seabed.
Synthesised echogram displaying the three scattering types; fish (pink), A. aurita (green) and macrozooplankton (mysids and L. macrurus copepods; yellow) during hypoxic/normoxic and day/night acoustic transects using three frequencies (70, 120 and 200 kHz) from a towed body in the Gulf of Finland August 2012. Echogram is overlain with the CTD casts showing oxygen (solid line) and temperature (dotted line) profiles. Labelling (a, b, c) identifies location of trawl sites and corresponds to Fig. 3
The vast majority of fish echoes occurred in normoxic sea waters both day and night, but occasional single fish were detected at depth, even in anoxic sea water (Fig. 2). The only gelatinous zooplankton collected were medusae of A. aurita. Grid cells with the backscatter characteristic of medusae occurred mostly in the upper 20 m both day and night (Fig. 2), and the trawl net sampled an average density of 0.2 ± 0.1 indiv m−3 (mean ± SE; Fig. 3). The manner in which the net was fished (nets one and three open from/to the surface) means that only the second of the three nets provides a sample clean from contamination from other depths. In Fig. 3, the clean net samples are those at 85, 75 and 24 m for Fig. 3a–c, respectively. Crustacean macrozooplankton were found spread throughout the water column but were concentrated in a dense layer in the hypoxic zone from 75 to 85 m both day and night (Fig. 2). This layer was confirmed by trawl data to consist of mysids, dominated by M. mixta at 1.7 ± 0.8 indiv m−3 (mean ± SE) and high densities (66 ± 28 indiv m−3; mean ± SE) of the copepod L. macrurus (Fig. 3). When the trawls (Fig. 3a, b) that targeted the acoustic backscattering layer were deployed, the layer was at 75 and 80 m, respectively (see also Fig. 2).
Results from the trawl nets towed in the Gulf of Finland from R/V Aranda in August 2012. Data were log (x + 1)-transformed. Labelling (a, b, c) corresponds to location of trawl sites and is marked on Fig. 2
Statistical modelling of acoustic data
From 16 parameters (see ‘Materials and methods’ section), our final best-fit GAM used just two variables and described 38 % (r 2adj = 0.38) of the variation in crustacean zooplankton backscatter in space and timer:
where Sv is is S v values at time i at depth s, f are smoothing functions and ɛ is are residuals independently, normally distributed with expectation 0 and variance σ 2. The GAM predicted a strong positive effect of hypoxic sea water on crustacean zooplankton (mysids and L. macrurus) backscatter (i.e. as oxygen concentration declined, zooplankton backscatter increased; F = 70, df = 5, p < 0.001; Fig. 4a). Intermediate levels of %PAR (6–8 %) had a positive effect on crustacean zooplankton backscatter (Fig. 4b; F = 64, df = 3, p < 0.001). Note that neither a day/night factor nor date/time explained a significant amount of the variation in crustacean zooplankton backscatter.
Results from the final best-fit general additive model (GAM) which used two variables, oxygen (a) and percentage of photosynthetically available radiation (%PAR; b) to explain 38 % of zooplankton backscatter as derived from acoustic data collected from a towed body in the Gulf of Finland in August 2012
Oxidative biomarker responses
The measured values of ORAC (M. mixta: 205 ± 30 and M. relicta: 209 ± 29 Trolox equivalents in mg protein−1) and TBARS (M. mixta: 19 ± 2 and M. relicta: 18 ± 2 nmol MDA mg protein−1) were similar between the species. However, the regression slopes between the biomarkers and explanatory variables (species, oxygen status and body size) were significantly different for the two species of mysids, with both two-way and three-way interactions. The sample composition was highly skewed towards M. mixta so the effect of oxygen concentration was evaluated for each species separately. Oxygen concentrations of bottom water (73–96 m) were hypoxic, between 0.1 and 1.9 mL L−1. In both species, significant effects of oxygen status on ORAC values were observed, indicating that average ORAC levels were significantly higher in the animals from the areas with anoxic bottom water (Table 1). In M. relicta, a significantly higher slope of the relationship between ORAC and body size was found for the animals collected at the stations with anoxic bottom water (Table 1). No significant oxygen status effects on TBARS were observed in either species (Table 2). Body size (BL) was the only significant variable affecting levels of TBARS (i.e. larger individuals had higher levels of TBARS), with the effect size being similar between the species.
