Abstract
Feeding of fish depends on a spatial and temporal match with prey, and since larval and juvenile feeding can be highly selective, their preferences for given prey sizes and taxa should be considered when quantifying the actual availability of potential prey. We investigated the diet and prey preferences of the early-life stages of Atlantic cod (Gadus morhua) to quantify the availability of prey during a spring-summer season in a West Greenlandic fjord. We hypothesized that abundances of larval and juvenile cod at size were synchronized to optimal availability of preferred prey in space and time. The present analysis is based on nine cruises each covering 5 stations visited between 24 May and 5 August 2010 comparing zooplankton abundance, cod gut content and distribution patterns. Cod 4–25 mm in length preferred prey of about 5 % of their own length. During ontogeny, their preferences changed from calanoid nauplii towards Pseudocalanus spp. and Calanus spp. copepodites. The larvae/juvenile had an exceptionally high dietary contribution from cladocerans, which were highly preferred by cod larger than 9 mm, while the abundant Metridia longa and the non-calanoid copepods contributed less. These findings stress the importance of focusing on abundance of preferred prey when assessing the actual prey availability to young fish. We found a spatio-temporal overlap between cod and their preferred prey, and observations suggest that advection of both zooplankton and cod contributed to this overlap. Hence, the larval feeding opportunities might be sensitive to climate-related changes affecting the circulation patterns in this fjord.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The availability of suitable prey during the early life of fish is believed to be a bottle neck for the recruitment to the adult population (Hjort 1914; Cushing 1990; Houde 2008). To avoid starvation, larval and juvenile fish need to overlap spatially with their prey (Cushing 1990; Platt et al. 2003). Starving fish are more vulnerable to predation from spending more time searching for prey and less on predator avoidance (Dill and Fraser 1984; Munk 1995), and because they have lower escape capabilities (Hossain et al. 2002; Takasuka et al. 2003). Good feeding conditions facilitate fast growth by reducing the duration of the larval stage where predation mortality is highest (Chambers and Leggett 1987; Houde 1987; Leggett and Deblois 1994). A thorough understanding of the processes involved in the spatio-temporal synchronization in predator and prey distribution is therefore essential to assess the linkages between prey availability and fish recruitment.
Predators are selective in their feeding; a trait that has received increasing scientific attention (e.g. Rowlands et al. 2008; Robert et al. 2011; Demontigny et al. 2012). This partially stems from the necessity to resolve the prey field of a predator in order to determine which fraction of potential prey organisms is available as food. The principal mechanisms constructing the prey field are visibility and catchability of the prey. For young fish, prey becomes easier to locate but more difficult to catch with increasing size (Munk 1997; Pepin and Penney 1997). Furthermore, the prey field changes with ontogeny as foraging capabilities improve (Voss et al. 2003; Robert et al. 2008; Rowlands et al. 2008). Consequently, only a restricted part of the zooplankton community is available as food to young fish at any given time during their development. Resolving the prey field is further complicated by species-specific differences in traits; e.g. prey morphology, visibility, motility, behaviour, predator avoidance and escape capabilities (Buskey et al. 1993; Heath 1993; Petrik et al. 2009). A high taxonomic resolution is therefore required when determining the diet and the zooplankton community composition to ascertain the relative taxonomic preferences (e.g. Robert et al. 2013). Furthermore, these traits change with the development of the prey (e.g. Titelman and Kiørboe 2003b). Hence, prey field investigations need to account for the interactive effects of prey taxon and size (Mayer and Wahl 1997). Ignoring these effects might result in misinterpretation of the prey availability.
The diet of a predator and its relative preferences for specific suitable prey species can also be affected by the relative abundance of prey species in the environment. For instance, larval and juvenile Atlantic cod (Gadus morhua) change their diet or modify their preferences for certain species of prey across their geographical distribution in response to changes in the zooplankton community composition (Heath and Lough 2007). Substantial differences in the zooplankton community may occur over small geographical scales (Munk et al. 2003; Arendt et al. 2010) across which, the young planktonic stages of fish or their prey may be transported over time (Fortier and Leggett 1983). Lastly, over the year there is a continuous succession in the plankton communities both in terms of species and developmental stages. Consequently, trophodynamics can be highly dynamic over temporal and spatial scales, and a fine spatio-temporal resolution is important when ascertaining the prey selection and diet of fish.
