Abstract
Shark nursery areas are widely regarded as essential habitats for the growth and survival of young individuals. The effective management and protection of shark nursery habitat are contingent upon a clear understanding of how individual species utilize such habitat both spatially and temporally. Although shark nurseries have been identified in the Caribbean, this information is generally lacking. From 2006 to 2012, we used passive acoustic telemetry to monitor the presence, movements, and habitat use of 65 juvenile blacktip sharks (Carcharhinus limbatus) and 42 juvenile lemon sharks (Negaprion brevirostris) within Fish Bay and Coral Bay, two shark nurseries in St. John, United States Virgin Islands. Both species were present in each bay during all months of the year, but abundance peaked during the summer (June–September). Although telemetry data indicated that blacktip and lemon sharks moved throughout each embayment, each species exhibited strong site fidelity to core areas across all years of the study. Habitat partitioning was observed in both nurseries as blacktip sharks generally occurred in areas characterized by water depths of 1.5–6 m with seagrass and sand/mud substrate, while lemon sharks remained in close proximity to or within shallow (<1 m), mangrove-fringed seagrass habitat. Blacktip sharks were also observed to exhibit greater activity space during the nighttime hours (1900–0659 h) within Coral Bay. The results of this study indicate that Fish Bay and Coral Bay are nursery areas that warrant designation as essential fish habitat and exemplify the need for additional focused management measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sharks are important members of marine communities capable of exerting strong top-down forces over large spatial and temporal scales (Ferretti et al. 2010). Within tropical marine communities, they have been shown to be integral to ecosystem health (Stevenson et al. 2006) as their removal from coral reefs has been linked to reef degradation and shifts to an alternate algae-dominated state (Jackson et al. 2001; Pandolfi et al. 2005; Newman et al. 2006; Myers et al. 2007). In the greater Caribbean, the broad-scale absence of sharks on reefs has been attributed to fishing mortality, with contemporary shark aggregations mostly occurring in areas with low human populations, strong fishing regulations, and/or well-enforced marine reserves (Ward-Paige et al. 2010). In the United States Virgin Islands (USVI), there is currently no territorial management of sharks, and despite federal shark fishing regulations, young sharks (i.e., sharks smaller than the 54″/137 cm minimum size limit; NMFS 2009) are often harvested in nearshore territorial waters for personal consumption (DeAngelis et al. 2008). To complicate matters, little information exists on the relative abundance or essential fish habitat (EFH) of these young sharks around these islands (DeAngelis et al. 2008). Hence, the impact of such fishing practices on nearshore shark populations remains unknown.
The delineation of shark nursery areas/habitats is critical for proper species management (Casey and Taniuchi 1990; Pratt and Otake 1990; NMFS 2006; Heithaus 2007). Such areas are traditionally thought to foster the growth of juveniles to maturity and, therefore, play an important role in the maintenance of populations (Heithaus 2007). However, beyond the geographic delineation of these areas, effective management of shark nursery habitat is contingent upon a clear understanding of how individual species utilize such habitat both spatially and temporally (Heithaus 2007). Such information can be used to delineate EFH and, perhaps, establish marine protected areas (MPAs; Heupel and Simpfendorfer 2005a, b), which ultimately protect shark populations from habitat destruction and overfishing (Heupel et al. 2007). While nearshore/coastal shark nursery areas have been identified and characterized extensively throughout the US Atlantic Ocean and greater Caribbean (e.g., Gruber et al. 1988; Heupel et al. 2007; Murchie et al. 2010; Kneebone et al. 2012), very limited information on such habitats exists in the USVI.
In recent years, efforts have been initiated to identify and delineate shark nursery habitat throughout St. Thomas and St. John, USVI (e.g., DeAngelis 2006, 2008). Among the numerous areas in which juvenile sharks were captured during standardized bottom longline surveys, two areas of St. John (Fish Bay and Coral Bay) emerged as highly productive, multi-species habitats containing an abundance of young of the year (YOY) and juvenile blacktip (Carcharhinus limbatus) and lemon (Negaprion brevirostris) sharks. Based on the relative abundance and long-term (i.e., inter-annual) site fidelity of each species within Fish Bay (using tag recapture and catch rate analyses, and limited active acoustic tracking), there is compelling evidence that this embayment provides important nursery habitat for both species (DeAngelis et al. 2008). There is also some evidence that portions of Coral Bay support nursery habitat; however, additional data on spatial and temporal habitat use throughout the entire embayment are warranted (DeAngelis 2006). Furthermore, given that each of these embayments is markedly smaller than nursery areas described for the species in other geographic locations throughout the US Atlantic and greater Caribbean (e.g., Morrissey and Gruber 1993; Chapman et al. 2009; Henderson et al. 2010; Heupel et al. 2007; Hueter and Tyminski 2007; Heithaus 2007; Abel et al. 2007; Parsons and Hoffmayer 2007; Steiner et al. 2007; Ulrich et al. 2007), additional data are required to examine whether the spatiotemporal behaviors of each species are consistent with other nursery areas.
The EFH amendment to the fishery management plan of the US Caribbean states that the waters and substrates that make up EFH in the USVI are readily susceptible to a number of human activities including overexploitation, sedimentation, pollution, and commercial and industrial development (Caribbean Fishery Management Council 1998a, b). Given the critical role sharks play in tropical marine communities and their susceptibility to these anthropogenic impacts (reviewed by Knip et al. 2010), information on the spatiotemporal dynamics of habitat use within such areas is pivotal for effective fisheries and habitat management. The objectives of this study were to employ passive acoustic telemetry to document and compare the presence, habitat use, and site fidelity of YOY and juvenile blacktip and lemon sharks within Fish Bay and Coral Bay, St. John, USVI, and to assess their relative importance as shark nursery habitats.
Materials and methods
Study site
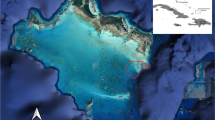
St. John (32 km2) is one of the three major islands constituting the USVI (Fig. 1). It is surrounded by narrow shelves (<16 km wide) on all sides, with sharp drop-offs and deep water (>3,600 m at deepest points) beyond the shelf along its north and south shores. The coastline of St. John is characterized by numerous bays containing diverse habitat comprising coral reefs, sea grasses, and mangroves. Although more than 75 % of the coastline falls within the USVI National Park (Fig. 1), fishing is allowed by rod and reel, handlines, fish traps, and baitfish nets (United States Virgin Islands Department of Planning and Natural Resources 2012).
Fish Bay and Coral Bay study sites in St John, United States Virgin Islands (USVI). Individual receiver stations (numbers), including location of temperature loggers used for analysis (red numbers), and zones (Coral Bay only) are presented. Seagrass (light gray area) and mangrove (red) habitat, along with the National Park (hatch) and Coral Monument boundary (crosshatch), are presented
Fish Bay is a relatively small embayment (0.34 km2) located on the southern coast of St. John (Fig. 1). A small portion of its eastern shore occurs within the USVI National Park. It is a fairly shallow embayment (0.5–10 m, X = 1.5 m), with bottom substrate consisting of predominantly continuous (>70 % coverage) to patchy (50–70 % coverage) seagrass (Thalassia testidinum) and patchy (<30 %) macroalgae. The northeastern shore is lined with partially submerged red (Rhizophora mangle), black (Avicennia germinans), and white (Laguncularia racemosa) mangroves (DeAngelis et al. 2008). The southern mouth of the bay is bordered by fringing reefs. Anthropogenic impacts to Fish Bay are relatively minor and include a small number (<3) of live-aboard vessels, a developed shoreline (private homes), and large amounts of sediment run-off from the watershed (Ramos-Scharrón and MacDonald 2005). Human activity (e.g., boat traffic and shoreline activity) within the embayment is relatively uncommon, although recreational fishing activity has been observed on several occasions (B. Legare and B. DeAngelis, pers observations).