Discussion
Hypoxia and anoxia were found in deep (>75 m) western areas, in keeping with expectations regarding the inflow of hypoxic Baltic Sea water into the Gulf of Finland. As expected, non-larval fish were aggregated above the oxycline in normoxic surface waters. Fish aggregations were found in the upper 40 m (approx. 40 % of the available water column), with the well-defined aggregation edge marking the start of the oxycline. This is in line with other Baltic Sea studies on cod, herring and sprat (Neuenfeldt and Beyer 2003; Ekau et al. 2010). Indeed, everywhere that hypoxia at depth has been studied, this spatial distribution pattern of fish is revealed (Chesapeake Bay: Costantini et al. 2008; Ludsin et al. 2009; the northwestern Gulf of Mexico: Zhang et al. 2009; Hazen et al. 2009; Craig and Bosman 2013; and freshwater lakes: Baldwin et al. 2002; Vanderploeg et al. 2009). In addition, research into midwater hypoxia—oxygen minimum zones (OMZ) and layers (OML)—reports fish avoidance of hypoxia, which, as in our study, reduces overlap with target prey species such as euphausiids and other crustacean that are able to tolerate low oxygen conditions (Ekau and Verheye 2005; Prince and Goodyear 2006; Parker-Stetter and Horne 2009; Bertrand et al. 2010). Although most fish were found in normoxic waters in our study, a few individual fish were found in the hypoxic zone. Previous studies have shown that fish are capable of hunting forays into hypoxic waters (Ludsin et al. 2009; Kaartvedt et al. 2009), but hypoxic conditions would be expected to disrupt schooling behaviour (Brierley and Cox 2010).
Aurelia aurita are more tolerant of hypoxia than fish (Purcell et al. 2001). In our study, A. aurita medusae were all within the upper 20 m and there was no evidence from acoustic data of jellyfish in deeper hypoxic waters. This is consistent with Möller’s (1980) observation that A. aurita in the Baltic Sea do not descend towards the bottom until they begin to reduce their swimming movements and die before winter (Möller 1980). Net data showing A. aurita at 85 m (Fig. 3b) are most likely due to the open style of the net upon ascending which will have caught medusae in the upper 20 m. Even though A. aurita medusae were not evident in hypoxic waters in our study, hypoxia may still benefit A. aurita populations by increasing planulae settlement through decreased benthic competitors at hypoxic settlement sites (Miller and Graham 2012). The volumetric density of jellyfish we detected, although high (0.2 ± 0.1 indiv m−3) (Schneider and Behrends 1994), was not quite at bloom levels, such as A. aurita blooms in Tokyo Bay or Taiwan (0.3–2.4 indiv m−3; Toyokawa et al. 2000; Lo et al. 2008). Any population response of A. aurita due to low oxygen concentrations will probably be linked with a response to low salinity in the Gulf of Finland because the number of ephyrae from strobilae has been shown to decline with decreasing salinity (Palmén 1954).
In keeping with our third null hypothesis, which was that crustacean zooplankton would not avoid hypoxic waters, we found that mysids and L. macrurus formed dense aggregations in the hypoxic layer rather than throughout the rest of the normoxic water column. An increased biomass of crustacean zooplankton has been detected previously in hypoxic layers in both the northwestern Gulf of Mexico and in Chesapeake Bay (Pierson et al. 2009; Kimmel et al. 2010) which they suggest is a predator avoidance strategy. In the Gulf of Mexico, Zhang et al. (2014) report very similar hypoxic conditions, varying from 5 to 19 % of the total water column (averages) in 2003 and in 2006 (cf. 18 % in this study), whereas in Chesapeake Bay, hypoxia can be up to 75 % of the total water column (Ludsin et al. 2009). Zhang et al. (2014) found that at these relatively low fractions of water column hypoxia, there was not a significant relationship between habitat quality for fish and hypoxia; however, habitat quality for fish was significantly influenced by zooplankton concentration. Whilst hypoxia may not affect the concentration or abundance of zooplankton directly (Roman et al. 2012), our study shows that even at relatively low vertical extents, hypoxia can cause a vertical separation of fish and their zooplankton prey, which effectively reduces the abundance of zooplankton prey available to predatory fish. The use of hypoxic zones by zooplankton as a refuge from fish predation has been reported (Vanderploeg et al. 2009), but it is not clear why in our study, zooplankton were inhabiting hypoxic conditions, whilst there was still a vertical gap of almost 30 m separating them from aggregated adult fish predators. It is possible, however, that as fish make the aforementioned short hunting forays into hypoxic waters, zooplankton would still be exposed to a considerable predation risk if they occupied a shallower depth (cf. Holliland et al. 2012). Also, in late summer, the population of L. macrurus cease their vertical migrations due to high temperatures in the upper water layers (Bityukov 1960), and mysids could have been using their antennal mechanosensors and sensitive eyes to hunt the L. macrurus within this depth layer (Lindström 2000).