In the present study, we focused on the trophodynamics of larval and juvenile Atlantic cod in the sub-Arctic fjord branch Kapisigdlit within the Godthåbsfjord system. This system contains the largest inshore population of cod in Western Greenland, and Kapisigdlit has previously been identified as a principal spawning site (Hansen 1949; Smidt 1979). Interestingly, inshore populations have remained at relatively constant size during the past 70 years, while the West Greenlandic offshore stock largely disappeared around 1970 due to a climatic cold period leading to failure in larval recruitment to the stock (Pedersen and Rice 2002; Buch et al. 2004; Stein and Borovkov 2004; ICES 2013). This indicates that the physics and biology in fjords have been more stable. Therefore, we carried out a comparative study on the little investigated inshore cod population in Kapisigdlit with the overall aim to determine which processes govern early-life success. In the present study, we investigate the diet and prey preferences of cod to quantify the prey availability, and test the hypothesis that abundances of larval and juvenile cod at size are synchronized to optimal availability of preferred prey in space and time.
Materials and methods
Study site
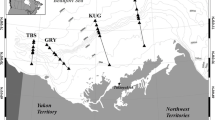
Sampling was carried out in the fjord branch Kapisigdlit in the Godthåbsfjord system, West Greenland. We established a transect of 6 stations along the 26 km long fjord branch, which were visited during 15 cruises, 7–10 days apart (each of 1–2 days duration), from 24 March to 5 August 2010. The vessel “Lille Masik” was used during all cruises except on June 17–18 where sampling was carried out from RV Dana (National Institute for Aquatic Resources, Denmark). Station (St.) 2 was located close to the mouth of the fjord branch, while St. 6 was located at the end of the fjord, in a shallow inner creek (Fig. 1). St. 2–4 covered deeper parts of the fjord, while St. 5 was located on a slope leading up to the shallow inner creek. During the study period, daylight hours increased from 13 to 21 h.
Physical measurements
For every sampling, vertical profiles of water temperature and salinity were recorded by CTD casts down to approximately 15 m above the sea floor. On all cruises, a SBE 19 plus was used, except from June 17–19 when a 911 plus SeaCat was used. Due to technical problems during few sampling events, a SBE 25 SM MicroCat was used to fill in missing data points. All CTDs were calibrated against each other and against salinity samples collected with a Niskin bottle at 1, 10, 20, 50, 75, 100, 150 and 250 m depth on May 24 and July 6 analysed on a Portosal salinometer.
Sampling of zooplankton
Mesozooplankton was sampled by vertical net tows using a Hydrobios Multinet (type Mini, 0.125 m2 opening) with five 50-µm mesh nets, or a 60-cm-diameter WP-2 net 50-µm mesh size equipped with a non-filtering cod end. The Multinet was used on every sampling date at St. 4, and on March 24, April 22, May 18, June 17 and July 6 at St. 2 and 5. The WP-2 net was used on all other sampling events. The nets were hauled at a speed of 0.2–0.3 m s−1 from a maximum depth of 100, 75 and 50 m depth to the surface at St. 2, 4, 5 and 6, respectively. No zooplankton sampling was done on St. 3. The content of the codend was immediately preserved in buffered formalin (4 % final concentration). All samples were analysed by the Plankton Sorting and Identification Center in Szczecin, Poland (www.nmfri.gdynia.pl). Samples containing high numbers of zooplankton were split into subsamples. All copepods and other zooplankton were identified to lowest possible taxonomic level (approximately 400 per sample), length measured and counted. Copepods were sorted into development stages (nauplii stage 1—copepodite stage 6) using morphological features and sizes, and up to 10 individuals of each stage were length measured. Data collected on May 24 (St. 6) and June 3 (St. 2, 5, 6) were not included since the depth strata sampled may have been incorrect, as the WP-2 net was towed at an angle of 30–45° due to bad weather conditions. The biomass of the different zooplankton species was calculated from length measurements. For copepodites, the prosome length was measured, except for Microsetella norvegica where the combined length of the prosome and urosome were measured. The diameter of eggs, bivalve and gastropod larvae was measured on the longest axis. For all other organisms, the full body length was measured excluding any hairs, appendages and spines. Carbon conversion factors used were derived from the literature (Table S1). Average abundances and biomasses were calculated for different time periods by integrating over the period and dividing by the number of days. Average abundances of different organisms for the whole fjord branch were calculated by integrating over the transect and dividing with the length of the transect. Oncaea spp. includes members of Triconia borealis which were also identified in the fjord (Maria Grazia Mazzocchi, pers. com.), but will be referred to as Oncaea spp.