Coral Bay is a comparably larger bay (1.3 km2) located on the southeastern side of St. John outside of the USVI National Park (Fig. 1). It is a relatively shallow embayment (0.5–10 m; X = 2 m) with bottom substrate in the center of the bay consisting of expansive soft bottom mud, macroalgae, sand, and patch reefs. Like Fish Bay, Coral Bay is partially bordered by submerged red, black, and white mangroves along the northern shoreline and supports a shallow (<1.5 m) seagrass bed (>70 % coverage; T. testudinum) that extends along most of the western shore. Anthropogenic impacts to Coral Bay include a large live-aboard vessel community (>50 boats), extensive riparian development, and considerable road run-off and sedimentation (Ramos-Scharrón and MacDonald 2005; Brooks et al. 2007; Smith et al. 2008). The large live-aboard community generates considerable human activity, with constant boat traffic and shoreline activity. Recreational fishing, particularly for small sharks, has been regularly observed in Coral Bay (B. Legare and B. DeAngelis, pers observations).
Shark tagging
Blacktip and lemon sharks were captured on benthic longlines in Fish Bay and Coral Bay during seven sampling trips conducted from 2006 to 2011 (Table 1). All longline sampling was conducted as described in DeAngelis et al. (2008), with the exception that hooks were baited with mackerel (Scomber scombrus), Atlantic bonito (Sarda sarda), little tunny (Euthynnus alleratus), and barracuda (Sphyraena barracuda). Soak times ranged from 0.5 to 1.0 h. On one occasion, a custom seine, approximately 8 m long by 2 m high, was also used to corral lemon sharks in the shallow waters of Coral Bay during the 2011 sampling trip.
Once landed, each shark was brought onboard and placed ventral side up in a V-shaped table to induce tonic immobility (Watsky and Gruber 1990). A 1.9-cm-diameter flexible tube connected to a submersible bilge pump (Rule Industries Inc., Gloucester, Massachusetts) was inserted into the mouth to deliver fresh seawater to the gills during tagging. An individually coded transmitter (models V9-2L-R64 K: delay 60–180 s, battery life 738 days; or V13-1-R64 K: delay 60–180 s, battery life 1,140 days; Vemco Division, AMIRIX Systems Inc., Halifax, Nova Scotia) was surgically implanted into the body cavity through a small incision on the ventral side of the shark along the midline, anterior to the pelvic fin. The incision was closed with 3–4 interrupted sutures (2–0 PDS II, Ethicon Inc., New Jersey). Transmitters, sutures, and surgical equipment were disinfected in 95 % ethanol between surgeries. All sharks were also fitted with NMFS conventional rototags (Kohler and Turner 2001) through the dorsal fin. Prior to release, sex and length (cm fork length, FL) were recorded. The entire procedure lasted approximately 5–10 min. All sharks were held in the water at the side of the vessel prior to release with some individuals being walked/swam briefly (in <1 m of water) to ensure full recovery. Sharks were designated as either YOY (age 0) or juveniles (age 1+) based on the size at capture, the published growth curves for each species (lemon: Freitas et al. 2009; blacktip: Passerotti and Baremore 2012) and the presence of fresh (open) umbilical scars (Merson 1998).
Acoustic monitoring
Movements of tagged individuals within Fish Bay and Coral Bay were monitored using a fixed array of acoustic receivers (Models VR2 and VR2 W, Vemco Division, AMIRIX Systems Inc., Halifax, Nova Scotia) deployed in each embayment (Fig. 1). All receivers were attached to sand screws and anchored directly to the seabed; receivers were generally positioned vertically 0–0.25 m off the substrate. Receivers were deployed in Fish Bay and Coral Bay from August 2006 to May 2012 and August 2008 to May 2012, respectively. However, the total number and specific locations of receiver stations within each embayment varied throughout the study period (Table 2). Receivers were downloaded and cleaned every 6 months. Movements of tagged individuals outside of Fish Bay and Coral Bay were monitored by a series of acoustic receivers deployed throughout the USVI (see Pittman et al. 2014 for information on receiver deployment locations). Data from these receivers were obtained opportunistically and incorporated into the analysis. Prior to analysis, all transmitter data were examined individually, and false detections rejected using criteria established by the manufacturer (Pincock 2012).
Ambient water temperature was monitored using temperature loggers (Model UA-001-64 Hobo Pendant and Hobo Pro, Onset Computer Corporation, Onset, Massachusetts) affixed to several receivers throughout each bay. Loggers recorded temperature every 30 min with an accuracy of ±0.7 °C (range 20–70 °C) and were maintained on the same schedule as the acoustic receivers. To examine the effect of temperature on shark presence within each embayment, average daily temperature was calculated only from loggers positioned in the areas of greatest blacktip and lemon shark activity (Fig. 1).
Detection range testing was performed on all receivers within each bay. A transmitter (Model V13-1-R64 K: delay 60–180 s, battery life 1,140 days, Vemco Division, AMIRIX Systems Inc., Halifax, Nova Scotia) was submerged 1 m below the surface, or at approximately half the water depth in waters <1 m, along transects in at least three cardinal directions at distances of 0, 25, 50, 100, 150, 200, 250, 300, 350, 400, and 450 m (or until land was reached) from the receiver for 15 min. The maximum detection range for each receiver was measured as the maximum distance from the receiver at which multiple transmitter detections were obtained during the 15-min deployment period. A handheld global positioning system unit (Model Garmin 76, Olathe, Kansas, USA) was utilized to plot coordinates near the periphery of a receiver’s detection radius; coordinates were subsequently utilized to approximate the total coverage area for each receiver (Supplementary Fig. 1). Receiver detection radius ranged from 25 to 400 m, depending on depth and bottom type. Shallow, soft bottoms tended to have smaller detection ranges (25–100 m) when compared to deeper, harder bottom areas (75–400 m).
Presence and residency
To examine temporal trends in blacktip and lemon shark presence within Fish and Coral bays, the number of sharks detected within each bay during each month of the year (January–December) was calculated annually and over the entire study period. To quantify residency within each bay, the presence of each tagged individual (within the bay in which it was tagged) was examined daily. Individuals were considered present if two or more detections occurred on at least one receiver within an embayment on a given day. Daily detection histories were then plotted for each individual, and the total number of days monitored (i.e., the number of days from the first to last detection in an embayment), number of days present, number of consecutive days present, and number of consecutive days absent (prior to a return) were calculated. To examine the relative amount of time spent within each embayment, a residency index was calculated for each individual as the ratio between the numbers of days present to the total number of days monitored (Knip et al. 2012); residency indices ranged from 0 (low residence) to 1 (high residence).
Habitat use and site fidelity
To elucidate patterns in habitat use by each species within Fish Bay and Coral Bay, a detection index was calculated for each receiver station as total number of detections logged divided by the total number of days deployed during which at least one tagged individual (of that species) was present in the array. Due to the low number of detections logged by some receivers throughout the study period, to enhance comparison, all receiver detection indices were multiplied by ten (detections 10 days−1). In addition, to examine the relative amount of time that sharks were monitored within each bay, a detection index was calculated for each tagged individual as the total number of detections logged divided by the number of days detected (detections day−1).
The preliminary analysis of individual receiver detection ranges, receiver detection indices, and individual detection indices provided strong evidence that the receiver array in Fish Bay was not efficient at monitoring the habitat use of lemon, and to a lesser extent blacktip, sharks throughout the entire embayment (i.e., limited spatial coverage). Consequently, site fidelity and activity space were not assessed for Fish Bay (see Discussion section, for justification).
To assess habitat use within Coral Bay, the center of activity (COA) of each tagged individual was calculated every 30 min using the mean position algorithm originally described in Simpfendorfer et al. (2002). The COA position represents the average geographic position of an individual within the 30-min period and provides a more realistic depiction of the habitat used by an individual than raw receiver locations. All COA positions were utilized to calculate the activity space of tagged sharks within Coral Bay.