The relative frequency response classification procedure is the most effective for identifying layers or multiple targets (Korneliussen and Ona 2003). The production of the synthesised echogram, clearly identifying the layer of crustacean zooplankton within the hypoxic zone, requires the spatial overlap of the beams to be over 85 %, which in our study was achieved at a depth of 12 m (Korneliussen et al. 2008). Another potential source of error is that each volume segment or cell can only be identified as one type of scatterer, meaning weaker scatterers may be underestimated when adult fish are present in large numbers. This did not affect the identification of the weakly scattering crustacean zooplankton in the hypoxic layer as adult fish were largely absent. In addition, the identification of the layer was supported by ‘clean’ net samples from the middle trawl net which provided species information and density estimates.
In our study, mysids from hypoxic areas (between 0.1 and 1.9 mL L−1) had significantly higher antioxidant capacity than the conspecifics from normoxic areas, and there was no evidence of significant oxidative damage. Higher antioxidant capacity and no sign of oxidative damage are consistent with mysid migration from hypoxic to normoxic waters; these observations support our fifth hypothesis. A similar response was observed in some myctophid fishes inhabiting oxygen minimum zones and frequently experiencing shifts from hypoxic to normoxic areas (Lopes et al. 2013). Although we found no evidence of a significant DVM signal for crustacean zooplankton, we suggest that mysids may be undertaking non-synchronised vertical migration or the foray type behaviour of crustacean zooplankton described by Cottier et al. (2006) in a high Arctic fjord from June to September. Alternatively, our mysid DVM signal could have been masked by the aforementioned high densities of non-migrating L. macrurus. Although day/night did not significantly affect crustacean zooplankton distribution in this study, photosynthetically available radiation (%PAR) did have a significant influence on zooplankton distribution. Intermediate levels of %PAR (6–8 %) had a positive effect on zooplankton backscatter, whilst higher levels (8–12 %) had a negative influence on zooplankton backscatter. At intermediate levels, %PAR probably enabled zooplankton to hunt their own pelagic prey more effectively (Viherluoto and Viitasalo 2001), whilst the negative effect at higher levels of %PAR fits with the generally accepted hypothesis that zooplankton avoid the predation risks associated with higher light levels (Pearre 2003).
The two species of mysids (M. mixta and M. relicta) may differ in the mechanisms of their response to hypoxia in the Baltic Sea, as indicated by the changes in antioxidant capacity as a function of body size. Other studies show that many biomarkers, including those involved in regulating redox state, vary with ontogeny and body size (reviewed in Metcalfe and Alonso-Alvarez 2010). Whereas the body size effect on lipid oxidation levels (TBARS) was similar between the two species (Table 2), its effect on the antioxidant levels (ORAC) was nearly twofold higher in M. mixta compared to M. relicta (Table 1), which indicates stronger ontogenetic influence on antioxidative status in the former species. Moreover, we observed significantly higher slope in the ORAC body size regression for M. relicta collected in hypoxic areas compared to that in normoxic areas (Table 2). The altered slope suggests that physiological and/or behavioural adaptations to these habitats may induce changes in total antioxidant capacity that are associated with higher maintenance or maturation costs, which may translate into lower investment to other functions, such as somatic growth, immune defence and progeny quality. The data on such physiological strategies for adaptation to oxygen variability in Baltic mysids are valuable for understanding how different species might respond to the impacts of environmental stressors (e.g. expanding bottom and mesopelagic hypoxia) coupled with variation in fish stocks.