Sampling of Atlantic cod larvae and juveniles
In May and June, the cod (Gadus morhua) larvae/juveniles were collected using double-oblique tows of 60-cm-diameter bongo with one 300-µm and one 500-µm mesh net. Later in July and August, the collection was done by double-oblique tows of a MIK ring net (ring of 2-m-diameter and a 14-m-long white net of 600-µm mesh size). Both net types were fitted with a flowmetre recording water flow, and a CTD (MicroCat SBE 25 SM) recording vertical hydrographical profiles. At the two innermost St. 5 and 6, the net tows were conducted down to 75 and 50 m, respectively; 35–50 m above the sea floor due to variable bathymetry. At stations 2–4, tows were performed to a maximum depth of 100 m. Ships speed was about 1.6 knots. Sampling at St. 6 on June 18 was carried out using a WP-2 net (200-µm mesh size), and these larvae were immediately preserved in ethanol (95 % final concentration). Bongo net samples were preserved either in buffered formalin (for the 300-µm net, 4 % final concentration) or in ethanol (for the 500-µm net, min 50 % final concentration). MIK net samples were split in two subsamples, and one of these was preserved in formalin and the other in ethanol. Occasionally, the total zooplankton content of the MIK net samples were very large, and therefore, only a subsample was preserved, while the remaining part was kept cold (~5 °C) and inspected for cod within 48 h of sampling. All larvae/juveniles found in these samples were preserved in 95 % ethanol.
All sampled cod were sorted and identified under a dissecting microscope. Up to a maximum of 40 larvae/juveniles per sample were length measured (standard length) to the nearest 0.2 mm. In total, 706 cod were measured out of 1,257 caught. Before length measurements, cod were soaked in freshwater for approximately 2 min to minimize bending of the body due to preservation. Standard lengths were corrected for shrinkage due to handling and preservation using Eq. 1 from Theilacker (1980):
where L is the standard length (mm) prior to handling and preservation, X 1 is the standard length of the preserved cod, and X 2 is the time from death to fixation (which were set at 20 min in the present study). Cod were divided into three size groups: 4–8-, 9–15- and 16–25-mm standard length. Conversion from standard length (L) into dry weight (DW) was based on Eq. 2 from Munk (1997):
where L is in mm and DW is in mg. We assumed 43 % carbon content of the dry weight (Harris et al. 1986).
Gut content analysis
Guts from all or a max of 15 ethanol preserved larvae/juveniles (preserved immediately after catch) from each station at each date were included in the analysis (144 individuals in total). Guts were removed and emptied using fine needles under a dissecting microscope. The content of prey organisms were length measured and identified to lowest possible taxonomic level. As many prey items found were partially digested, identification was mainly done based on size, shape and other morphological characteristics. Copepodites and nauplii were primarily identified from the size and shape of the prosome and urosome, and from the number and size of individual segments and appendages. It was not possible to distinguish between Calanus spp. and Metridia spp. copepodite stages 1–3 so these stages were treated as one group. Some prey taxa found in the guts could only be identified to e.g. cladocera, calanoid, copepod or crustacea. A maximum of 20 individuals per taxa and developmental stage (e.g. nauplii or copepodite) were measured, and the rest were counted.
Carbon weights of prey items identified to appropriate taxonomic groups were estimated using the conversion factors in supplementary Table S1. Conversion for Calanus spp. and Metridia spp. C1–C3 was carried out using an average between the conversion for C. finmarchicus and C. glacialis from Madsen et al. (2001) and Metridia spp. from Hirche and Mumm (1992). Calanoid nauplii found in the gut were all converted to carbon weight using the conversion factor from Hygum et al. (2000). Carbon weight of prey, where parts of the main body (prosome for copepodites) were missing, was calculated as the average of other individuals of the same taxa found in the gut.
Prey size and taxonomic preferences
The dietary preferences were described using the Chesson (1978) α-selectivity index calculated by Eq. 3:
where d i and z i is the abundance of prey item i in the gut and environment, respectively, and N is the number of prey items considered. The index was calculated for individual larvae/juveniles and then averaged for the three size groups. All arthropods positively identified to class, order or genus depending on the organism (see Table 1 and S2), bivalves, gastropods and polychaetes are included in the analysis. All taxa were divided into 6 log-scaled length classes with the following mid-points: 100, 200, 400, 800, 1,600 and 3,200 µm, based on body length. Eight zooplankton taxonomic groups were considered (the more abundant and ingested); hence, a total of 48 prey categories (N) were used. Neutral preference is found at 1/N.
Modelled prey size preferences
In order to estimate availability of prey (at size) in the environment, we calculated the theoretical prey size spectra of cod in the three size groups. Using a Gaussian distribution of the spectra, we estimated the prey length of maximum preference (preymax) and the width of spectra (b) by nonlinear fit to the data. We assume the frequency distribution of α to be normal over the prey length classes. The relative preference (p) for the ith prey length could then be estimated from Eq. 4:
and i is the length interval, and N is the number of prey length classes considered. Preferred prey length to cod length ratio was estimated in the same way for each cod size group, by dividing the prey length (mid-points of the six prey length classes) with the standard lengths of each individual fish.
Prey availability (preyavailable) could then be calculated based on the relative prey length preference of the cod larvae/juvenile, for the biomass of each length class of zooplankton in the environment (z) by Eq. 5:
where p i is the relative preference for the ith prey length interval (Simonsen et al. 2006).