Given the irregular boundary of Coral Bay, as well as the deployment pattern of the acoustic receivers therein, a lattice-based density estimator (Barry and McIntyre 2011) was utilized to generate estimates of 50 and 95 % total activity spaces (TAS) for all tagged fish while in the bay. To examine diel patterns in activity space, 50 and 95 % day (defined as 0,700–1,859 h) and night (defined as 1,900–0659 h) activity spaces were also estimated. Only sharks monitored for at least five total days were included in the activity space analyses. The empirical estimation of the optimal smoothing parameter (k) using unbiased cross-validation was problematic due to the dispersion of COA positions (i.e., many positions in the same location and/or in very close proximity). Instead, a fixed k value, obtained by visual inspection of resulting density estimates generated by varying k values, was utilized for all lattice-based density analyses (Kneebone et al. 2012). All lattice-based activity space estimates were obtained using the ‘latticeDensity’ package in R (Barry and McIntyre 2011). To investigate the effects of shark sex and size (FL) on TAS, linear models were applied to log-transformed total activity space estimates in the ‘lme4’ (Bates et al. 2011) library in R (R Development Team 2012). Diel differences in 50 and 95 % activity spaces were examined using a paired t test in R; statistical significance was accepted at P < 0.05.
To examine site fidelity, three zones were created in Coral Bay (Fig. 1) based on preliminary 95 % activity space estimates for each species. Site fidelity to each zone was assessed using a site fidelity index (SFI) (March et al. 2010), which was calculated by dividing the total number of days an individual was detected on any receiver within that zone by the total number of days an individual was detected in the entire array. SFI values ranged from 0 (no fidelity) to 1 (high fidelity) with SFI = 0.5 set as the lower limit for ‘strong’ site fidelity. Only sharks tracked for at least 5 days were included in the analysis. A Bray–Curtis similarity matrix of SFI values by bay was computed between individual sharks as samples for comparison between species, and non-metric multidimensional scaling (nMDS) ordinations were performed to graphically depict differences in site fidelity of individual sharks of each species. An analysis of similarity (ANOSIM) was used to identify whether SFI varied by zone between species. All nMDS and ANOSIM analyses were performed using the software package Primer-e (Primer-e 6, Primer-E ltd, Plymouth, England).
Results
Shark tagging
Fish Bay
From 2006 to 2011, 30 YOY and one juvenile blacktip sharks (11 females and 20 males) ranging in size from 42 to 69 cm (X ± SD = 51 ± 6 cm, n = 31) and 14 YOY and three juvenile lemon sharks (10 females and 7 males) ranging in size from 52 to 103 cm (X ± SD = 61 ± 13 cm, n = 17) were acoustically tagged in Fish Bay during six annual tagging trips (Table 1). Individual blacktip sharks logged 8–172,358 (X ± SD = 9,920 ± 31,318, n = 31) detections (total = 307,534 detections) and 3–471 (X ± SD = 114 ± 122, n = 31) detections day−1. Tagged lemon sharks were detected 3–3,194 (X ± SD = 614 ± 863, n = 14) times (total = 10,430 detections) logging 1–200 (X ± SD = 29 ± 47, n = 14) detections day−1. No individuals of either species tagged in Fish Bay experienced immediate post-release mortality.
Coral Bay
From 2008 to 2011, 32 YOY and one juvenile blacktip sharks (20 females and 13 males) ranging in size from 45 to 63 cm (X ± SD = 51 ± 4, n = 33) and 21 YOY and four juvenile lemon sharks (13 females and 12 males) ranging in size from 48 to 70 cm (X ± SD = 59 ± 6, n = 25) were tagged within Coral Bay during four annual tagging trips (Table 1). Tagged blacktip sharks logged from 12 to 94,844 (X ± SD = 15,184 ± 21,303, n = 33) detections (total = 485,910 detections) and 2–2,107 (X ± SD = 255 ± 389, n = 33) detections day−1. Tagged lemon sharks logged a total of 187,240 detections with individuals logging 1–49,032 (X ± SD = 7,801 ± 12,325, n = 25) detections and 1–449 (X ± SD = 65 ± 95, n = 25) detections day−1. Based on continuous detections at a single receiver (i.e., lack of movement), two blacktip sharks were believed to have died within a day of being released; data from these fish were not included in any analysis. No immediate post-release mortality was observed in lemon sharks.
Presence and residency
Fish Bay
Blacktip sharks were monitored within Fish Bay during all months of the year with the greatest number of tagged individuals detected between June and October (Figs. 2 and 3). Monitoring periods ranged from 1 to 1,052 (X ± SD = 80 ± 189, n = 31) days, with individuals present within the bay for 1–1,043 (X ± SD = 74 ± 189, n = 31) days. Blacktip residency indices were generally high for each year of the study (X ± SD = 0.90 ± 0.22, n = 31), and sharks were present for periods of 1–431 (X ± SD = 22 ± 33, n = 31) consecutive days; some periodic movement in and out of the bay was evident during each year of the study, although individuals were absent for no more than 35 consecutive days (Table 3).
Lemon sharks were monitored within Fish Bay during all months of the year with the greatest number of individuals detected from May to August (Figs. 2 and 3). A relatively sharp drop in presence was observed in September. Individual monitoring periods ranged from 1 to 340 (X ± SD = 66 ± 95, n = 17) days during which individuals were present for 1–243 (X ± SD = 32 ± 58, n = 17) days (Table 3). Residency indices were broad, ranging from 0.01 to 1.00 (X ± SD = 0.73 ± 0.33, n = 17) and the number of consecutive days present varied from 1 to 42 (X ± SD = 5 ± 4, n = 17) throughout each year of the study. Some periodic movement outside of the bay was apparent, although sharks were absent for no more than 32 days.
Coral Bay
Blacktip sharks were detected within Coral Bay throughout all months of the year (Figs. 3 and 4) with the greatest number of sharks observed from May to September. Peak abundance appeared to occur in June and July, with gradual decreases in presence from July to October. Throughout the study, individuals were monitored for periods of 2–261 (X ± SD = 61 ± 62, n = 32) days and were present within the bay for 2–193 (X ± SD = 54 ± 51, n = 32) days (Table 3). Residency index values were generally high for each year of the study (X ± SD = 0.95 ± 0.17, n = 32). Some periodic movement in and out of Coral Bay was evident during each year of the study; the number of consecutive days present and absent ranged from 1 to 193 (X ± SD = 29 ± 28, n = 32) and from 0 to 48 (X ± SD = 4 ± 6, n = 32) days, respectively.
Lemon sharks were detected within Coral Bay during all months of the year with the greatest number of sharks observed during the summer months (June to September) (Figs. 3 and 4). A gradual reduction in shark presence was observed from July to October. Individuals were monitored for periods of 1–429 (X ± SD = 143 ± 122, n = 24) days and observed to be present within the bay for 1–326 (X ± SD = 116 ± 108, n = 24) days (Table 3). Residency indices were generally high for each year of the study (X ± SD = 0.86 ± 0.20, n = 24), although five individuals exhibited weak residency in the bay. The number of consecutive days present varied from 1 to 165 (X ± SD = 15 ± 10, n = 24) days throughout each year of the study. Some periodic movement outside of the study site was apparent, although sharks were absent for no more than 40 consecutive days.
Habitat use and site fidelity
Fish Bay
Juvenile blacktip and lemon sharks were detected on nearly all of the receivers deployed within Fish Bay, although individual receiver detection indices varied considerably within and between species (Table 2). Blacktip sharks were detected more frequently than lemon sharks and were observed most often by the northernmost receiver (Station 1), and a group of three receivers (Stations 4, 5, and 6) deployed in (relatively) deeper water (0.9–3.0 m) near the center of the bay. Lemon sharks were detected most frequently by two receivers deployed in shallow water (0.9–1.2 m) on an extensive seagrass bed that occurs at the northern and eastern extent of the bay (Table 2; Fig. 1).
Coral Bay
Juvenile blacktip and lemon sharks were detected on nearly all receivers deployed within Coral Bay with each species being detected most frequently by different receivers (Table 2). In general, lemon shark activity spaces were located within Zone 3 at the southern extent of Coral Bay, while blacktip activity space was generally distributed throughout Zone 1, and to a lesser extent Zone 2, near the northern reaches of the bay (Figs. 1 and 5). Blacktip activity spaces were generally slightly larger than those of lemon sharks (Table 4); there was no effect of sex or FL on 50 % (GLM-sex: t = −1.14, P = 0.27; FL: t = −0.31, P = 0.27) and 95 % (GLM-sex: t = –1.14, P = 0.76; FL: t = −0.32, P = 0.75) TAS estimates for either species. There was no evidence of diel patterns in lemon shark 50 % (t Test: t21 = −1.04, P = 0.31) or 95 % (t Test: t21 = −0.35, P = 0.73) activity spaces; however, blacktip sharks exhibited greater nighttime 50 % (t Test: t26 = −5.40, P < 0.05) and 95 % (t Test: t26 = −4.93, P < 0.05) activity spaces (Table 4).