In summary, during August in the Gulf of Finland, A. aurita medusae were not in hypoxic waters but were almost at bloom levels of abundance in the upper 20 m. Fish were aggregated in normoxic upper water layers (<40 m), with evidence of a few individual forays into deeper hypoxic water. Crustacean zooplankton were found aggregated within this deep hypoxic layer, possibly as a refuge from fish predation. Coupled with the vertical heterogeneity of oxygen concentrations, mysid shrimp physiology showed a preparatory antioxidant response with no evidence of oxidative stress, yet there was no evidence of a synchronised vertical migration. We suggest that non-synchronous mysid vertical migration occurred from hypoxic to normoxic water layers, but the statistical significance of this was masked by the high densities of non-migrating L. macrurus copepods, which shared their hypoxic refuge.
References
Akaike H (1974) New look at statistical-model identification. Trans Autom Control 19:716–723
Angel MV, Pugh PR (2000) Quantification of diel vertical migration by micronektonic taxa in the northeast Atlantic. Hydrobiologia 440:161–179
Appletans W, Hannouti A, Van Damme S, Soetaert K, Vanthomme R, Tackx M (2003) Zooplankton in the Schelde estuary (Belgium/The Netherlands). The distribution of Eurytemora affinis: effect of oxygen? J Plankton Res 25:1441–1445
Baldwin CM, Beauchamp DA, Gubala CP (2002) Seasonal and diel distribution and movement of cutthroat trout from ultrasonic telemetry. Trans Am Fish Soc 131:143–158
Barz K, Hirche HJ (2005) Seasonal development of scyphozoan medusae and the predatory impact of Aurelia aurita on the zooplankton community in the Bornholm Basin (central Baltic Sea). Mar Biol 147:465–476
Bertrand A, Ballon M, Chaigneau A (2010) Acoustic observation of living organisms reveals the upper limit of the oxygen minimum zone. PLoS One 5:4
Bityukov EP (1960) The ecology of Limnocalanus grimaldii (Guerne) in the Gulf of Finland English summ. Zool Zhur 39:1783–1789
Brierley AS (2014) Diel vertical migration. Curr Biol 24:1074–1076
Brierley AS, Cox MJ (2010) Shapes of krill swarms and fish schools emerge as aggregation members avoid predators and access oxygen. Curr Biol 20:1758–1762
Brierley AS, Boyer DC, Axelsen BE, Lynam CP, Sparks CAJ, Boyer HJ, Gibbons MJ (2005) Towards the acoustic estimation of jellyfish abundance. Mar Ecol Prog Ser 295:105–111
Burd AB, Hansell DA, Steinberg DK, Anderson TR, Aristegui J, Baltar F, Beaupre SR, Buesseler KO, Dehairs F, Jackson GA, Kadko DC, Koppelmann R, Lampitt RS, Nagata T, Reinthaler T, Robinson C, Robison BH, Tamburini C, Tanaka T (2010) Assessing the apparent imbalance between geochemical and biochemical indicators of meso-and bathypelagic biological activity: what the @$#! is wrong with present calculations of carbon budgets? Deep Sea Res Part II 57:1557–1571
Colombo GA, Mianzan H, Madirolas A (2003) Acoustic characterization of gelatinous-plankton aggregations: four case studies from the Argentine continental shelf. ICES J Mar Sci 60:650–657
Conley DJ, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hansson LA, Rabalais NN, Voss M, Zillen L (2009) Tackling hypoxia in the Baltic Sea: is engineering a solution? Environ Sci Technol 43:3407–3411
Costantini M, Ludsin SA, Mason DM, Zhang X, Boicourt WC, Brandt SB (2008) Effect of hypoxia on habitat quality of striped bass (Morone saxatilis) in Chesapeake Bay. Can J Fish Aquat Sci 65:989–1002
Cottier FR, Tarling GA, Wold A, Falk-Petersen S (2006) Unsynchronized and synchronized vertical migration of zooplankton in a high arctic fjord. Limnol Oceanogr 51:2586–2599
Craig JK, Bosman SH (2013) Small spatial scale variation in fish assemblage structure in the vicinity of the northwestern Gulf of Mexico hypoxic zone. Estuar Coast 36:268–285