Statistics
All statistical analyses were performed in SYSTAT version 13. Analysis of total gut content (prey numbers and carbon weight) and prey size preferences was done on log-transformed data using ANCOVA, followed by a Tukey’s post hoc test. Analysis on the relative contribution of different prey taxa to the diet was done using Kruskal–Wallis followed by a pairwise comparison test. Test of difference in length of specific prey taxa was done by ANOVA when comparing three cod size groups, and t test when comparing two. Assumptions of normality and homogeneity of variance in all parametric tests performed were tested using Shapiro–Wilk and Levene’s test, respectively.
Results
Hydrography
The physical conditions in the fjord branch changed during the study period. The upper 30 m of the water column, in the inner half of the fjord branch, became warmer by the end of April. By early June, a thermocline had formed at around 20 m depth, and temperature had increased from 4.5° to 7.8° C (average in upper 30 m), moving inwards from the mouth of the fjord branch to St. 6. Following the ice break-up after June 20 in Kapisigdlit River, located at the end of the fjord branch, the stratification became stronger by the formation of a halocline at 10 m depth. By early July, the average temperature below the pycnocline (10–50 m depth) increased from 2.8° to 4.4° C moving from St. 2 to St. 6. The temperature continued to increase at St. 6 until the end of the study period. Moreover, salinity decreased from 33 down to 16 near the surface. Further details on hydrography are given in Riisgaard et al. (2014).
Zooplankton distribution
The zooplankton community structure varied temporally and spatially. Throughout the investigation, rotifers accounted for approximately half the total abundance of zooplankton which included all crustaceans, molluscs, polycheates and free spawned eggs (supplementary Table S2). In terms of biomass, their significance was, however, lower. Microsetella norvegica nauplii and copepodites, which numerically dominated the copepod community (see also Koski et al. 2013), were approximately one magnitude lower than rotifer abundance. Other zooplankton that in terms of abundance remained important throughout the study were Pseudocalanus spp. and free eggs (mainly from copepods), while important contributors to the biomass included calanoid nauplii and Calanus spp. and Metridia longa copepodites. In the last half of the study period, M. norvegica (both nauplii and copepodites), Evadne spp., Podon spp., bivalve larvae, Oithona similis (see also Zamora-Terol et al. 2014) and Oncaea spp. had become increasingly important (Table S2). Biomass of protozoans (mainly ciliates) was comparable to other zooplankton from mid-June until the end of the study (Riisgaard et al. 2014).
The variability in the abundance of zooplankton taxa at the different stations is illustrated for seven taxa, which were all abundant and important prey items for larval cod (Fig. 2). In May–June, Evadne spp. and Podon spp. were only found at St. 5 and 6 (only qualitative at St. 6), while bivalve larvae, O. similis and Pseudocalanus spp. increased in abundance and biomass towards the entrance of the fjord (from St. 5 to 2, Fig. 2a, b). Later in the season, Evadne spp. and Podon spp. were present at all stations, but increased significantly in abundance towards the end of the fjord (from St. 2 to 6, Fig. 2c–f). O. similis, Pseudocalanus spp., calanoid nauplii, cirripedia (nauplii and cypris, likely from Balanus spp.) and bivalve larvae had generally a low abundance (and biomass) at St. 6, but increased towards the entrance of the fjord (Fig. 2c–f).
Bars: Relative abundance (a, c, e) and biomass (b, d, f) of 7 selected taxa at four stations covering the length of the fjord during three time periods: a, b May 24–June 17. c, d June 18–July 12 and e, f July 13–August 5. Shown for 4 areas. Connected symbols: Relative contribution of selected taxa to all zooplankton taxa (grey squares), and the abundance (n m−2) and biomass (mg C m−2) of all zooplankton taxa (white circles) is shown on the second Y-axis, note different scales
Cod larval and juvenile diet
Of the 144 cod examined, 131 had prey in their guts. In total, 1,849 prey items were found and 1,421 were identified to order (copepod nauplii), genus (copepodites and cladocerans) and larvae (cirripedia and bivalves) (Table 1). Some prey items could not be identified or only to copepoda (nauplii, copepodite) or cladocera. In addition, 89 eggs were found likely from copepods and euphausiids. Another 32 prey items were identified as Amphipoda, Decapoda, Euphausiacea, Gastropoda, Ostracoda, Polycheata larvae or Rotifera and Tintinnida.