Map of activity spaces for all blacktip and lemon sharks monitored within Coral Bay for at least 5 days. Top panels compare 95 and 50 % activity space and core blacktip shark activity space a was located within inner Coral Harbor and core lemon shark activity space b within Johnson’s Bay. Panel c is 95 % Day and 95 % Night activity space for blacktip sharks. Night activity space was significantly larger than day activity space for Blacktip sharks. Panel d is 95 % Day and 95 % Night activity space for lemon sharks with no difference between Day and Night activity spaces. Seagrass (light gray area) and mangrove (red) habitat, along with the National Park (hatch)
Tagged YOY and juvenile blacktip and lemon sharks exhibited strong site fidelity to areas/zones of Coral Bay that were consistent with locations of 50 % activity space and tagging locations for each species All blacktip sharks were tagged in Zone 1 (n = 33) and exhibited strong site fidelity to that zone, while 92 % (23/25) of lemon sharks tagged in zones 1 (n = 4) and 3 (n = 21) exhibited strong fidelity to their tagging zone. Non-metric multidimensional scaling plots generated using all SFI data from each year revealed distinct patterns of site fidelity, with blacktip sharks displaying strong site fidelity to Zones 1 and 2 and lemons sharks to Zone 3 (Fig. 6). Results of an ANOSIM indicated that blacktip and lemon sharks exhibited significantly different site fidelity to the designated zones of Coral Bay (ANOSIM, r = 0.90, N = 39, P = 0.1 %), thereby providing evidence for habitat partitioning between these two species in this embayment. For blacktip sharks, SFI values ranged from 0.50 to 1.00 (X ± SD = 0.97 ± 0.02, n = 26) within Zone 1, from 0.00 to 1.00 (X ± SD = 0.59 ± 0.02, n = 26) within Zone 2, and from 0.00 to 0.66 (X ± SD = 0.07 ± 0.03, n = 26) within Zone 3, resulting in 100 % (n = 26), 58 % (n = 15), and 4 % (n = 1) of individuals exhibiting strong site fidelity (i.e., SFI ≥ 0.5) to these zones, respectively. Lemon shark SFI values ranged from 0.00 to 1.00 (X ± SD = 0.14 ± 0.08, n = 13) within Zone 1, from 0.00 to 0.14 (X ± SD = 0.02 ± 0.0.1, n = 13) within Zone 2, and from 0.00 to 1.00 (X ± SD = 0.85 ± 0.08, n = 13) within Zone 3, resulting in 15 % (n = 2), 0 % (n = 0), and 85 % (n = 11) of individuals exhibiting strong site fidelity to these zones, respectively.
Non-metric multidimensional scaling plot generated using site fidelity index (SFI) data from blacktip sharks (n = 26) and lemon sharks (n = 13) monitored greater than 5 days. Distinct clusters are visible, comprising groups of blacktip sharks that displayed strong site fidelity to Zones 1 and Zone 2 and lemon sharks that displayed strong site fidelity to Zone 3. Dispersal of points around these clusters is indicative of sharks that displayed varying degrees of site fidelity to multiple zones (i.e., SFI ≈ 0.1–0.4)
Discussion
This manuscript presents the results of a complex 6-year study that examined the spatial and temporal dynamics of habitat use by YOY and juvenile blacktip and lemon sharks within two coastal embayments, Fish Bay and Coral Bay, in St John, USVI. Collectively, our findings suggest that each area supports nursery habitat for young individuals and, therefore, plays an integral role in the health of both blacktip and lemon shark populations in the USVI. We first provide a separate discussion of our results relative to each embayment, followed by a broader discussion of general trends that were observed within both embayments, and finally a discussion of potential management implications.
Fish Bay
Both blacktip and lemon sharks exhibited relatively high residency within Fish Bay, although some periodic movement in and out of the nursery was evident. In general, periods of absence were relatively brief (2–3 days); however, some individuals spent extended periods (weeks) outside of the nursery during which they were detected in adjacent bays (Pittman et al. 2014) along the south shore of St. John (Fig. 2) before returning to Fish Bay. Interestingly, YOY sharks generally did not commence these excursions until the mid- to late summer (July to September), suggesting that newborn individuals do not regularly leave core habitats during the first weeks/months of life (Heupel et al. 2007; Chapman et al. 2009). It should be noted, however, that our inability to monitor individuals throughout the entirety of Fish Bay (discussed below) likely resulted in an underestimation of the number of days individuals were present within the embayment and may have precluded our ability to determine whether the lack of detections during a given time period was indicative of absence (from Fish Bay) or simply the movement to areas devoid of receiver coverage (e.g., shallow seagrass habitat at the northern and eastern edge of the bay). Close examination of individual shark detection histories revealed that receiver detections immediately preceding and following short periods of absence (i.e., hours to days) occurred almost exclusively within the interior of the bay in close proximity to these shallow, unmonitored areas. Given the lack of detection on receivers in the deeper water near the mouth of Fish Bay during these periods, it is likely that individuals remained within the bay during brief periods of absence/lack of detection. Interestingly, during the latter stages of an individual’s residency within Fish Bay, the last detections prior to a prolonged (i.e., days) absence occurred more frequently on receivers closer to the mouth of the bay, suggesting that sharks may have undergone forays outside of Fish Bay more frequently during the latter stages of their residency. Regardless, we are confident that the receiver array within Fish Bay was sufficient to monitor the general presence/absence of both species throughout the study period and confirm the results of DeAngelis et al. (2008) that Fish Bay serves as an important nursery area.
The analysis of receiver detection ranges within Fish Bay provided strong evidence that the array was insufficient to capture the fine-scale habitat use of blacktip and lemon sharks throughout all available habitats. Originally designed to monitor movements of queen conch (Strombus gigas), the receivers within Fish Bay were generally deployed in deeper waters near the center of the embayment (Doerr and Hill 2010), resulting in incomplete coverage in the shallower (<1 m) areas of the bay (Supplementary Fig. 1) that are utilized by both species, particularly lemon sharks (DeAngelis 2006; DeAngelis et al. 2008; Legare 2011). For example, lemon sharks that were present in Fish Bay for at least five total days logged an average of 33 (range 3–200) detections day−1 and were observed nearly four times less often (on average) than blacktips [mean (range) 130 (36–471) detections day−1]. By comparison, both species were detected twice as often (on average) within the more extensive receiver array in Coral Bay [blacktip 255 (2–2,107) detections day−1; lemon 69 (8–449) detections day−1]. Thus, although approximately 56 % of the available habitat in Fish Bay was within the detection range of the receiver array, the lack of coverage in the shallow seagrass habitat at the periphery of bay precluded the detailed comprehensive assessment of fine-scale habitat use and site fidelity of blacktip and lemon sharks throughout the entirety of the bay.
Despite limited receiver coverage in Fish Bay, the available data provided additional evidence of habitat partitioning between blacktip and lemon sharks within this nursery area as originally suggested by DeAngelis et al. (2008). In their study, longline catch data and limited active acoustic tracking indicated that young blacktip sharks utilized a wide range of depths (0.5–13 m) and substrates (e.g., seagrass, macroalgae, reef, and sand) over a broad area of Fish Bay, while YOY and juvenile lemon sharks remained almost exclusively within shallow (<1 m) mangrove-fringed seagrass habitat along the shoreline of the bay. In this study, we did not monitor these shallow seagrass areas, but the paucity of overall detections from the majority of lemon sharks suggests that these sharks occurred primarily in areas devoid of receiver coverage. In contrast, the detection of blacktip sharks nearly four times more frequently at numerous receiver stations (Table 2) suggests that blacktip sharks occupied deeper habitat near the center of the bay considerably more often than lemon sharks. Taken together, these observations suggest that tagged individuals exhibited patterns of habitat use similar to that described by DeAngelis et al. (2008).