Dandapat J, Chainy GBN, Rao KJ (2003) Lipid peroxidation and antioxidant defence status during larval development and metamorphosis of giant prawn, Macrobrachium rosenbergii. Comp Biochem Physiol C: Toxicol Pharmacol 135:221–233
De Robertis A, Mckelvey DR, Ressler PH (2010) Development and application of an empirical multifrequency method for backscatter classification. Can J Fish Aquat Sci 67:1459–1474
Desprez M, Rybarczyk H, Wilson JG, Ducrotoy JP, Sueur F, Olivesi R, Elkaim B (1992) Biological impact of eutrophication in the Bay of Somme and the induction and impact of anoxia. Neth J Sea Res 30:149–159
Ekau W, Verheye HM (2005) Influence of oceanographic fronts and low oxygen on the distribution of ichthyoplankton in the Benguela and southern Angola currents. Afr J Mar Sci 27:629–639
Ekau W, Auel H, Poertner HO, Gilbert D (2010) Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 7:1669–1699
Gorokhova E, Löf M, Reutgard M, Lindström M, Sundelin B (2013) Exposure to contaminants exacerbates oxidative stress in amphipod Monoporeia affinis subjected to fluctuating hypoxia. Aquat Toxicol 127:46–53
Greenlaw CF (1979) Acoustical estimation of zooplankton populations. Limnol Oceanogr 24:226–242
Hamming RW (1973) Numerical methods for scientists and engineers. McGraw-Hill, New York
Hastie T, Tibshirani R (1990) Exploring the nature of covariate effects in the proportional hazards model. Biometrics 46:1005–1016
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503:163–170
Hazen EL, Craig JK, Good CP, Crowder LB (2009) Vertical distribution of fish biomass in hypoxic waters on the Gulf of Mexico shelf. Mar Ecol Prog Ser 375:195–207
HELCOM (1988) Guidelines for the Baltic monitoring programme for the third stage. Part D. Biological determinands. In: Balt Sea Environ Proceedings of 27D. http://helcom.fi/action-areas/monitoring-and-assessment/manuals-and-guidelines/combine-manual
Hermes-Lima M, Zenteno-Savín T (2002) Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Phys C 133:537–556
Hermes-Lima M, Storey JM, Storey KB (2001) Antioxidant defenses and animal adaptation to oxygen availability during environmental stress. In: Storey KB, Storey JM (eds) Cell and molecular responses to stress, vol 2. Elsevier Press, Amsterdam, pp 263–287
Hernandez-Leon S, Franchy G, Moyano M, Menendez I, Schmoker C, Putzeys S (2010) Carbon sequestration and zooplankton lunar cycles: could we be missing a major component of the biological pump? Limnol Oceanogr 55:2503–2512
Hidaka K, Kawaguchi K, Murakami M, Takahashi M (2001) Downward transport of organic carbon by diel migratory micronekton in the western equatorial Pacific: its quantitative and qualitative importance. Deep-Sea Res Part II 48:1923–1939
Hoguet J, Key PB (2007) Activities of biomarkers in multiple life stages of the model crustacean, Palaemonetes pugio. J Exp Mar Biol Ecol 353:235–244
Holliday DV, Pieper RE (1980) Volume scattering strengths and zooplankton distributions at acoustic frequencies between 0.5 and 3 MHz. J Acoust Soc Am 67:135–146
Holliland PB, Ahlbeck I, Westlund E, Hansson S (2012) Ontogenetic and seasonal changes in diel vertical migration amplitude of the calanoid copepods Eurytemora affinis and Acartia spp. in a coastal area of the northern Baltic Proper. J Plankton Res 34:298–307
Kaartvedt S, Rostad A, Klevjer TA (2009) Sprat Sprattus sprattus can exploit low oxygen waters for overwintering. Mar Ecol Prog Ser 390:237–249
Kimmel DG, Boicourt WC, Pierson JJ, Roman MR, Zhang X (2010) The vertical distribution and diel variability of mesozooplankton biomass, abundance and size in response to hypoxia in the northern Gulf of Mexico USA. J Plankton Res 32:1185–1202
Kloser RJ, Ryan T, Sakov P, Williams A, Koslow JA (2002) Species identification in deep water using multiple acoustic frequencies. Can J Fish Aquat Sci 59:1065–1077
