Abstract
Marine predators may undergo remarkable dietary changes through time as a result of both anthropogenic and natural changes in the environment, but this variability is often difficult to tackle and seldom incorporated into ecosystem models. This paper uses the stable isotope ratios of carbon and nitrogen in skeletal material of South American sea lions from Brazilian scientific collections to investigate whether these animals modified their diet from 1986 to 2009, as reported for other marine predators in the region. Stable isotope ratios indicated that demersal potential prey were always enriched in 13C as compared with pelagic prey. Accordingly, the absence of any statistically significant correlation between stranding year and the δ13C values of adult males indicated no major increase in the consumption of pelagic prey from 1986 to 2009. Likewise, the results of the mixing model SIAR revealed a mixed diet including pelagic and demersal prey, with a central role for demersal fishes throughout the whole period. Furthermore, SIAR suggested no major changes in the proportion of pelagic and demersal prey in the diet of adult male South American sea lions during the past three decades. Demersal fishes were also relevant prey for juvenile South American sea lions during the whole period, but they always consumed a larger proportion of pelagic prey than the adults did. These results suggest no major changes in the diet of male South American sea lions during the past three decades in southern Brazil, contrary to what has been reported for other to predators in the regions and for the species in northern Patagonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human activities have impacted most of the marine ecosystems around the world (Halpern 2008), and only retrospective studies can give us a full account of the magnitude of the change (Jackson et al. 2001). This approach has revealed that some marine predators have undergone remarkable dietary changes through time as a result of natural changes in food web structure (e.g., Trites et al. 2007; Páez-Rosas et al. 2012) and interaction with fisheries (e.g., Drago et al. 2009a; Hanson et al. 2009; Gómez-Campos et al. 2011). Ecosystem models need to account for those changes to produce realistic reconstructions of historical changes in ecosystem dynamics, but this is often impossible due to the absence of retrospective studies on the diet of marine predators.
Otariids inhabiting the southeastern coast of South America were heavily exploited since the arrival of western settlers and exploitation lasted till the second half of the twentieth century (Pérez Fontana 1943; Godoy 1963; Rodríguez and Bastida 1998; Ponce de León 2000). The northernmost rookeries of the South American sea lion (Otaria flavescens) are found in Uruguay, where <15,000 South American sea lions were estimated to survive in 1995 and the production of sea lion pups would be descending at a rate of 4.5 % per year (Páez 2006). Conversely, the numbers of South American fur seals (Arctocephalus australis) breeding in the same colonies increased since the end of commercial sealing (Vaz-Ferreira 1982; Lima and Páez 1997; Franco-Trecu et al. 2012).
The reason for the differences in the post-harvest dynamics of these two species is unknown, although Costa et al. (2004, 2006) have argued that pelagic foragers recover faster than demersal ones after exploitation because pelagic resources are usually less exploited by humans than demersal ones. South American sea lions breeding in Uruguay forage over a large area spanning from southern Brazil to northern Argentina (Rodríguez et al. 2013), with high levels of individual variability in the foraging grounds used (Zenteno et al. 2013). The same region supports important demersal fisheries, but landing biomass, catch per unit effort and mean trophic level of landings declined in the mid-1990s in some areas and currently many stocks are fully exploited or overexploited (Haimovici 1998; Vasconcellos and Gasalla 2001; Jaureguizar and Milessi 2008; Milessi and Jaureguizar 2013). As a response to the above reported changes, franciscana dolphins (Pontoporia blainvillei) decreased the consumption of some demersal sciaenid fishes (Pinedo 1994; Secchi et al. 2003; Crespo and Hall 2002) and the overall contribution of demersal fishes to the diet of marine birds declined over the past 30 years (Bugoni 2008).
Available dietary information for the South American sea lion in the region is based on scats and stomach contents analysis and revealed no evident temporal changes off southern Brazil (Rosas, 1989; Oliveira et al. 2008; Machado, 2013) and Uruguay [Riet-Sapriza et al. (2012), but see Naya et al. (2000); Szteren et al. (2004)]. However, most of the information has been collected only recently, and scats and stomach contents are not appropriate to test long-term variation in food resources, since these methods provide only a single “snapshot” of the diet of each individual just before sampling (Iverson et al. 2004). Furthermore, repeated sampling of large animals for stomach content analysis is extremely difficult and assigning scats to particular individuals is highly unlikely in crowded rookeries (Drago et al. 2010a).
Stable isotope analysis offers an alternative method to reconstruct dietary changes in marine predators over long periods of time (e.g., Drago et al. 2009a; Hanson et al. 2009; Newsome et al. 2010a). The method is based on the assumption that the stable isotope ratio in the consumer’s tissues integrates the stable isotope ratio of its prey items in a predictable manner over a long period of time, although stable isotope ratios experience a stepwise enrichment in the heavier isotope relative to prey (DeNiro and Epstein 1978; Kelly 2000). This increase is more pronounced in δ15N values (3–5 ‰), which consequently are used to assess trophic level (DeNiro and Epstein 1978; Minagawa and Wada 1984). Trophic enrichment in 13C is smaller (0.5–1.1 ‰) (Fry and Sherr 1984; Wada et al. 1991), and as a consequence, animal δ13C values are useful to identify consumption of prey with different δ13C values at a local scale, as well as foraging areas at larger geographic scales (Rau et al. 1982; Hobson et al. 1997).
Here, we use stable isotope ratios in skeletal material of South American sea lions available at scientific collections from Brazil to investigate whether major dietary shifts have occurred during the past three decades.
Materials and methods
Study site and sample collection
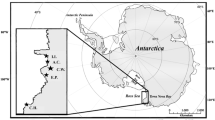
The scientific collection of Universidade Federal do Rio Grande (FURG) stores skeletal material from South American sea lions dead stranded in southern Brazil (29°S–32°S; Fig. 1) from 1986 to 1988, whereas the collection of Grupo de Estudos de Mamíferos Aquáticos do Rio Grande do Sul (GEMARS) stores skeletal material from animals dead stranded in the same area from 1994 to 2009.