Coral Bay
Our acoustic detection data in conjunction with catch data reported by DeAngelis (2006) provide strong evidence that Coral Bay serves as a nursery area for both YOY and juvenile blacktip and lemon sharks. Heupel et al. (2007) proposed three criteria for an area to be considered a shark nursery: (1) sharks are more commonly encountered in the area when compared to other areas; (2) sharks have a tendency to remain or return for extended periods; and (3) the area or habitat is repeatedly used across years. In this study, YOY individuals of both species were observed in Coral Bay during each year of sampling, indicating that new cohorts recruit into the bay annually. In addition, tagged individuals were also observed to remain within the embayment for extended periods (days to months) and to return to Coral Bay (for extended periods) after being absent for several weeks/months. Capture data from this study and longline survey records from DeAngelis (2006) also suggest that both blacktip and lemon sharks were observed more frequently within Coral Bay than adjacent areas of St. John. Taken together, these findings suggest that Coral Bay meets the proposed criteria of Heupel et al. (2007) for a shark nursery. Clearly, this embayment provides secondary nursery habitat (as defined by Bass 1978) for both species, but the extent to which Coral Bay serves as a primary nursery remains unknown because gravid sharks and/or parturition has yet to be observed (DeAngelis 2006, 2008; Legare 2011).
Various metrics provided evidence of habitat partitioning between blacktip and lemon sharks within Coral Bay. Despite being detected throughout the majority of the bay, each species’ core habitat (i.e., 50 % TAS) occurred in markedly different areas with individuals displaying relatively high site fidelity to those areas. For example, YOY and juvenile blacktip core habitat occurred primarily in the northern portion of Coral Bay (i.e., Zones 1 and 2), which is characterized by water depths of 1.5–6 m, seagrass, and sand/mud substrate. In contrast, the majority (76 %) of lemon sharks were monitored almost exclusively in close proximity to or within shallow (<1 m), mangrove-fringed seagrass habitat in Zone 3 at the southern extent of Coral Bay. Although numerous blacktip sharks were also detected in Zone 3, only one individual spent more than three consecutive days in this Zone, and no individuals were detected in the area with the greatest lemon shark presence (Station 21). This pattern of habitat use and space partitioning is very similar to that observed in Fish Bay. The habitat selection observed in Fish Bay and Coral Bay is consistent with those previously described for both species (Morrissey and Gruber 1993; Ward-Paige et al. 2014).
Blacktip and lemon sharks were detected relatively infrequently by receiver stations 12–18 within Zone 2 of Coral Bay (Table 2). While there are several factors that may contribute to the limited use of this area, this behavior is likely linked to predator avoidance. These receiver stations, which are positioned in the center of Coral Bay, occurred in deeper water (mean 5.1 m; range 2.7–9.1 m) than those receivers that detected individuals of both species more regularly (i.e., stations 1–5, 8, 20, 21; X = 1.7 m; range 0.9–2.7 m). Several studies have suggested that depth is a major factor influencing the distribution of YOY and juvenile blacktip and lemon sharks with individuals avoiding deeper water as a means of predator avoidance (e.g., Morrissey and Gruber 1993; Heupel and Hueter 2001; DeAngelis et al. 2008; Ward-Paige et al. 2014). In addition, stations 11, 13, and 16 were located in close proximity to the mouth of Coral Bay where larger sharks have been observed (e.g., Carcharhinus acronotus, Carcharhinus perezi, Galeocerdo cuvier; DeAngelis 2006, B. DeAngelis pers observation). Regardless of the factors, both species did not spend large amounts of time within this area of Coral Bay, with the observed detections likely occurring during movement between more favorable habitats in Zones 1 and 3 or emigration from the embayment.
General trends in habitat use
Despite variability in the timing of annual tagging trips, peak blacktip and lemon shark presence within each bay occurred during the period from May to August, immediately following the purported timing of parturition in both species (i.e., May–early June; DeAngelis 2006, 2008). This observation, coupled with the fact that the majority of blacktip (97 %) and lemon (83 %) sharks tagged during this study were YOY, suggests that the apparent peak in shark abundance was driven by the annual recruitment of new YOY cohorts into each embayment during the late spring. However, despite the apparent reduction in shark abundance during the fall months, tagged sharks were present in both bays year round, with some individuals remaining for several years following tagging.
The small tropical bays investigated in this study, which represent habitats common throughout the Caribbean, differ from other blacktip and lemon shark nurseries previously studied. For example, lemon sharks tagged in Bimini (The Bahamas) occupy much larger nursery areas for multiple years (Morrissey and Gruber 1993; Chapman et al. 2009), while YOY lemon sharks in subtropical southwest Florida utilize expansive estuarine nursery habitat only in the summer and fall (Hueter and Tyminski 2007; Steiner et al. 2007). Based on catch data, Henderson et al. (2010) also reported short-term residency of juvenile lemon sharks over broader spatial scales in the nearshore nurseries of the Turks and Caicos Islands (Henderson et al. 2010). In addition, blacktip shark nursery areas in the subtropical continental USA from North Carolina to Texas are orders of magnitude larger than those in the current study (i.e., 10–900 km2; Heupel et al. 2007; Hueter and Tyminski 2007; Heithaus 2007; Abel et al. 2007; Parsons and Hoffmayer 2007; Steiner et al. 2007; Ulrich et al. 2007), with individuals exhibiting seasonal residency. The size of the embayments may play a large role in the presence of both blacktip and lemon sharks, and the limited availability of similar habitat emphasizes the importance of protecting these areas.
The decrease in the number of both species of sharks detected in both bays during the late summer and early fall (August–October) may be associated with emigration, mortality, or both. Emigration may be driven by a variety of biotic and abiotic factors including temperature (e.g., Castro 1993; Hopkins and Cech 2003; Heupel et al. 2007; Hueter and Tyminski 2007; Carlisle and Starr 2009; Kneebone et al. 2012), photoperiod (Grubbs et al. 2007; Kneebone et al. 2012), and seasonal prey abundance (Simpfendorfer and Milward 1993; Heupel et al. 2007). In this study, no direct statistical analyses were performed to examine the effect of these factors, yet the limited seasonal fluctuations in day length (11–13 h) and ambient water temperature (25–32 °C) observed in these bays suggest that neither of these environmental factors greatly influenced the seasonal reduction in abundance. Although we did not assess prey abundance within Fish Bay and Coral Bay, teleosts, which comprise the majority of young blacktip and lemon sharks’ diets (Cortes and Gruber 1990; Bethea et al. 2004; Newman et al. 2011), exhibit seasonal shifts in abundance within mangrove and seagrass habitats on neighboring St. Thomas (Boulon 1992) and Puerto Rico (Rooker and Dennis 1991). Should similar trends in teleost abundance occur in Fish Bay and Coral Bay, it is possible that decreases in prey abundance during the late summer and early fall increase both intra- and inter-species competition and elicit the movement of individuals out of nursery habitat. The additional investigation of factors that influence the emigration of sharks from these nurseries is warranted.
Mortality is another major factor that may influence the decrease in shark abundance in these bays. In this study, 24 and 28 % of the tagged sharks emigrated from Fish Bay and Coral Bay (based on detection by receivers outside the bays), respectively, while the balance of the tagged sharks was last detected within each bay, suggesting that they may have died within the nurseries. While it is difficult to distinguish the relative contribution of emigration vs. mortality on the observed trends in shark abundance, high rates of natural mortality observed in YOY blacktip (e.g., 61–92 %, Heupel and Simpfendorfer 2002) and lemon sharks (35–76 %, Gruber et al. 2001; Freitas et al. 2009) while occupying nursery habitat suggest that mortality is likely a factor.