The gut contents increased significantly in number of prey and carbon weight from the smaller to the larger cod size group (df = 2, F = 7.217, p = 0.001 and df = 2, F = 7.327, p = 0.001, respectively; Table 1). There was no effect of time of day when the cod were caught on number of prey or carbon weight (df = 1, F = 1.252, p = 0.265 and df = 1, F = 2.118, p = 0.148, respectively), and no interaction between time and cod size group in number of prey or carbon weight (df = 2, F = 1.109, p = 0.333 and df = 2, F = 0.629, p = 0.535, respectively). Calanoid nauplii accounted for most of the diet in small 4–8-mm cod larvae, but their relative contribution, and the contribution of Calanus spp. and/or M. longa copepodite stages 1–3 (C1–C3) and cirripedia, generally decreased with increasing cod size (Table 2). The contribution of Pseudocalanus spp. was highest in the 9–15-mm size group, while the contribution of Calanus spp. C4–C6, cladoceran and bivalve larvae generally increased with cod size (Table 2). Other important components of the cod diet were eggs (mainly from copepods), O. similis and Centropages spp. (Table 1). The contribution of copepodite stages to the diet was, however, underestimated, since 1/3 of them could not be identified to genus and were therefore not included in the analysis. Most of the unidentified copepodites were calanoids. Significant increases in prey length with cod size were found for Podon spp. and Evadne spp., cirripedia and O. similis (Table 2).
Spatial differences in larval and juvenile gut contents were observed. Since relatively few cod were caught in the later part of the investigation, data were merged from St. 2 and 3 in the further analysis (Fig. 3). The 9–15-mm larvae/juveniles collected at St. 6 had significantly fewer prey in their gut compared to St. 4 and 5 and less prey carbon than at St. 5 (p < 0.003 and p = 0.008, respectively; Fig. 3c, d). In 4–8- and 16–25-mm cod, the gut content did, however, not differ between stations in terms of numbers of prey (p = 0.287 and p = 0.966, respectively) or carbon weight (p = 0.053 and p = 0.267, respectively; Fig. 3a, b, e, f). The relative contribution of 7 prey taxa accounting for most of the diet is shown in Fig. 3. The contribution of Pseudocalanus spp. to the diet generally increased moving further into the fjord. In small 4–8-mm larvae, the contribution of calanoid nauplii was equal at all stations, except St. 4 where it was lower (Fig. 3a, b), while in 9–15-mm cod, it increased moving out the fjord (Fig. 3c, d). The contribution from cladocerans was highest at St. 4 and 5 (Fig. 3c–f).
Bars: Relative contribution in numbers (a, c, e) and carbon weight (b, d, f) of 7 selected prey taxa in the gut for three size groups of cod: a, b 4–8 mm, c, d 9–15 mm and e, f 16–25 mm. Connected symbols: Relative contribution of selected prey taxa to all prey found in the gut (grey squares), and the total gut content in numbers and carbon weight (white circles ± SE) is shown on the second Y-axis, note different scales
Prey preference
Cod larvae and juvenile prey preferences were calculated for the following eight selected zooplankton taxonomic groups: Pseudocalanus spp., Oithona similis, cirripedia nauplii–cypris, bivalve larvae, calanoid nauplii, Podon spp. and Evadne spp. Cod showed preference for specific prey sizes and taxa, and their preferences changed during ontogeny. Rotifers and protozoans were likely underrepresented in the guts, as they generally were more degraded and only few could be identified. They were therefore omitted from the analysis. Small 4–8-mm larvae showed the highest preference for prey in the 400-µm length class (Table 3), and calanoid nauplii were the preferred prey. The larger 9–15-mm larvae/juveniles showed highest preference for prey in the 400- and 800-µm length class. Calanoid nauplii and Podon spp. were preferred in the 400-µm length class, and Pseudocalanus spp. in the 800-µm length class. The large 16–25-mm juveniles had the highest preference towards the 800- and 1,600-µm prey length classes. Podon spp. and others were preferred in the 800-µm length class and others in the 1,600-µm length class. These other prey taxa were mainly Centropages spp. in the 800-µm length class and Calanus spp. C4–C6 in the 1,600-µm length class. At the outer margins of the prey size spectrum, taxa that were most abundant in the environment were in most cases also the preferred prey of all cod size groups (Table 3).
When comparing the three cod size groups, a decline in the importance of calanoid nauplii and an increase in importance of cladocerans were apparent during ontogeny (Table 3). The 9–15-mm larvae/juveniles showed high preference for Pseudocalanus spp., while the 16–25-mm juveniles preferred larger calanoid species. Prey length of maximum preference increased with cod length being 352, 505 and 835 µm for the 4–8-, 9–15- and 16–25-mm size groups, respectively (Fig. 4a). This corresponds to a preferred prey length of 6, 5 and 4 % the larval length within the three respective larval size groups (Fig. 4b).