Study area. South American sea lion samples were collected along the dashed line. Potential prey were collected along northern Argentina and southern Brazil. The triangles show the main breeding rookeries of South American sea lions in Uruguay, whereas the circles show the main haul-outs sites occupied by South American sea lions in southern Brazil. Potential prey were collected within the dotted polygons (Source: www.seaturtle.org)
Although the South American sea lion is one of the most frequently pinniped species observed off Brazil, there are no breeding colonies of the species in the area (Pinedo 1990; Simões-Lopes et al. 1995), and South American sea lions are thought to come from the breeding colonies in Uruguay, 300 km south of Rio Grande do Sul (Pinedo 1990; Rosas et al. 1994). Satellite telemetry has revealed that during the breeding season South American sea lions forage in a wide area ranging from southern Brazil to northern Argentina, but stable isotopes of oxygen have revealed limited exchange of adult male South American sea lions with other regions in the southwestern Atlantic Ocean (Zenteno et al. 2013).
Additional South American sea lion samples (bone and vibrissae) were collected from the scientific collection of Centro Nacional Patagónico (Puerto Madryn, Argentina) to calculate diet-to-predator discrimination factors (see below).
Maxillo-turbinal bones were initially selected for the present study, as sampling them preserved the collected skulls for further study. However, only the canine teeth of the earlier specimens had been preserved in the collection. Since stable isotope ratios of carbon and nitrogen may vary between tissues [Koch (2007), but see Riofrío-Lazo and Aurioles-Gamboa (2013)], differences in δ13C and δ15N values in paired samples of bone and dentine (all the layers after the second annuli) from 12 individuals were tested. Additional paired samples of vibrissae and bone from eight adult individuals were also analyzed to calculate a diet-to-bone and diet-to-dentine discrimination factors (see below).
South American sea lions may forage over a wide area including southern Brazil, Uruguay and northern Argentina (Rodríguez et al. 2013). The stable isotope ratios of some South American sea lion prey from southern Brazil, Uruguay and northern Argentina have been reported by Abreu et al. (2006), Bugoni et al. (2010), Botto et al. (2011) and Franco-Trecu et al. (2013a). Additional potential prey previously identified by stomach and scat analysis (Naya et al. 2000; Szteren et al. 2004, 2006; Suárez et al. 2005; Oliveira et al. 2008; Machado 2013) was collected. Samples were obtained from fishermen from Brazil (Santa Catarina and Rio Grande do Sul province) and northern Argentina (Buenos Aires province) in 2009 and 2010 (Fig. 1; Table 2). White dorsal muscle was sampled from fishes and mantle from cephalopods. All samples were stored in a freezer at −20 °C until analysis.
Sex and age determination
Sex was determined based on the external morphology (presence of bacullum bone) during sampling collection and eventually assessed according to secondary sexual characteristics of skull following Crespo (1984, 1988). Only males were considered for this study, due to the scarcity of females in the scientific collections. South American sea lions had previously been aged by counting growth layers in the dentine of the canines (assuming annual deposition) in thin ground sections or acid-etched highlighted teeth (Hohn 1980; Perrin and Myrick 1980; Crespo 1988). The life span of South American sea lions is around 20 years (Crespo 1988), and they become physiologically mature between 4 and 6 years, although mate for the first time when they are 9 years old or more (Crespo 1988; Grandi et al. 2010). Furthermore, skull growth stops at the age of 9 years (Drago et al. 2009b). Based on these data, South American sea lions 2–8 years old were considered juveniles and adolescents and those older than 8 years were considered adults. All the analysis was done independently for adults and for younger animals. Furthermore, individual age was included in the correlation analysis conducted for each age class.
Stable isotope analysis
Bone, dentine (all the layers for FURG samples) and muscle samples were thawed, dried in a stove at 60 °C for 36 h and grounded into a fine powder using a mortar and pestle. Since lipids can bias the analyses by decreasing δ13C values (DeNiro and Epstein 1977), they were removed from the samples using a sequential soak in a chloroform/methanol (2:1) solution (Bligh and Dyer 1959) and shaken with a rotator to accelerate the lipid extraction. Vibrissa was soaked in a chloroform/methanol (2:1) solution for 15 min in an ultrasonic bath. Any remaining residue on vibrissae was scrubbed off with a brush and the soaking process repeated. The samples were then dried again for 48 h at 60 °C. Vibrissae were cut into 3-mm-long consecutive sections starting from the proximal end and each section analyzed separately. This is because each section integrates diet during 1 month (Hirons et al. 2001; Cherel et al. 2009; Kernalégen et al. 2012) and the results will be used latter in a different study aiming to reconstruct monthly changes in the diet of sea lions (Zenteno, unpublished data). Here, only the average values of individual vibrissa were used, because they integrate approximately the same time span than bone (Riofrío-Lazo and Aurioles-Gamboa 2013). As bones and teeth samples contain a high concentration of inorganic carbon that may add undesirable variability to δ13C (Lorrain et al. 2003), they were previously treated by soaking for 24 h in 0.05 N hydrochloric acid (HCl) to decarbonise them (Ogawa and Ogura 1997). Since acidification may modify δ15N values (Bunn et al. 1995), samples were divided into two subsamples, one used to measure δ13C values following acidification and the other to measure δ15N values prior to acidification.
Approximately 0.3 mg of vibrissae, 0.4 mg of dentine, 0.8 mg of bone and 0.3 mg of white muscle from fish and mantle from cephalopods were weighed into tin capsules (3.3 × 5 mm), combusted at 900 °C and analyzed in a continuous flow isotope ratio mass spectrometer (Flash 1112 IRMS Delta C Series EA; Thermo Finnigan, Bremen, Germany). Atropine was used as a system check for elemental analyses. Samples were processed at Centres Cientifics i Tecnològics de la Universitat de Barcelona.
The abundances of stable isotopes, expressed in delta (δ) notation, were the relative variations of stable isotope ratios expressed as per thousand (‰) deviations from predefined international standards as:
where X is 13C or 15N, and R sample and R standard are the 13C/12C and 15N/14N ratios in the sample and standard, respectively. The δ13C standard was Vienna PeeDee Belemnite (VPDB) calcium carbonate, and δ15N standard was atmospheric nitrogen (N2). International standards (ammonium sulfate, potassium nitrate, glutamic acid for δ15N and polyethylene, sucrose and glutamic acid for δ13C) were inserted after every 12 samples to calibrate the system and compensate for any drift over time. Precision and accuracy for δ13C and δ15N measurements were 0.1 and 0.3 ‰, respectively.