The patterns of YOY and juvenile blacktip and lemon shark habitat use and site fidelity observed in this study suggest that sharks inhabit core areas of these St. John nurseries to seek refuge from predators. It has been demonstrated that both species exhibit strong site fidelity to shallow habitats and make infrequent excursions beyond core habitats as a means of predator avoidance (e.g., Gruber et al. 1988; Morrissey and Gruber 1993; Heupel et al. 2004; Heupel and Simpfendorfer 2005b; Wetherbee et al. 2007; Yeiser et al. 2008; Murchie et al. 2010). In addition, we observed conspecific groups of young blacktip and lemon sharks swimming in shallow water, a behavior associated with predator avoidance (Heupel and Simpfendorfer 2005b; Guttridge et al. 2009). Collectively, these observations suggest that predatory avoidance may also dictate habitat use in these nurseries.
In Fish Bay, we hypothesize that the limited availability of suitable habitat (i.e., the bay is relatively small) also likely exacerbates competition for both space and prey resources and drives the apparent habitat partitioning between adjacent and seemingly connected habitats. In contrast, due to the greater spatial extent of Coral Bay, blacktip and lemon sharks are not forced to partition habitats on a fine scale, but instead appear to minimize competition by occupying two separate areas of the nursery. However, it is important to note that habitat partitioning may not be solely driven by competition. For example, inherent morphological (e.g., coloration) and physiological (e.g., respiratory needs) adaptations may dictate the habitat use of a species more so than any external factor. It has also been shown that the presence of dominant individuals of the same or different species can cause shifts in habitat use (Romey 1995; Morrell and Romey 2008).
Management implications
The results of this study hold important implications for the management and conservation of blacktip and lemon sharks in Fish and Coral bays as well as the habitat on which they depend. Clearly, both Fish Bay and Coral Bay warrant designation as EFH under the Fishery Management Plan of the US Caribbean. However, additional management measures focused at minimizing adverse anthropogenic impacts on these sensitive ecosystems (reviewed by Knip et al. 2010) are warranted. For example, these results indicate the importance of the transitional land-sea habitats (e.g., shallow sea grass flats and mangrove flats) as being the most critical areas where clear habitat dependencies exist for both species. Degradation and/or destruction of shallow seagrass and mangrove habitat may result in localized reduction in the abundance of fish and invertebrate species that serve as the main prey items of young blacktip and lemon sharks in these nearshore areas (Cortes and Gruber 1990; Heupel and Hueter 2002; Hoffmayer and Parsons 2003) and, ultimately, lead to the displacement of sharks from their core habitats or the embayments as a whole. Such displacement would be especially problematic for these species given the general lack of suitable nursery habitat (i.e., extensive shallow, protected bays) throughout St. John and other parts of the Caribbean (DeAngelis et al. 2008). Similar declines in the quantity and quality of seagrass and mangrove habitats have already adversely affected blacktip and lemon shark habitat in several geographic areas (Ellison and Farnsworth 1996; Nagelkerken et al. 2000; Feldheim and Edren 2002; Jennings et al. 2008; Dibattista et al. 2011) and accentuate the importance of protecting these habitats within Fish Bay and Coral Bay.
The finding that both blacktip and lemon sharks exhibited high site fidelity to core habitats within Fish Bay and Coral Bay suggests that enhanced protection of these areas may be achieved in part through the implementation of marine protected areas (MPAs). MPAs, which function by prohibiting or restricting any or all anthropogenic activity within a defined area, may be effective for the management and conservation of shark nursery habitat (Bonfil 1997; Heupel and Simpfendorfer 2005a; Kinney and Simpfendorfer 2009), particularly for site-attached species (Garla et al. 2006). The creation of small MPAs that prohibit or highly restrict coastal development within the northern and eastern portions of Fish Bay and within Inner Coral Harbor (Zone 1) and Johnson’s Bay (Zone 3) of Coral Bay would seemingly be highly effective at minimizing negative anthropogenic impacts on core shark habitat. However, beyond the protection of these critical habitats, increased public awareness and enforcement of shark fishing regulations is critical for the conservation of these shark nurseries given the extent to which illegal fishing for these undersized sharks (i.e., <54″/137 cm FL; NMFS 2009) occurs within each embayment (DeAngelis et al. 2008) and the broader surrounding region.
Given the consistent coastal geology throughout the Caribbean islands, it is likely that blacktip and lemon shark populations rely heavily on nursery habitat similar to that in Fish Bay and Coral Bay throughout the greater Caribbean. Consequently, the health of these species’ populations in the Caribbean may be linked to a limited number of small nurseries, many of which have yet to be identified and studied. Given the relative importance of shark nursery areas to the maintenance of populations (Heithaus et al. 2007; Heithaus et al. 2007), the threat of continual habitat alteration and/or destruction within the USVI (Ramos-Scharrón and MacDonald 2005; Brooks et al. 2007; Smith et al. 2008) and greater Caribbean (Ellison and Farnsworth 1996; Causey et al. 2002), and the lack of regulation enforcement, future efforts should focus on the identification and characterization of potential shark nursery areas and the implementation of focused management and conservation plans to ensure the long-term productivity of the shark nurseries in this broad region.
References
Abel DC, Young RF, Garwood JA, Travaline MJ, Yednock BK (2007) Habitat utilization, relative abundance, and seasonality of sharks in the estuarine and nearshore waters of South Carolina. In: McCandless C, Kohler N, Pratt Jr HL (eds) American Fisheries Society Symposium 50:110–125
Barry RP, McIntyre J (2011) Estimating animal densities and home range in regions with irregular boundaries and holes: a lattice-based alternative to the kernel density estimator. Ecol Model 222:1666–1672. doi:10.1016/j.ecolmodel.2011.02.016
Bass A (1978) Problems in studies of sharks in the southwest Indian Ocean. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates and rays. Office of Naval Research, Department of the Navy, Arlington, pp 545–594
Bates R, Davidson D, Bates D (2011) lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-38, http://CRAN.R-project.org/package=lme4
Bethea DM, Buckel JA, Carlson JK (2004) Foraging ecology of the early life stages of four sympatric shark species. Mar Ecol Prog Ser 268:245–264. doi:10.3354/meps268245
Bonfil R (1997) Status of shark resources in the southern Gulf of Mexico and Caribbean: implications for management. Fish Res 29:101–117. doi:10.1016/S0165-7836(96)00536-X
Boulon R (1992) Use of mangrove prop root habitats by fish in the northern U.S. Virgin Islands. Proc Gulf Carib Fish Inst 41:189–204
Brooks GR, Devine B, Larson RA, Rood BP (2007) Sedimentary development of Coral Bay, St. John, USVI: a shift from natural to anthropogenic influences. Caribb J Sci 43:226–243
Caribbean Fishery Management Council (1998) Essential fish habitat (EFH) generic amendment to the fishery management plans (FMPs) of the U.S. Caribbean including a draft environmental assessment, vol 1. Caribbean Fishery Management Council, San Juan, Puerto Rico
Carlisle A, Starr R (2009) Habitat use, residency, and seasonal distribution of female leopard sharks Triakis semifasciata in Elkhorn Slough, California. Mar Ecol Prog Ser 380:213–228. doi:10.3354/meps07907