Knudsen HP (2009) Long-term evaluation of scientific-echosounder performance. ICES J Mar Sci 66:1335–1340
Korneliussen RJ, Ona E (2003) Synthetic echograms generated from the relative frequency response. ICES J Mar Sci 60:636–640
Korneliussen RJ, Diner N, Ona E, Berger L, Fernandes PG (2008) Proposals for the collection of multifrequency acoustic data. ICES J Mar Sci 65:982–994
Kowaltowski AJ, Vercesi AE (1999) Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26:463–471
Lindstrom M (2000) Eye function of Mysidacea (Crustacea) in the northern Baltic Sea. J Exp Mar Biol Ecol 246:85–101
Lo WT, Purcell JE, Hung JJ, Su HM, Hsu PK (2008) Enhancement of jellyfish (Aurelia aurita) populations by extensive aquaculture rafts in a coastal lagoon in Taiwan. ICES J Mar Sci 65:453–461
Lopes AR, Truebenbach K, Teixeira T, Lopes VM, Pires V, Baptista M, Repolho T, Calado R, Diniz M, Rosa R (2013) Oxidative stress in deep scattering layers: heat shock response and antioxidant enzymes activities of myctophid fishes thriving in oxygen minimum zones. Deep-Sea Res Part I 82:10–16
Ludsin SA, Zhang X, Brandt SB, Roman MR, Boicourt WC, Mason DM, Costantini M (2009) Hypoxia-avoidance by planktivorous fish in Chesapeake Bay: implications for food web interactions and fish recruitment. J Exp Mar Biol Ecol 381:121–131
Metcalfe NB, Alonso-Alvarez C (2010) Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol 24:984–996
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Miller MEC, Graham WM (2012) Environmental evidence that seasonal hypoxia enhances survival and success of jellyfish polyps in the northern Gulf of Mexico. J Exp Mar Biol Ecol 432:113–120
Modig H, Olafsson E (1998) Responses of Baltic benthic invertebrates to hypoxic events. J Exp Mar Biol Ecol 229:133–148
Möller H (1980) Population dynamics of Aurelia aurita medusae in Kiel Bight, Germany. Mar Biol 60:123–128
Mutlu E (1996) Target strength of the common jellyfish (Aurelia aurita): a preliminary experimental study with a dual-beam acoustic system. ICES J Mar Sci 53:309–311
Neuenfeldt S, Beyer JE (2003) Oxygen and salinity characteristics of predator–prey distributional overlaps shown by predatory Baltic cod during spawning. J Fish Biol 62:168–183
Ogonowski M, Hansson S, Duberg J (2013) Status and vertical size-distributions of a pelagic mysid community in the northern Baltic proper. Boreal Environ Res 18:1–18
Palmén E (1954) Seasonal occurrence of ephyrae and subsequent instars of Aurelia aurita in the shallow waters of Tvärminne, S. Finland. Arch Soc Vanamo 8:2
Parker-Stetter SL, Horne JK (2009) Nekton distribution and midwater hypoxia: a seasonal, diel prey refuge? Estuar Coast Shelf S 81:13–18
Pearre S (2003) Eat and run? The hunger/satiation hypothesis in vertical migration: history, evidence and consequences. Biol Rev 78:1–79
Peltonen H, Balk H (2005) The acoustic target strength of herring (Clupea harengus) in the northern Baltic Sea. ICES J Mar Sci 62:803–808
Pierson JJ, Roman MR, Kimmel DG, Boicourt WC, Zhang X (2009) Quantifying changes in the vertical distribution of mesozooplankton in response to hypoxic bottom waters. J Exp Mar Biol Ecol 381:74–79
Prior RL, Hoang H, Gu LW, Wu XL, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang DJ, Ou BX, Jacob R (2003) Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J Agric Food Chem 51:3273–3279
Prince ED, Goodyear CP (2006) Hypoxia-based habitat compression of tropical pelagic fishes. Fish Oceanogr 15:451–464
Purcell JE, Breitburg DL, Decker MB, Graham WM, Youngbluth MJ, Raskoff KA (2001) Pelagic cnidarians and ctenophores in low dissolved oxygen environments: A review. In: Rabalais NN (ed) Coastal and Estuarine Sciences, Coastal Hypoxia: Consequences for Living Resources and Ecosystems, Vol 58
Rajasilta M, Hanninen J, Vuorinen I (2014) Decreasing salinity improves the feeding conditions of the Baltic herring (Clupea harengus membras) during spring in the Bothnian Sea, northern Baltic. ICES J Mar Sci 71:1148–1152