Suess effect correction
The content of 13C in atmospheric CO2 has decreased 0.022 per mil/year since 1960, due largely to fossil fuel burning (Francey et al. 1999; Indermühle et al. 1999). For that reason, we have corrected the original δ13C values of the skeletal material shown in Table 1 to account for such a decrease and allow comparison among samples from different periods. All the corrected δ13C values were referenced to 2009.
Stable isotope discrimination factors
The use of appropriate diet–tissue discrimination factors is one of the most important basic requirements when applying stable isotope mixing models to predict the dietary sources of a consumer and the trophic position relative to primary consumers (Newsome et al. 2010a). In pinnipeds, previous studies have assessed discriminating factors between diet and blood, skin and vibrissae (Hobson et al. 1996), but nothing is known about the diet-to-bone discrimination factor. Here, we calculated two discrimination factors using different approaches.
The first discrimination factor was calculated using previously published information about diet composition from northern Patagonia (Koen-Alonso et al. 2000), stable isotope ratios of potential prey from that area (Drago et al. 2010b) and stable isotope ratios in the bone of South American sea lions from the same area (Drago et al. 2009a). The second discrimination factor was calculated using previously published information about diet-to-vibrissa discrimination in marine mammals (Hobson et al. 1996; Newsome et al. 2010b) and the stable isotope ratios in paired samples of vibrissa and skull from the CENPAT scientific collection. This latter diet-to-bone fractionation was computed as follows:
Data analysis
Data are presented as mean ± standard deviation (SD), and significance was assumed at the 0.05 level. All statistical analyses were carried out with PASW Statistics (version 17.0 for Windows, SPSS). As long as the assumptions of normality (tested using Lilliefors’s test) and homoscedasticity (tested using Levene’s test) were met, parametric approaches (Pearson’s correlation and ANCOVA) were used.
Two-way ANOVA was used to compare the stable isotope ratios of potential prey in southern Brazil and northern Argentina. Potential prey from Uruguay was not included in the analysis because only average and standard deviation values have been published (Franco-Trecu et al. 2013a). Temporal trends in the isotopic signal of the bones and teeth of South American sea lion were investigated using partial correlation coefficients controlling for ages. Although bone and dentine integrate dietary information over long periods, stranding year was used as a temporal reference, without any attempt to calculate the central year of the time span integrated by each individual. δ13C values were corrected for the Suess effect.
Finally, SIAR, a Bayesian mixing model Stable Isotope Analysis in R (Parnell et al. 2010) package for software R (R Development Core Team 2009), was used to assess the relative contributions of potential prey species to the diet of South American sea lion males dead stranded before 1990 and after 1999. There were two reasons for that partitioning. First, only dentine samples were available before 1989 and only bone samples were available since 1994 (Table 1). Second, fisheries operating in the adjoining Argentinean–Uruguayan Common Fishing Zone suffered major changes in the average trophic level of landings during the mid-1990s (Jaureguizar and Milessi 2008; Milessi and Jeureguizar 2013). Although the significance of those changes for the availability of potential prey for South American sea lions in southern Brazil remains unknown, the exclusion from the analysis of those specimens that lived during that period aims to control such a possible influence.
SIAR estimates the probability distributions of multiple source contributions to a mixture while accounting for the observed variability in source and mixture isotopic compositions, dietary isotopic fractionation and elemental concentration. The model included prey species that were clumped into ecological groups: All the demersal fishes together, small pelagic fishes from Brazil, small pelagic fishes from Argentina, demersal pelagic cephalopods from Brazil and demersal pelagic cephalopods from Argentina. The species included in each group were selected according to previous studies analyzing stomach contents and scats from the region (Oliveira et al. 2008; Machado 2013; Naya et al. 2000; Szteren et al. 2004; Riet-Sapriza et al. 2012; Suárez et al. 2005), although they may not give full coverage of the diet due to seasonal biases in sampling. Data within each group fitted a normal distribution, as this is required by SIAR (Parnell et al. 2010). The model was run twice, using the two sets of fractionation factors obtained in this study.
Results
The stable isotope ratios of potential prey from northern Argentina and southern Brazil are shown in Table 2. Potential prey from northern Argentina was usually depleted in 13C and enriched in 15N when compared with the same species from southern Brazil (two-way ANOVA; δ13C: F (11, 48) = 37.41, P < 0.001; δ15N: F (11, 48) = 32.15, P < 0.001). However, the species–area interaction term was statistically significant in both cases (δ13C F (11, 48) = 8.12, P < 0.001; δ15N: F (11, 48) = 12.3, P < 0.001), thus indicating that some species departed from that pattern. Nevertheless, demersal fishes from the two regions were more enriched in 13C than any other group of potential prey and small pelagic fishes from both regions were more depleted in 15N that any other group (Fig. 2). For further analysis, prey was pooled into ecological groups differing in average stable isotope ratios: demersal fishes, medium-size pelagic fishes, small pelagic fishes from Brazil, small pelagic fishes from Argentina, demersal pelagic cephalopods from Brazil and demersal pelagic cephalopods from Argentina.
Bivariated stable isotope ratios of prey and South American sea lion males from southern Brazil after correcting them with the indirect vibrissa–bone discrimination factor (a) and the direct discrimination factor (b) and their main prey from southern Brazil and northern Argentina. Bone and dentine samples are denoted by circles and triangles, respectively. Open symbols represent adult South American sea lions older than 9 years, and solid symbols represent adult South American sea lions younger than 8 years
Paired samples of bone and dentine from adult South American sea lions did not differ in average δ13C values (δ13C bone = −11.9 ± 0.4 ‰; δ13C dentine = −12.0 ± 0.5 ‰; paired t test; t = 0.571, P = 0.574, n = 12 for each tissue), but dentine was depleted in 15N when compared with bone from the same individual (δ15N bone = 22.2 ± 0.8 ‰, δ15N dentine = 21.4 ± 0.6 ‰; paired t test; t = 2.763, P = 0.011, n = 12 for each tissue). Accordingly, only the δ13C values from the whole data set can be considered to analyze temporal changes while analysis of δ15N values had to be limited to the 1994–2009 period (bone samples).