Casey JG, Taniuchi T (1990) Recommendations for future shark tagging programs. NOAA Tech Rep NMFS 90:511–512
Castro JI (1993) The shark nursery of Bulls Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. Environ Biol Fish 38:37–48. doi:10.1007/bf00842902
Causey B, Delaney J, Diaz E, Dodge D, Garcia J, Higgins J, Keller B, Kelty R, Jaap W, Matos C, Schmahl C, Rogers C, Miller M, Turgeon D (2002) Status of coral reefs in the US Caribbean and Gulf of Mexico: Florida, Puerto rico, US Virgin Islands, Navassa. In: Wilkinson CR (ed) Status of coral reefs of the world: 2002: GCRMN Report. Australian Institute of Marine Science, Townsville, pp 251–276
Chapman DD, Babcock EA, Gruber SH, Dibattista JD, Franks BR, Kessel SA, Guttridge T, Pikitch EK, Feldheim KA (2009) Long-term natal site-fidelity by immature lemon sharks (Negaprion brevirostris) at a subtropical island. Mol Ecol 18:3500–3507. doi:10.1111/j.1365-294X.2009.04289.x
Cortés E, Gruber SH (1990) Diet, feeding habits and estimates of daily ration of young lemon sharks, Negaprion brevirostris (Poey). Copeia 1990:204–218
DeAngelis B (2006) The distribution of elasmobranchs in St. Thomas and St. John, United States Virgin Islands with an emphasis on shark nursery areas. Masters Thesis, University of Rhode Island, Kingston Rhode Island’
DeAngelis B, McCandless C, Kohler N, Recksiek C, Skomal GB (2008) First characterization of shark nursery habitat in the United States Virgin Islands: evidence of habitat partitioning by two shark species. Mar Ecol Prog Ser 358:257–271. doi:10.3354/meps07308
DiBattista JD, Feldheim KA, Garant D, Gruber SH, Hendry AP (2011) Anthropogenic disturbance and evolutionary parameters: a lemon shark population experiencing habitat loss. Evol Appl 4:1–17. doi:10.1111/j.1752-4571.2010.00125.x
Doerr JC, Hill RL (2010) A preliminary analysis of habitat use, movement, and migration patterns of queen conch, Strombus gigas, in St. John, USVI, using acoustic tagging techniques. Proc Gulf Carib Fish Inst 60:509–515
Ellison A, Farnsworth E (1996) Anthropogenic disturbance of Caribbean mangrove ecosystems: past impacts, present trends, and future predictions. Biotropica 28:549–565
Feldheim KA, Gruber SH, Ashley MV (2002) The Breeding Biology of lemon sharks at a tropical nursery lagoon. Proc Biol Sci 269:1655–1661
Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK (2010) Patterns and ecosystem consequences of shark declines in the ocean. Ecol Lett 13(8):1055–1071
Freitas RHAD, Rosa RS, Wetherbee BM, Gruber SH (2009) Population size and survivorship for juvenile lemon sharks (Negaprion brevirostris) on their nursery grounds at a marine protected area in Brazil. Neotrop Ichthyol 7:205–212
Garla R, Chapman D, Wetherbee B, Shivji M (2006) Movement patterns of young Caribbean reef sharks, Carcharhinus perezi, at Fernando de Noronha Archipelago, Brazil: the potential of marine protected areas for conservation of a nursery ground. Mar Biol 149:189–199. doi:10.1007/s00227-005-0201-4
Grubbs RD, Musick JA, Conrath CL, Romine JG (2007) Long-term movements, migration, and temporal delineation of summer nurseries for juvenile sandbar sharks in the Chesapeake Bay region. In: McCandless C, Kohler N, Pratt Jr HL (eds) Shark nursery grounds of the Gulf of Mexico and the East Coast waters of the United States, vol 50. American Fisheries Society Symposium, p 87–108
Gruber SH, Nelson DR, Morrissey JF (1988) Patterns of activity and space utilization of lemon sharks, Negaprion Brevirostris, in a shallow Bahamian lagoon. Bull Mar Sci 43:61–76
Gruber SH, De Marignac JRC, Hoenig JM (2001) Survival of juvenile lemon sharks at Bimini, Bahamas, estimated by mark-depletion experiments. Trans Am Fish Soc 130:376–384. doi:10.1577/1548-8659
Guttridge TL, Gruber SH, Gledhill KS, Croft DP, Sims DW, Krause J (2009) Social preferences of juvenile lemon sharks, Negaprion brevirostris. Anim Behav 78:543–548. doi:10.1016/j.anbehav.2009.06.009
Heithaus MR (2007) Shark nursery grounds of the Gulf of Mexico and the East Coast waters of the United States. In: McCandless C, Kohler N, Pratt Jr HL (eds), vol 50. American Fisheries Society Symposium, p 1–2
Heithaus MR, Burkholder D, Hueter RE, Heithaus LI, Pratt HL Jr, Carrier JC (2007) Spatial and temporal variation in shark communities of the lower Florida Keys and evidence for historical population declines. Can J Fish Aquat Sci 64:1302–1313. doi:10.1139/f07-098
Henderson A, Katherine M, Calosso M (2010) Preliminary assessment of a possible lemon shark nursery in the Turks and Caicos Islands, British West Indies. Caribb J Sci 46:29–38
Heupel MR, Hueter RE (2001) Use of an automated acoustic telemetry system to passively track juvenile blacktip shark movements. In: Sibert JR, Nielsen JL (eds) Electronic tagging and tracking in marine fisheries. Kluwer Academic Publishers, Netherlands, pp 217–236
Heupel M, Hueter R (2002) Importance of prey density in relation to the movement patters of juvenile blacktip sharks (Carcharhinus limbatus) within a coastal nursery area. Mar Freshw Res 53:543–550. doi:10.1071/MF01132
Heupel MR, Simpfendorfer CA (2002) Estimation of mortality of juvenile blacktip sharks, Carcharhinus limbatus, within a nursery area using telemetry data. Can J Fish Aquat Sci 59:624–632. doi:10.1139/f02-036
Heupel MR, Simpfendorfer CA (2005a) Using acoustic monitoring to evaluate MPAs for shark nursery areas: the importance of long-term data. Mar Tech Soc J 39:10–18. doi:10.4031/002533205787521749
Heupel M, Simpfendorfer C (2005b) Quantitative analysis of aggregation behavior in juvenile blacktip sharks. Mar Biol 147:1239–1249. doi:10.1007/s00227-005-0004-7
Heupel M, Simpfendorfer C, Hueter R (2004) Estimation of shark home ranges using passive monitoring techniques. Environ Biol Fish 71:135–142. doi:10.1023/B:EBFI.0000045710.18997.f7
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297. doi:10.3354/meps337287
Hoffmayer ER, Parsons GR (2003) Food habits of three shark species from the Mississippi Sound in the northern Gulf of Mexico. Southeast Nat 2:271–280. doi:10.1656/1528-7092
Hopkins TE, Cech JJ Jr (2003) The influence of environmental variables on the distribution and abundance of three elasmobranchs in Tomales Bay, California. Environ Biol Fish 66:279–291. doi:10.1023/A:1023907121605
Hueter RE, Tyminski JP (2007) The nurse shark, mating and nursery habitat in the Dry Tortugas, Florida. In: McCandless C, Kohler N, Pratt Jr HL (eds) American Fisheries Society Symposium, vol 50. p 194–225
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–637. doi:10.1126/science.1059199
Jennings DE, Gruber SH, Franks BR, Kessel ST, Robertson AL (2008) Effects of large-scale anthropogenic development on juvenile lemon shark (Negaprion brevirostris) populations of Bimini, Bahamas. Environ Biol Fish 83:369–377
Kinney MJ, Simpfendorfer CA (2009) Reassessing the value of nursery areas to shark conservation and management. Conserv Lett 2:53–60. doi:10.1111/j.1755-263X.2008.00046.x
Kneebone J, Chisholm J, Skomal GB (2012) Seasonal residency, habitat use, and site fidelity of juvenile sand tiger sharks Carcharias taurus in a Massachusetts estuary. Mar Ecol Prog Ser 471:165–181. doi:10.3354/meps09989
Knip DM, Heupel MR, Simpfendorfer CA (2010) Sharks in nearshore environments: models, importance, and consequences. Mar Ecol Prog Ser 402:1–11. doi:10.3354/meps08498
Knip DM, Heupel MR, Simpfendorfer CA (2012) Habitat use and spatial segregation of adult spottail sharks Carcharhinus sorrah in tropical nearshore waters. J Fish Biol 80:767–784. doi:10.1111/j.1095-8649.2012.03223.x
Kohler NE, Turner PA (2001) Shark tagging: a review of conventional methods and studies. Envir Biol Fish 60:191–224. doi:10.1023/a:1007679303082
Legare BJ (2011) Juvenile Blacktip shark (Carcharhinus Limbatus) and lemon shark (Negaprion Brevirostris) movements within two nursery areas of St. John, United States Virgin Islands (USVI). Masters Thesis, University of the Virgin Islands, St. Thomas