Prey preference index estimated for 3 larval size groups fitted a Gaussian 3 parameter normal distribution function. a On a log-scaled axis of prey lengths, and b on an axis of relative prey lengths. a Prey length of maximal preference (Log(preymax)) and b was 2.55 and 0.17 in 4–8-mm larvae (r 2 = 0.34), 2.70 and 0.21 in 9–15-mm larvae (r 2 = 0.21) and 2.92 and 0.28 in 16–25-mm larvae (r 2 = 0.21), respectively. b Prey length to larval length ratio of maximal preference (Log(preymax)) and b was −1.21 and 0.18 in 4–8-mm larvae (r 2 = 0.31), −1.31 and 0.24 in 9–15-mm larvae (r 2 = 0.22) and −1.42 and 0.29 in 16–25-mm larvae (r 2 = 0.23), respectively
Cod distribution and preferred prey availability
The availability of prey, calculated as available biomass from the prey size preferences of the individual cod size groups (Fig. 4a), was higher in June–July compared to the other two periods (3–7 times, Fig. 5). However, availability of preferred taxa differed between periods. The available biomass of calanoid nauplii was largest in May–June, Podon spp., Evadne spp. and Pseudocalanus spp. in June–July, while Calanus spp. and Pseudocalanus spp. was largest in July–August.
Average prey availability (bars) and cod larval/juvenile biomass (connected symbols) at different stations covering the length of the fjord calculated from the prey size spectrum of three larval size groups: a–c 4–8 mm, d–f 9–15 mm and g–i 16–25 mm, during three time periods: May 24–June 17 (a, d, g), June 18–July 12 (b, e, h) and July 13–August 5 (c, f, i). Zooplankton was not collected at St. 3. Due to sampling problems in May–June at St. 6, prey availability was calculated as an average between May 18 and June 17 (hatched bars)
The small 4–8-mm cod larvae were primarily found in the May–June (43 %) and June–July (50 %) periods and mainly at St. 6 (81 and 65 %, respectively, Fig. 5a–c). Early in the season, when cod larvae hatched and started feeding (late March to mid-June), availability of prey biomass increased from St. 2 towards the inner fjord (Fig. 5a). Due to sampling problems, the prey availability on St. 6 in the May–June period was calculated as an average between May 18 and June 17 (Fig. 5a, d, g). However, prey distribution changed dramatically between these dates. On May 18 at St. 6, prey availability was 2–6 times higher than at St. 2–5, but by June 17, St. 6 had become 2–3 times lower. Later in June–July, when more of the 4–8-mm cod larvae had dispersed out into the fjord the availability of prey was now highest at St. 5 (3–12 times, Fig. 5b). By July–August, the distribution of prey had switched, increasing with increasing distance from St. 6 (3–10 times, Fig. 5c). At this time, 89 % of the larvae were located outside St. 6. A similar relationship was observed in the biomass of calanoid nauplii with sizes within the prey size spectra of the 4–8-mm larvae. On May 18, these were more available at St. 6 compared to the other stations (3–8 times), while by June 17, calanoid nauplii were almost absent at St. 6 but highly available at the other stations. In June–July, calanoid nauplii continued to be more available at St. 2–5 than at St. 6 (4 times), and by July–August, their availability increased with increasing distance from St. 6 (7–72 times).
Larger 9–15-mm larvae and juveniles were found in all time periods, although mainly in June–July and July–August (29 and 57 %, respectively, Fig. 5d–f). Initially, 50 % of the 9–15-mm cod were found at St. 6, but this percentage gradually decreased to 11 % by July–August. The distribution of prey biomass largely followed that of the cod. In May–June, prey availability was higher at St. 6 compared to the other stations (2 times, Fig. 5d). Later in June–July, prey had become equally available at St. 5 and 6 and still 3–7 times higher than at St. 2–4 (Fig. 5e). By July–August, the distribution of prey had reversed and was now higher at St. 2–5 compared to St. 6 (2–4 times, Fig. 5f). A slightly different relationship was seen in the distribution of preferred Pseudocalanus spp. that were within the prey size spectra of the 9–15-mm cod. In May–June, these were up to 2 times more available at St. 6, while in the two consecutive periods Pseudocalanus spp. had become 2–3 times more available at St. 2–5. Initially, calanoid nauplii were equally available within the fjord, but in June–July, they were more available at St. 2–5 (3–4 times) and by July–August they were almost exclusively found at St. 2. Cladocerans, another preferred group of prey, first appeared on June 17 and only at St. 5–6, although most available at St. 6 (3 times). Their restricted distribution largely persisted in June–July where they accounted for 58 % of the available prey biomass at St. 5–6, but by July–August, they had become more equally distributed in the fjord and were now most available at St. 5 (2–4 times).
Large 16–25-mm juvenile cod were almost exclusively found in July–August (97 %), although in terms of abundance 25 % of them were around in June–July (Fig. 5g–i). These juveniles were largely found outside St. 6. In June–July, the availability of prey biomass was highest at St. 6 and tended to decrease with increasing distance (2–11 times, Fig. 5h), but by July–August, prey were equally available at St. 2–5 and higher compared to St. 6 (2 times, Fig. 5i). The distribution of the preferred cladocerans that were within the prey size spectra of the 16–25-mm cod was almost identical to the distribution of those sizes preferred by the 9–15-mm cod. Only few Centropages spp. were caught within the fjord, but Calanus spp. C4–C6, the other preferred group of prey, was generally more available at the central St. 4–5 in the last two periods compared to St. 2 and 6 (2–7 times more in June–July, 2 times more in July–August).