When the whole data set of males South American sea lions older than 9 years was considered (years 1986–2009), stranding year and δ13C values were uncorrelated (Fig. 3a; δ13C: partial correlation, r = 0.0.038, N = 34, P = 0.834) and the same was true for the juvenile and adolescent males (Fig. 3b; δ13C: partial correlation, r = 0.332, N = 20, P = 0.165). This result is unlikely to be an artifact of combining dentine and bone δ13C values, not only because the absence of statistically significant differences above reported, but also because the variability of the δ13C values was similar in the three decades (Table 3). The coefficient of variation was always <10 % of the mean, and δ15N values were also uncorrelated when only the bone data set (1994–2009) was considered, both for adult males more than 9 years old (Fig. 3c; δ15N: partial correlation, r = −0.201, N = 26, P = 0.336) and juvenile males <8 years old (Fig. 3d; δ15N: partial correlation, r = 0.219, N = 12, P = 0.519). The variability of the δ15N values was similar in the three decades (Table 3). The coefficient of variation was always <10 % of the mean.
Temporal changes in the ratios of stable isotopes of carbon and nitrogen in South American sea lions dead stranded along the coast of southern Brazil. The lighter area represents the period of low demersal fishing intensity (LDFI-years 1975–1989) and the darker area represents the period of increasing demersal fishing intensity (HDFI-years 1990–2010), accordingly by Haimovici (1998) and Milessi and Jaureguizar (2013). Left panels presents bone and tooth dentine δ13C values from specimens older than 9 years (a) and younger than 8 years (b) stranded between 1986 and 2009. Right panels present bone δ15N values from specimens older than 9 years (c) and younger than 8 years (d) stranded between 1992 and 2009. The δ13C values were corrected for the Suess effect. See Table 2 for the original data
The expected stable isotope ratios of the diet of South American sea lions from Northern Patagonia were δ13C = −16.6 ‰ and δ15N = 17.0 ‰ (Table 4), and the stable isotope ratios of male South American sea lions bone from the same area were δ13C = −12.2 ± 0.8 ‰ and δ15N = 22.3 ± 1.3 ‰. This resulted into a diet-to-bone discrimination factor of 4.4 ± 0.8 ‰ for δ13C and 5.3 ± 1.3 ‰ for δ15N. Vibrissae of South American sea lions from northern Patagonia were depleted both in 13C and 15N relative to bone (mean δ13C: vibrissae = −13.1 ± 0.8; bone = −12.3 ± 0.8; mean δ15N: vibrissae = 21.2 ± 0.9; bone = 22.5 ± 1.5), which resulted into a diet-to-bone discrimination factor of 3.5 ± 0.8 ‰ for δ13C and 4.4 ± 0.8 ‰ for δ15N when combined with the published diet-to-vibrissa discrimination factors.
Figure 2 shows the position of potential prey and South American sea lions within the regional isoscape once the stable isotope ratios of the predator have been corrected for the Suess effect and diet-to-predator stable isotope discrimination. Most of the South American sea lion samples, independently on the tissue, were close to demersal prey when the indirect vibrissa–bone discrimination factor was used, although a few South American sea lion samples had stable isotope ratios consistent with pelagic foraging (Fig. 2a; Table 5). Conversely, the stable isotope ratios of South American sea lion samples were intermediate between those of demersal and medium-size pelagic prey when the direct prey–bone discrimination factor was used, thus suggesting more mixed diets (Fig. 2b; Table 5).
The output of SIAR confirmed that demersal and medium-size pelagic fishes dominated the diet of South American sea lions older than 9 years during the whole considered period, although the actual proportions varied according to the discrimination factor used and the importance of medium-size pelagic fishes might have increased slightly after 1994 (Figs. 4, 5; Table 5). On the other hand, pelagic prey was always more relevant for the diet of juveniles males younger than 8 years than for adults, and no major dietary shift was observed during the period considered, although the actual proportion of pelagic and demersal prey depended on the fractionation factor used (Figs. 4b, d and 5b, d).
Diet composition of male South American sea lions off southern Brazil according to SIAR mixing model and the indirect vibrissa–bone discrimination factor. The contribution of each prey to the diet is shown with 95, 75 and 50 % credibility intervals. The δ13C values of South American sea lions were corrected for the Suess effect, to allow comparison with modern preys. See Table 2 for the original data
Diet composition of male South American sea lions off southern Brazil according to SIAR mixing model and the direct bone discrimination factor. The contribution of each prey to the diet is shown with 95, 75 and 50 % credibility intervals. The δ13C values of South American sea lions were corrected for the Suess effect, to allow comparison with modern preys. See Table 2 for the original data
Discussion
South American sea lions have been reported as broad-spectrum predators (Aguayo and Maturana 1973; Koen-Alonso et al. 2000; Naya et al. 2000) and diet often overlaps, at least partially, with fisheries catch in most of their range (Koen-Alonso et al. 2000; Hückstädt and Antezana 2003; Oliveira et al. 2008; Romero et al. 2011; Riet-Sapriza et al. 2012; Machado 2013). Nevertheless, they are often considered to have a low vulnerability to the development of demersal fisheries because of a high trophic plasticity (Koen-Alonso et al. 2000; Müller 2004; Szteren et al. 2004). The data presented here confirm that adult male South American sea lions from southern Brazil had mixed demersal/pelagic diets through the study period and hence suggest that no major dietary changes happened since the 1980s.
Nevertheless, historical changes in the isotopic baseline may hinder the interpretation of retrospective studies on trophic level and food web structure (Casey and Post 2011), and thus, the interpretation of isotopic signals without relevant ecological data can be challenging. In this study, δ13C values were corrected to account for the Suess effect (Francey et al. 1999; Indermühle et al. 1999), but reference samples from historical fish and invertebrates were not available, and hence, other sources of variation were not controlled. For instance, an increase in the arrival of sewage during the last decades might have enhanced primary productivity and simultaneously increased the δ15N values of the coastal food web (Calvert et al. 1992; Wu et al. 1997). However, available evidence revealed no major changes in primary productivity in southern Brazil from 1998 to 2006 (Heileman and Gasalla 2008), and accordingly, no major change in the δ15N baseline is expected, as both parameters are strongly correlated along the coasts of the southwestern Atlantic (Saporiti et al. 2014). In any case, access to historical samples of potential prey will be extremely useful to be completely rule out changes in the stable isotope baseline during the period considered.