March D, Palmer M, Alos J, Grau A, Cardona F (2010) Short-term residence, home range size and diel patterns of the painted comber Serranus scriba in a temperate marine reserve. Mar Ecol Prog Ser 400:195–206. doi:10.3354/meps08410
Merson R (1998) Nursery grounds and maturation of sandbar sharks in the western North Atlantic. Dissertation, University of Rhode Island, Kingston Rhode Island
Morrell LJ, Romey WL (2008) Optimal individual positions within animal groups. Behav Ecol 19:909–919. doi:10.1093/beheco/arn050
Morrissey J, Gruber S (1993) Home range of juvenile lemon sharks. Copeia 2:425–434
Murchie K, Schwager E, Cooke S, Danylchuk A, Danylchuk S, Goldberg T, Suski C, Philipp D (2010) Spatial ecology of juvenile lemon sharks (Negaprion brevirostris) in tidal creeks and coastal waters of Eleuthera, The Bahamas. Envir Biol Fish 89:95–104. doi:10.1007/s10641-010-9693-y
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory dharks from a coastal ocean. Science 315:1846–1850. doi:10.1126/science.1138657
Nagelkerken I, Dorenbosch M, Verberk WCEP, Cocheret de la Moriniere E, van der Velde G (2000) Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: patterns in biotope association, community structure and spatial distribution. Mar Ecol Prog Ser 202:175–192. doi:10.3354/meps202175
Newman MJH, Paredes GA, Sala E, Jackson JBC (2006) Structure of caribbean coral reef communities across a large gradient of fish biomass. Ecol Lett 9:1216–1227. doi:10.1111/j.1461-0248.2006.00976.x
Newman S, Handy R, Gruber S (2011) Ontogenetic diet shifts and prey selection in nursery bound lemon sharks, Negaprion brevirostris, indicate a flexible foraging tactic. Environ Biol Fish 95:115–126. doi:10.1007/s10641-011-9828-9
NMFS (2006) Final consolidated atlantic highly migratory species fishery management plan. NOAA, NMFS, Office of Sustainable Fisheries, Highly Migratory Species Management Division, Silver Spring
NMFS (2009) Amendment 1 to the consolidated atlantic highly migratory species fishery management plan: essential fish habitat, highly migratory species management division, office of sustainable fisheriesdivision. NMFS, Silver Spring
Pandolfi JM, Jackson JBC, Baron N, Bradbury RH, Guzman HM, Hughes TP, Kappel CV, Micheli F, Ogden JC, Possingham HP, Sala E (2005) Are U.S. Coral reefs on the slippery slope to slime? Science 307:1725–1726. doi:10.2307/3841811
Parsons GR, Hoffmayer ER (2007) Shark nursery areas of central Louisiana’s nearshore coastal waters. In: McCandless C, Kohler N, Pratt Jr HL (eds) American Fisheries Society Symposium, vol 50. p 302–317
Passerotti M, Baremore I (2012) Updates to age and growth parameters for blacktip shark, Carcharhinus limbatus, in the Gulf of Mexico SEDAR29-WP-18. SEDAR, North Charleston
Pincock DG (2012) False detections: what they are and how to remove them from detection data Vemco, Amirix System Inc Doc-004691-08 Halifax. Nova Scotia, Canada
Pittman SJ, Monaco ME, Friedlander AM, Legare B, Nemeth RS, Kendall MS, Poti M, Clark RD, Wedding LM, Caldow C (2014) Fish with chips: tracking reef fish movement to evaluate size and connectivity of Caribbean marine protected areas. PLoS One 9(5):e96028. doi:10.1371/journal.pone.0096028
Pratt H, Otake T (1990) Recommendations for work needed to increase our knowledge of reproduction relative to fishery management. NOAA Tech Rep NMFS 90:509–510
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org/
Ramos-Scharrón CE, MacDonald LH (2005) Measurement and prediction of sediment production from unpaved roads, St John, US Virgin Islands. Earth Surf Process Landf 30:1283–1304. doi:10.1002/esp.1201
Romey WL (1995) Position preferences within groups: do whirligigs select positions which balance feeding opportunities with predator avoidance? Behav Ecol Sociobiol 37:195–200. doi:10.1007/BF00176717
Rooker JR, Dennis GD (1991) Diel, lunar and seasonal changes in a mangrove fish assemblage off southwestern Puerto Rico. Bull Mar Sci 49:684–698
Simpfendorfer CA, Milward NE (1993) Utilization of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrnidae. Environ Biol Fish 37:337–345. doi:10.1007/bf00005200
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59:23–32
Smith TB, Nemeth RS, Blondeau J, Calnan JM, Kadison E, Herzlieb S (2008) Assessing coral reef health across onshore to offshore stress gradients in the US Virgin Islands. Mar Pol Bull 56:1983–1991
Steiner PA, Michel M, O’Donnell PM (2007) Effects of Tidal Current on the Movement Patterns of Juvenile Bull Sharks and Blacktip Sharks. In: McCandless C, Kohler N, Pratt Jr HL (eds) American Fisheries Society Symposium, vol 50. p 252–265
Stevenson C, Katz LS, Micheli F, Block B, Heiman KW, Perle C, Weng K, Dunbar R, Witting J (2006) High apex predator biomass on remote Pacific Islands. Coral Reefs 26:47–51. doi:10.1007/s00338-006-0158-x
Ulrich GF, Jones CM, Driggers I, William B., Drymon JM, Oakley D, Riley C (2007) Shark nursery grounds in Sapelo Island National estuarine research reserve, Georgia. In: McCandless C, Kohler N, Pratt Jr HL (eds) American Fisheries Society Symposium, vol 50. p 126–141
United States Virgin Islands Department of Planning and Natural Resources (2012) U.S. Virgin Islands commercial & recreational Fisher’s information booklet. United States Virgin Islands department of planning and natural resources, St Thomas, VI
Ward-Paige CA, Mora C, Lotze HK, Pattengill-Semmens C, McClenachan L, Arias-Castro E, Myers RA (2010) Large-scale absence of sharks on reefs in the greater-Caribbean: a footprint of human pressures. PLoS One 5:e11968
Ward-Paige CA, Britten GL, Bethea DM, Carlson JK (2014) Characterizing and predicting essential habitat features for juvenile coastal sharks. Mar Ecol Prog Ser. doi:10.1111/maec.12151
Watsky M, Gruber S (1990) Induction and duration of tonic immobility in the lemon shark, Negaprion brevirostris. Fish Physiol and Biochem 8:207–210. doi:10.1007/bf00004459
Wetherbee BM, Gruber SH, Rosa RS (2007) Movement patterns of juvenile lemon sharks Negaprion brevirostris within Atol das Rocas, Brazil: a nursery characterized by tidal extremes. Mar Ecol Prog Ser 343:283–293. doi:10.3354/meps06920
Yeiser B, Heupel MR, Simpfendorfer CA (2008) Occurrence, home range and movement patterns of juvenile bull (Carcharhinus leucas) and Lemon (Negaprion brevirostris) sharks within a Florida estuary. Mar Freshw Res 59:489–501. doi:10.1071/MF07181
Acknowledgments
This research was supported by funding from grants from the University of Puerto Rico Sea Grant (Project No. R-31-1-10), Peter and Carol Bouyoucos, and Federal Aid in Sportfish Restoration. We thank Ron Hill and Jennifer Doerr (NMFS, Galveston, TX) for sharing equipment and data; Sharon Coldren and the Coral Bay Community Council for support and logistics; Phil Strenger (R/V GEV) for vessel time and local expertise; Maho Bay Camps, Inc. for housing; Randy Brown, Randy Fish, and Jamie Irving of the Virgin Islands Environmental Research Station for the use of their equipment and field logistics; Dr. Richard Nemeth (University of the Virgin Islands) and Dr. Simon Pittman (NOAA Biogeography Branch) for local support and data sharing; Ian Bouyoucos, Zach Whitener, and Clayton Pollock for field assistance. This is UVI Center for Marine and Environmental Studies Contribution Number 123 and Massachusetts Division of Marine Fisheries Contribution Number 54.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Righton.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Legare, B., Kneebone, J., DeAngelis, B. et al. The spatiotemporal dynamics of habitat use by blacktip (Carcharhinus limbatus) and lemon (Negaprion brevirostris) sharks in nurseries of St. John, United States Virgin Islands. Mar Biol 162, 699–716 (2015). https://doi.org/10.1007/s00227-015-2616-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2616-x