Discussion
Zooplankton community and larval diet
The zooplankton community in the Kapisigdlit fjord was not reflected in the diet composition of larval and juvenile cod. This was especially clear from the low contribution of non-calanoid copepods. In situ biomasses of Oithona similis nauplii, Microsetella norvegica and Oncaea spp. nauplii and copepodites were 14-fold higher than the larger calanoid nauplii. Nevertheless, very few were found in the cod guts, while the less-abundant calanoid nauplii had a high contribution to the diet. Other studies have also found high importance of calanoid nauplii in cod larval and juvenile diet (Heath and Lough 2007, and references therein), while only one study has reported the presence of Microsetella spp. but in low numbers (Pepin and Penney 1997). Predation on Oncaea spp. has not previously been reported. Calanoid nauplii have a high lipid content (Jung-Madsen et al. 2013) and are therefore likely nutritional prey items for the young cod. Moreover, the ingested nauplii were generally large and expected to belong to the genera Pseudocalanus spp., Metridia longa or Calanus spp., as these genera contributed with >99 % of the calanoid nauplii biomass. These prey items contributed most to the diet of small 4–8-mm cod larvae, while cladocerans and Calanus spp. (C4–C6) were more important prey for the large 16–25-mm juveniles. The 9–15-mm larvae/juveniles displayed a more diverse diet, transitioning from nauplii to larger copepods and cladocerans.
Metridia longa was only found in a few cod, despite being a dominant copepod species in Kapisigdlit in terms of biomass (Riisgaard et al. 2014). A temporal and spatial mismatch left cod with few opportunities to predate on M. longa as its copepodites performed dial vertical migrations spending only 6–12 h per day in the upper water column (Hays 1995; Kjellerup unpubl; Daase et al. 2008), and only accounted for a small fraction of the zooplankton at the time where juveniles were present in the fjord. In line with these results, only few studies have reported on Metridia spp. nauplii and copepodites in cod diet (Heath and Lough 2007). No dial differences were found in the cod stomach content despite changes in light. However, light levels were never below the minimum required for cod larvae to successfully feed in the upper 50 m of the water column during the time of the study (Vollset et al. 2011).
Prey preferences
The present study showed an increase in the prey size of maximal preference as the cod grew, in accordance with other studies (Munk 1997; Rowlands et al. 2008; Robert et al. 2011) and other fish species (e.g. Munk 1992; Pepin and Penney 1997; Simonsen et al. 2006). Cod preferred prey size 4–6 % of their length well within the range found by Munk (1997). Marked prey preferences for specific taxa were observed in the larvae and juveniles that changed both with prey length and with size of the fish. This was clearly illustrated by the shift in preference from calanoid nauplii to cladocerans and calanoid copepodites as cod grew, in concurrence with other studies (Kane 1984; Rowlands et al. 2008; Robert et al. 2011). Most prominent was the switch from cladoceran in the 400-µm prey length class to Pseudocalanus spp. and larger calanoids in the 800-µm class, despite high availability of both prey taxa, demonstrating a marked change in taxonomic preference with prey size. Although found in several guts, sometimes in high numbers, we observed a low preference for O. similis in agreement with other studies (Kane 1984; Pepin and Penney 1997; Robert et al. 2011).
While the preference for certain calanoid species of copepods could be high, we found that larvae generally showed low preference for non-calanoid copepods. These findings are in agreement with other studies (Heath and Lough 2007, and references therein; Robert et al. 2011) and might stem from morphological or behavioural differences between species. Fish larvae prey preferences is affected by (1) visibility influenced by size and contrast of the prey; (2) catchability influenced by prey escape response and (3) encounter rate influenced by prey swimming mode (Buskey et al. 1993; Heath 1993; Hwang and Turner 1995). The swimming pattern in calanoid species tends to be more continuous, increasing the risk of predator encounters, compared to the jump-sink motility pattern of e.g. Oithona spp. and Oncaea spp. (Buskey et al. 1993; Hwang and Turner 1995; Titelman and Kiørboe 2003b). This would explain the observed high preferences for calanoid species. Conversely, the narrow body shape of Microsetella norvegica and its association with particles would make it less visible to the larvae, while the motility pattern of O. similis and Oncaea spp. would make them less likely to encounter. Moreover, we observed that late calanoid nauplii development stages (N3–N6) were selectively ingested. Most of these late stages were within the preferred prey size range. However, the increase in nauplii swimming activity as they start feeding (at N3) would also have made them more likely to encounter, compared to the jump-sink motility observed in early stages (Buskey et al. 1993; Titelman and Kiørboe 2003a, b). Petrik et al. (2009) also found that species-specific differences in escape response had an even greater impact on prey selection in larval cod and haddock, which explained their high preference for Pseudocalanus spp. relative to Calanus finmarchicus, Oithona similis and Centropages typicus.