A second limiting factor is the existence of two tissues integrating dietary information over different time spans. Pinniped bone has been claimed to integrate dietary information throughout ~5 years, whereas canine dentine integrates dietary information through life (Riofrío-Lazo and Aurioles-Gamboa 2013). The difference is because bone is metabolically active and undergoes constant turnover, whereas dentine is metabolically inert and new layers are settled throughout the life of the individual into the open pulp cavity of the canine teeth (Riofrío-Lazo and Aurioles-Gambioa 2013). However, the actual significance of these differences for diet reconstruction is probably limited to young individuals. Suckling pinniped pups are more enriched in 15N than their mothers, whereas the relationship between suckling pups and their mothers is less clear for 13C and may be species dependent (Ducatez et al. 2008; Drago et al. 2009b; Newsome et al. 2010a). The suckling signal remains forever in the dentine formed during the first year of life, but fades from bone after 1 or 2 years due to tissue turnover (Drago et al. 2009b; Newsome et al. 2010a). Accordingly, the dietary reconstructions using dentine and bone from individuals older than 2 years may lead to different conclusions about trophic level. However, the impact of the suckling signal on the overall stable isotope ratio of dentine decreases as new layers are settled and is expected to have a negligible impact on adults, where represents <1/9 of dentine.
Independently of these obscuring factors, the results here reported reveal a remarkable dietary stability of both adults and juveniles during 30 years, although there is a high level of individual variability during the whole time span of the study, independently on the age class and tissue considered. There are at least two possible caused for such variability. First, South American sea lions forage over a wide area including southern Brazil, Uruguay and northern Argentina (Rodríguez et al. 2013), and prey from those regions is known to differ in their stable isotope ratios (Abreu et al. 2006; Bugoni et al. 2010; Botto et al. 2011; Franco-Trecu et al. 2013a; this study). We are uncertain about the actual foraging area used by each individual and for how long they foraged off southern Brazil, but stable isotopes of oxygen suggest some individual differences in the foraging grounds used (Zenteno et al. 2013). Second, the existence of different individual foraging strategies cannot be excluded, as the stable isotope ratios of some adult males are closer to those of midsize pelagic fishes than to those of demersal ones. Nevertheless, stomach content analysis (Oliveira et al. 2008; Machado 2013) and stable isotope analysis (this study) agree in identifying demersal fishes as the staple food of South American sea lions in southern Brazil. Scat analysis indicates that females breeding in Uruguay also forage primarily on demersal fishes, at least during the breeding season (Riet-Sapriza et al. 2012).
There are at least two non-excluding explanations for the intense use of demersal prey by adult South American sea lions, despite of the high abundance of pelagic prey in the study area. Firstly, a selection based on prey size, as benthic prey is usually larger than pelagic prey. Secondly, a preference for benthic prey would be explained by their more sedentary behavior (Womble and Sigler 2006) and the permanent motion of pelagic prey (Gende and Sigler 2006). The first hypothesis is supported by the larger size of the demersal prey consumed by South American sea lions when compared with that of pelagic prey (Szteren et al. 2004; Riet-Sapriza et al. 2012), although pelagic prey has a higher energy density (Drago et al. 2009a).
Demersal fishes also had a central role in the diet of juvenile and adolescent males, but small and medium pelagic fishes represented the bulk of their diet. Ontogenic dietary changes in pinnipeds are often related to somatic growth and the associated improvement in diving performance (Gentry et al. 1986; Horning and Trillmich 1997; Costa et al. 2004). South American sea lions are not an exception, and they dive deeper (Rodríguez et al. 2013) and increase the consumption of demersal prey as they grow older (Drago et al. 2009b). This was also the pattern observed in the present study and suggests that the scats from unknown individuals with a high proportion of small pelagic fish (Naya et al. 2000; Szteren et al. 2004) likely represent the diet of juvenile and adolescent South American sea lions.
The dietary stability of the South American sea lion Otaria flavescens in southern Brazil is opposite to the dietary changes reported from northern and central Patagonia, where South American sea lions have increased the consumption of pelagic prey since the 1970 (Koen-Alonso et al. 2000; Drago et al. 2009a; Romero et al. 2011), in parallel to the development of the bottom trawling fishery but also to the increase in the South American sea lion population resulting from legal protection (Drago et al. 2009a). On the contrary, the population of the South American sea lion is decreasing in Brazil, Uruguay and northern Argentina (Páez 2006). This suggests that the per capita availability of demersal prey for the South American sea lion may have declined in northern Patagonia but remained stable in southern Brazil during the last three decades, which may explain why diet changed dramatically in the former (Drago et al. 2009a) but remained stable in the latter (this study). On the contrary, franciscana dolphins and sea birds from northern Argentina and southern Brazil have shifted diets during the past three decades (Pinedo 1994; Secchi et al. 2003; Crespo and Hall 2002; Bugoni 2008), which suggest species-specific responses to environmental changes, probably linked to differences in body size and diving performance (Páez-Rosas et al. 2012).
In conclusion, the results reported here do not support a major dietary shift for male South American sea lions during the past three decades in southern Brazil, opposite to the pattern reported in other top predators in the region which may be related to differences in body size and population dynamics (Drago et al. 2011). Certainly, females have not been considered in this study, but recent published information based on scat analysis and stable isotopes suggests a diet very similar to that of males (Riet-Sapriza et al. 2012; Franco-Trecu et al. 2013b).