Roman MR, Pierson JJ, Kimmel DG, Boicourt WC, Zhang X (2012) Impacts of hypoxia on zooplankton spatial distributions in the northern Gulf of Mexico. Estuar Coast 35:1261–1269
Rudstam LG, Hansson S (1990) On the ecology of Mysis mixta (Crustacea, Mysidacea) in a coastal area of the northern Baltic Proper. Ann Zool Fenn 27:259–263
Sarkar D (2008) Lattice: multivariate data visualization with R. Springer, New York
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Schneider G, Behrends G (1994) Population dynamics and the trophic role of Aurelia aurita medusae in the Kiel Bight and western Baltic. ICES J Mar Sci 51:359–367
Simmonds EJ, MacLennan DN (2005) Fisheries acoustics: theory and practice. Blackwell Science, London
Shoji J, Masuda R, Yamashita Y, Tanaka M (2005) Predation on fish larvae by moon jellyfish Aurelia aurita under low dissolved oxygen concentrations. Fish Sci 71:748–753
Stanton TK, Chu DZ, Wiebe PH, Clay CS (1993) Average echoes from randomly oriented random-length finite cylinders—zooplankton models. J Acoust Soc Am 94:3463–3472
Stanton TK, Wiebe PH, Chu DZ, Benfield MC, Scanlon L, Martin L, Eastwood RL (1994) On acoustic estimates of zooplankton biomass. ICES J Mar Sci 51:505–512
Toyokawa M, Furota T, Terazaki M (2000) Life history and seasonal abundance of Aurelia aurita medusae in Tokyo Bay, Japan. Plankton Biol Ecol 47:48–58
Tyler JE (1968) Secchi disc. Limnol Oceanogr 13:1–6
Vanderploeg HA, Ludsin SA, Cavaletto JF, Hoeoek TO, Pothoven SA, Brandt SB, Liebig JR, Lang GA (2009) Hypoxic zones as habitat for zooplankton in Lake Erie: refuges from predation or exclusion zones? J Exp Mar Biol Ecol 381:108–120
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci USA 105:15452–15457
Viherluoto M, Viitasalo M (2001) Effect of light on the feeding rates of pelagic and littoral mysid shrimps: a trade-off between feeding success and predation avoidance. J Exp Mar Biol Ecol 261:237–244
Viitasalo M (1992) Mesozooplankton of the Gulf of Finland and northern Baltic Proper—a review of monitoring data. Ophelia 35:147–168
Warren JD, Smith JN (2007) Density and sound speed of two gelatinous zooplankton: ctenophore (Mnemiopsis leidyi) and lion’s mane jellyfish (Cyanea capillata). J Acoust Soc Am 122:574–580
Watkins JL, Brierley AS (1996) A post-processing technique to remove background noise from echo integration data. ICES J Mar Sci 53:339–344
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall/CRC, Boca Raton
Zhang H, Ludsin SA, Mason DM, Adamack AT, Brandt SB, Zhang X, Kimmel DG, Roman MR, Boicourt WC (2009) Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. J Exp Mar Biol Ecol 381:80–91
Zhang H, Mason DM, Stow CA, Adamack AT, Brandt SB, Zhang X, Kimmel DG, Roman MR, Boicourt WC, Ludsin SA (2014) Effects of hypoxia on habitat quality of pelagic planktivorous fishes in the northern Gulf of Mexico. Mar Ecol Prog Ser 505:209–226
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We are indebted to the captain and crew onboard the R/V Aranda for the research cruises in August 2007 and 2012. We are very grateful to Pia Varmanen and Jere Riikonen for onboard laboratory results. This study was funded by the Finnish Environment Institute, the Nottbeck Walter and Andrée de Nottbeck Foundation and the Natural Environment Research Council, UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-O. Pörtner.
Rights and permissions
About this article
Cite this article
Webster, C.N., Hansson, S., Didrikas, T. et al. Stuck between a rock and a hard place: zooplankton vertical distribution and hypoxia in the Gulf of Finland, Baltic Sea. Mar Biol 162, 1429–1440 (2015). https://doi.org/10.1007/s00227-015-2679-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2679-8