References
Abreu PC, Costa CSB, Bemvenuti CE, Odebrecht C, Graneli W, Anesio AM (2006) Eutrophication processes and trophic interactions in a shallow estuary: preliminary results based on stable isotope analysis (δ13C and δ15N). Estuaries Coasts 29:277–285
Aguayo A, Maturana R (1973) Presencia del lobo marino común Otaria flavescens en el litoral chileno. I. Arica (18°20´S) a Punta Maiquillahue (39°27´S). Biología Pesq 6:45–75
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Botto F, Gaitan E, Mianzan H, Acha M, Giberto D, Schiariti A, Iribarne O (2011) Origin of resources and trophic pathways in a large SW Atlantic estuary: an evaluation using stable isotopes. Estuar Coast Shelf Sci 92:70–77
Bugoni L (2008) Ecology and conservation of albatrosses and petrels at sea off Brazil. Dissertation. University of Glasgow, Scotland
Bugoni L, McGill R, Furness RW (2010) The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. J Exp Mar Biol Ecol 391:190–200
Bunn SE, Loneragan NR, Kempster MA (1995) Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: implications for food-web studies using multiple stable isotopes. Limnol Oceanogr 40:622–625
Calvert SE, Nielsen B, Fontugne MR (1992) Evidence from nitrogen isotope ratios for enhanced productivity during the formation of eastern Mediterranean sapropels. Nature 359:223–225
Casey M, Post D (2011) The problem of isotopic baseline: reconstructing the diet and trophic position of fossil animals. Earth Sci Rev 106:131–148
Cherel Y, Kernaléguen L, Richard P, Guinet C (2009) Whisker isotopic signature depicts migration patterns and multi-year intra- and inter-individual foraging strategies in fur seals. Biol Lett 5:830–832
Costa DP, Kuhn CE, Weise MJ, Shaffer SA, Arnould JPY (2004) When does physiology limit the foraging behaviour of freely diving mammals? Int Congr Ser 1275:359–366
Costa DP, Weise MJ, Arnould JPY (2006) Potential influences of whaling on the status and trends of pinniped populations. In: Estes JA, Demaster DP, Doak DF, Williams TM, Brownell RL (eds) Whales, whaling and ocean ecosystems. University of California Press, Berkeley, pp 344–359
Crespo EA (1984) Dimorfismo sexual en los dientes caninos y en los cráneos del lobo marino del sur, Otaria flavescens (Pinnipedia, Otariidae). Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 13:245–254
Crespo EA (1988) Dinámica poblacional del lobo marino de un pelo Otaria flavescens (Shaw, 1800), en el norte del Litoral Patagonico. Dissertation, Universidad Nacional de Buenos Aires
Crespo EA, Hall MA (2002) Interactions between aquatic mammals and humans in the context of ecosystem management. In: Evans PGH, Raga JA (eds) Marine mammals: biology and conservation. Academic Publishers, New York, pp 463–490
DeNiro MJ, Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197:261–263
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Drago M, Cardona L, Crespo EA, Aguilar A (2009a) Ontogenic dietary changes in South American sea lions. J Zool 279:251–261
Drago M, Crespo EA, Aguilar A, Cardona L, García N, Dans SL, Goodall N (2009b) Historic diet change of the South American sea lion in Patagonia as revealed by isotopic analysis. Mar Ecol Prog Ser 384:273–289
Drago M, Cardona L, Aguilar A, Crespo EA, Ameghino S, García N (2010a) Diet of lactating South American sea lions, as inferred from stable isotopes, influences pup growth. Mar Mamm Sci 26:309–323
Drago M, Cardona L, Crespo EA, Ameghino S, Aguilar A (2010b) Change in the foraging strategy of female South American sea lions (Carnivora: Pinnipedia). Sci Mar 74:589–598
Drago M, Cardona L, García N, Ameghino S, Aguilar A (2011) Influence of colony size on pup fitness and survival in South American sea lions. Mar Mam Sci 27:167–181
Ducatez S, Dalloyau S, Richard P, Guinet C, Cherel Y (2008) Stable isotopes document winter trophic ecology and maternal investment of adult female southern elephant seals (Mirounga leonina) breeding at the Kerguelen Islands. Mar Biol 155:413–420
Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, Langenfelds RL, Michel E, Steele LP (1999) A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B 51:170–193
Franco-Trecu V, Aurioles-Gamboa D, Arim M, Lima M (2012) Prepartum and postpartum trophic segregation between sympatrically breeding female Arctocephalus australis and Otaria flavescens. J Mamm 93:514–521
Franco-Trecu V, Aurioles-Gamboa D, Inchausti P (2013a) Individual trophic specialisation and niche segregation explain the contrasting population trends of two sympatric otariids. Mar Biol 161:609–618
Franco-Trecu V, Drago M, Riet-Sapriza FG, Parnell A, Frau R, Inchausti P (2013b) Bias in diet determination: incorporating traditional methods in bayesian mixing models. PLoS ONE 8(11):e80019
Fry B, Sherr EB (1984) 13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Gende SM, Sigler MF (2006) Persistence of forage fish ‘hot spots’ and its association with foraging Steller sea lions (Eumetopias jubatus) in southeast Alaska. Deep Sea Res Part II 53:432–441
Gentry RL, Kooyman GL, Goebel ME (1986) Feeding and diving behaviour of northern fur seals. In: Gentry RL, Kooyman GL (eds) Fur seals: maternal strategies on land and at sea. Princeton University Press, Princeton, pp 61–78
Godoy JC (1963) Fauna Silvestre. Serie: evaluación de los Recursos Naturales de la Argentina. Consejo Federal de Inversiones, Buenos Aires
Gómez-Campos E, Borrell A, Cardona L, Forcada J, Aguilar A (2011) Overfishing of small pelagic fishes increases trophic overlap between immature and mature striped dolphins in the Mediterranean Sea. PLoS ONE 7:1–9
Grandi MF, Dans SL, García NE, Crespo EA (2010) Growth and age at sexual maturity of South American sea lions. Mamm Biol 75:427–436
Haimovici M (1998) Present state and perspectives for the Southern Brazil shelf demersal fisheries. Fish Manag Ecol 5:277–289
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Hanson NN, Wurster CM, Bird MI, Reid K, Boyd IL (2009) Intrinsic and extrinsic forcing in life histories: patterns of growth and stable isotopes in male Antarctic fur seal teeth. Mar Ecol Prog Ser 388:263–272
Heileman S, Gasalla MA (2008) South brazil shelf LME. In Sherman K and Hempel G (eds) The UNEP large Marine ecosystems report: a perspective on changing conditions in LMEs of the World’s regional seas, 2nd edn. United Nations Environmental Program (UNEP), Nairobi, pp 723–734
Hirons AC, Schell DM, St. Aubin DJ (2001) Growth rates of vibrissae of harbor seals (Phoca vitulina) and Steller sea lions (Eumetobias jubatus). Can J Zool 79:1053–1061
Hobson KA, Schell DM, Renouf D, Noseworthy E (1996) Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Can J Fish Aquat Sci 53:528–533
Hobson KA, Sease JL, Merrick RL, Piatt JF (1997) Investigating trophic relationships of pinnipeds in Alaska and Washington using stable isotope ratios of nitrogen and carbon. Mar Mamm Sci 13:114–132
Hohn AA (1980) Age determination and age related factors in the teeth of western north Atlantic bottlenose dolphins. Sci Rep Whales Res Inst Tokio 32:39–66
Horning M, Trillmich F (1997) Development of hemoglobin, hematocrit, and erythrocyte values in Galapagos fur seals. Mar Mamm Sci 13:100–113
Hückstädt LA, Antezana T (2003) Behaviour of the southern sea lion (Otaria flavescens) and consumption of the catch during purse-seining for jack mackerel (Trachurus symmetricus) off central Chile. ICES J Mar Sci 60:1003–1011
Indermühle A, Stocker TF, Joos F, Fischer H, Smith HJ, Wahlen M, Deck B, Mastroianni D, Tschumi J, Blunier T, Meyer R, Stauffer B (1999) Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica. Nature 398:121–126
Iverson SJ, Field C, Don Bowen W, Blanchard W (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol Monogr 74:211–235
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury R, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner R (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Jaureguizar AJ, Milessi AC (2008) Assessing the sources of the fishing down marine food web process in the Argentinean–Uruguayan common fishing zone. Sci Mar 72:25–36
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Kernaléguen L, Cazelles B, Arnould JPY, Richard P, Guinet C, Cherel Y (2012) Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PLoS ONE 7:e32916
Koch PL (2007) Isotopic study of the biology of modern and fossil vertebrates. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Publishing, Malden, pp 99–154
Koen-Alonso M, Crespo EA, Pedraza SN, García NA, Coscarella MA (2000) Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish Bull 98:250–263
Lima M, Páez E (1997) Demography and population dynamics of South American fur seals. J Mamm 78:914–920
Lorrain A, Savoye N, Chauvaud L, Paulet Y, Naulet N (2003) Decarbonation and preservation method for the analysis of organic C and N contents and stable isotope ratios of low-carbonated suspended particulate material. Anal Chim Acta 491:125–133
Machado R (2013) Conflicto entre o Leão-marinho sul-americano (Otaria flavescens) e a pesca costeira de emalhe no sul do Brasil: Uma análise ecológica e econômico. Universidade do Valle do Rios dos Sinos, Dissertation
Milessi AC, Jaureguizar AJ (2013) Evolución temporal del nivel trófico medio de los desembarques en la Zona Común de Pesca Argentino–Uruguaya años 1989–2010. Frente Maritimo 23:83–93
Minagawa M, Wada E (1984) Stepwise enrichment of δ15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Müller G (2004) The foraging ecology of South American Sea Lions (Otaria flavescens) on the Patagonian Shelf. Ph.D thesis, Christian-Albrechts-Universität
Naya DE, Vargas R, Arim M (2000) Preliminary analysis of southern sea lion (Otaria flavescens) diet in Isla de Lobos, Uruguay. Bol Soc Zool Urug 12:14–21
Newsome SD, Clementz MT, Koch PL (2010a) Using stable isotope biogeochemistry to study marine mammal ecology. Mar Mamm Sci 26:509–572
Newsome SD, Bentall GB, Tinker MT, Oftedal OT, Ralls K, Estes J, Fogel M (2010b) Variation in δ13C and δ15N diet-vibrissae trophic discrimination factors in a wild population of California sea otters. Ecol Appl 20:1744–1752
Ogawa N, Ogura N (1997) Dynamics of particulate organic matter in the Tamagawa Estuary and inner Tokyo Bay. Estuar Coast Shelf Sci 44:263–273
Oliveira LR, Ott PH, Malabarba LR (2008) Ecologia alimentar dos pinípedes do Sul do Brasil e uma avaliação de suas interações com atividades pesqueiras. In: Reis NR, Peracci AL, Santos GASD (eds) Ecologia de Mamíferos. Technical Booksed, Londrina, pp 97–116
Páez E (2006) Situación de la administración del recurso lobos y leones marinos en Uruguay. In: Menafra R, Rodríguez-Gallego L, Scarabino F, Conde D (eds) Bases para la conservación y el manejo de la costa uruguaya. Vida Silvestre, Sociedad Uruguaya para la Conservación de la Naturaleza, Montevideo, pp 577–583
Páez-Rosas D, Aurioles-Gamboa D, Alava JJ, Palacios DM (2012) Stable isotopes indicate differing foraging strategies in two sympatric otariids of the Galapagos Islands. J Exp Mar Biol Ecol 424–425:44–52
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5(3):e9672
Pérez Fontana H (1943) Informe sobre la industria lobera. Servicio Oceanográfico y de Pesca, Montevideo
Perrin WF, Myrick AC (1980) Age determination of toothed whales and sirenians. Rep Int Whal Comm Spec Issue 3:1–50
Pinedo MC (1990) Ocorrência de Pinípedes na costa brasileira. Garcia Orla Ser Zool 15:37–48
Pinedo MC (1994) Review of the status and fishery interactions of the franciscana, Pontoporia blainvillei, and other small cetaceans of the Southern Brazil. Rep Int Whal Comm Spec Issue 15:251–259
Ponce de León A (2000) Taxonomía, sistemática y sinopsis de la biología y ecología de los pinipedios de Uruguay. In: Rey M, Amestoy F (eds) Sinopsis de la biología y ecología de las poblaciones de lobos finos y leones marinos de Uruguay. Pautas para su manejo y Administración. Parte I, Biología de las especies, Uruguay, pp 9–36
Rau GH, Sweeney RE, Kaplan IR (1982) Plankton 13C:12C ratio changes with latitude: differences between northern and southern oceans. Deep Sea Res Part II 29:1035–1039
Riet-Sapriza FG, Costa DP, Franco-Trecu V, Marín Y, Chocca J, González B, Beathyate G, Chilvers L, Hückstädt LA (2012) Foraging behavior of lactating south American sea lions (Otaria flavescens) and spatial- temporal resource overlap with the Uruguayan fisheries. Deep Sea Res Part II 88:106–119
Riofrío-Lazo M, Aurioles-Gamboa D (2013) Timing of isotopic integration in marine mammal skull: comparative study between calcified tissues. Rapid Commun Mass Spectrom 27:1076–1082
Rodríguez D, Bastida R (1998) Four hundred years in the history of pinniped colonies around Mar del Plata, Argentina. Aquat Conserv 8:721–735
Rodríguez DH, Dassis M, Ponce de León A, Barreiro C, Farenga M, Bastida RO, Davis RW (2013) Foraging strategies of Southern sea lion females in the La Plata River Estuary (Argentina–Uruguay). Deep Sea Res II 88–89:120–130
Romero MA, Dans SL, González R, Svendsen GM, García N, Crespo EA (2011) Solapamiento trófico entre el lobo marino de un pelo Otaria flavescens y la pesquería de arrastre demersal del Golfo San Matías, Patagonia, Argentina. LAJAM 39:344–358
Rosas FCW (1989) Aspectos da dinâmica populacional e interações com a pesca do leão-marinho-do-sul, Otaria flavescens (Shaw, 1800) (Pinnipedia, Otariidae) no litoral sul do Rio Grande do Sul, Brasil. 1989. Dissertation, Universidade Federal do Rio Grande
Rosas FCW, Pinedo MC, Marmontel M, Haimovici M (1994) Seasonal movements of the South American sea lion (Otaria flavescens, Shaw) off the Rio Grande do Sul coast, Brazil. Mammalia 58:51–59
Saporiti F, Bala LO, Gómez Otero J, Crespo EA, Piana EL, Aguilar A, Cardona L (2014) Paleoindian pinniped exploitation in South America was driven byoceanic productivity. Quat Int. doi:10.1016/j.quaint.2014.05.015
Secchi ER, Ott PH, Danilewicz D (2003) Effects of fishing bycatch and the conservation status of the franciscana dolphin, Pontoporia blainvillei. In: Gales N, Hindell M, Kirkwood R (eds) Marine mammals: fisheries, tourism and management issues. Commonwealth scientific and industrial research organization (CSIRO) Publishing, Melbourne, pp 174–191
Silva-Costa A, Bugoni L (2013) Feeding ecology of Kelp gulls (Larus dominicanus) in marine and limnetic environments. Aquat Ecol 47:211–224
Simões-Lopes PC, Drehmer CJ, Ott PH (1995) Nota sobre os Otariidae e Phocidae (Mammalia: Carnivora) da costa norte do Rio Grande do Sul e Santa Catarina, Brasil. Biociências 3:173–181
Suárez AA, Sanfelice D, Cassini MH, Cappozzo HL (2005) Composition and seasonal variation in the diet of the South American sea lion (Otaria flavescens) from Quequén, Argentina. LAJAM 4:163–174
Szteren D (2006) Predation of Otaria flavescens over artisanal fisheries in Uruguay: opportunism or prey selectivity? LAJAM 5:29–38
Szteren D, Naya D, Arim M (2004) Overlap between Pinniped summer diets and artisanal fishery catches in Uruguay. LAJAM 2:119–125
Trites AW, Miller AJ, Maschner HDG, Alexander MA, Bograd SJ, Calder JA, Capotondi A, Coyle KO, DiLorenzo E, Finney BP, Gregr EJ, Grosch CE, Hare SR, Hunt GL, Jahncke J, Kachel NB, Kim H, Ladd C, Mantua NJ, Marzban C, Maslowski W, Mendelssohn R, Neilson DJ, Okkonen SR, Overland JE, Reedy-Maschner KL, Royer TC, Schwing FB, Wang JXL, Winship AJ (2007) Bottom–up forcing and the decline of Steller sea lions (Eumetopias jubatus) in Alaska: assessing the ocean climate hypothesis. Fish Oceanogr 16:46–67
Vasconcellos M, Gasalla MA (2001) Fisheries catches and the carrying capacity of marine ecosystems in southern Brazil. Fish Res 50:279–295
Vaz-Ferreira R (1982) Otaria flavescens (Shaw) South American sea lion. Mamm Seas FAO Fish Ser 4:477–495
Wada E, Mizutani H, Minagawa M (1991) The use of stable isotopes for food web analysis. Crit Rev Food Sci Nutr 30:361–371
Womble JN, Sigler MF (2006) Seasonal availability of abundant, energy-rich prey influences the abundance and diet of a marine predator, the Steller sea lion Eumetopias jubatus. Mar Ecol Prog Ser 325:281–282
Wu J, Calvert SE, Wong CS (1997) Nitrogen isotope variations in the subarctic northeast Pacific: relationships to nitrate utilization and trophic structure. Deep Sea Res Part I 44:287–314
Zenteno L, Crespo E, Goodall A, Aguilar A, de Oliveira L, Drago M, Secchi E, Garcia N, Cardona L (2013) Stable isotopes of oxygen reveal dispersal patterns of the South American sea lion in the southwestern Atlantic Ocean. J Zool 291:119–126
Acknowledgments
This research was funded by Fundación BBVA through the project “Efectos de la explotación humana sobre depredadores apicales y la estructura de la red trófica del Mar Argentino durante los últimos 6.000 años” (BIOCON 08-194/09 2009–2011); Agencia Nacional de Promoción Científica y Tecnológica (PICT N° 2110); Mohamed bin Sayed Conservation (0925516); and the Zoo d’Amneville, France. At the time this manuscript was written, L.Z. was supported by a Fellowship Comsión Nacional de Investigación Científica y Tecnológica (CONICYT-Chile), F.S. was supported by a Fellowship from Ministerio de Ciencia e Innovación (Spain), and D.G.V. and L.S. were supported by a Fellowship Program from National Research Council of Argentina (CONICET). E.R.S. is sponsored by the National Council for Technological and Scientific Development CNPq–Brazil (fellowship no. 307843/2011-4). Thanks are given to the National Agency for Research and Innovation (ANII) of Uruguay to supported M.D. through a Postdoctoral fellowship. The authors would like to thank Rodrigo Machado for his assistances with the collection of GEMARS and the collection of sample of prey species in southern Brazil, Nicolás Martínez for his careful work in teeth preparation and Florencia Grandi for her collaboration as reader in the process of age determination. The Research Group “Ecologia e Conservação da Megafauna Marinha–EcoMega/CNPq” contributed to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Cherel.
Rights and permissions
About this article
Cite this article
Zenteno, L., Crespo, E., Vales, D. et al. Dietary consistency of male South American sea lions (Otaria flavescens) in southern Brazil during three decades inferred from stable isotope analysis. Mar Biol 162, 275–289 (2015). https://doi.org/10.1007/s00227-014-2597-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2597-1