Preferences for cladocerans
We documented high preference of large cod (9–25 mm) for the cladocerans Podon spp. and Evadne spp. Hence, they contributed significantly to the diet, surpassing the contribution of the more abundant Pseudocalanus spp., Calanus spp. and other copepodites. Several studies have found that these copepods are key components in cod larval diet (Heath and Lough 2007); however, few studies have investigated the significance of cladocerans, and their importance in the diet was reported to be low (Pepin and Penney 1997; Voss et al. 2003; Robert et al. 2011). Podon spp. was generally preferred over Evadne spp. As Evadne spp. were larger than Podon spp., the difference in preference suggests a difference in predator avoidance such as diurnal migration (see Onbe and Ikeda 1995), pigmentation (i.e. size of compound eye, Zaret and Kerfoot 1975) or escape response between the two genera.
The high preference for cladocerans could partly be due to a patchy distribution and high catchability of this species. Marine cladocerans tend to aggregate close to the surface (Onbe and Ikeda 1995; Saito and Hattori 2000; Andersen and Nielsen 2002) or in association with subsurface blooms (Nielsen 1991). They rely on early maturation and parthenogenesis, producing large offspring to overcome predation, instead of good escape capabilities (Lynch 1980; Verity and Smetacek 1996). Although cladocerans are smaller and less fat (8–23 % of DW in freshwater species; Goulden et al. 1982; Goulden and Place 1993), compared to the larger Calanus spp. (9–74 % of DW; Swalethorp et al. 2011), their high catchability may compensate for a smaller energy gain. Moreover, cladoceran lipid content is comparable to that of Pseudocalanus spp. and Centropages spp. (Lee et al. 2006, and references therein).
Prey availability and larval distribution
Comparison of the seasonal distribution patterns in cod abundance and size, and the availability of their preferred prey, indicated a temporal and spatial synchronization between emergence of larvae and the development of the zooplankton community. In general, most of the zooplankton was located in the upper 25 m of the water column (Koski et al. 2013; Riisgaard et al. 2014; Zamora-Terol et al. 2014, Kjellerup unpubl.), where many young cod were likely located. Initially, prey availability was highest in the main cod spawning area at St. 6 and decreased with increasing distance from this area. Later in the season, when larger larvae and juveniles advected out following the ice break-up of Kapisigdlit River (June 20, Riisgaard et al. 2014), the distribution of prey had reversed for all three cod size groups. Cod spatial distribution patterns should not have been affected by our change in gear; however, biomass estimates may have been underestimated later in the season when we switched to the larger 2-m-diameter MIK net (Munk and Nielsen 1994). The towing speed of 1.6 knots could have resulted is a lower catch efficiency of larger individuals with improved escape capabilities, but larval condition and our determination of feeding indices may have been improved (Colton et al. 1980).
Our results suggest that changes in prey distribution were a combination of advection of the prey organisms and of spatio-temporal differences in abundance of specific prey taxa. Cladocerans which at times accounted for most of the available prey were not found in the outer fjord before the ice break-up of Kapisigdlit River around June 20. An aggregation near the surface layer (Onbe and Ikeda 1995; Andersen and Nielsen 2002) and an ability to cross a strong halocline (Pagano et al. 1993) might have positioned them in the top layer where there was an outward moving current (Swalethorp unpubl.). Despite being advected, fast reproduction allowed them to maintain a high biomass at St. 6, as observed in a Norwegian fjord (Nielsen and Andersen 2002). Furthermore, larger calanoid copepods preferred by the 9–25-mm cod were generally more abundant outside the main spawning area (St. 6), while calanoid nauplii preferred by the 4–8-mm larval cod were most abundant inside the spawning area when the larvae were present. Hence, our results show that during ontogeny, there was a consistent match between cod distribution (at the given time) and the availability of prey of the preferred size/taxa. However, it is unknown whether the shift in calanoid nauplii distribution from inner to outer fjord around mid-June was only due to advection, or if predation was also a factor as total fish larval abundance was highest at St. 6.
Conclusion and perspective
We found that during ontogeny cod larvae and juveniles preferred increasingly larger prey and that increases were proportional to the length increases of the cod. During ontogeny, taxonomic prey preferences also changed. Cod changed from calanoid nauplii towards Pseudocalanus spp. and Calanus spp. copepodites and cladocerans, while the contribution of otherwise dominating Metridia longa and non-calanoid copepods was low. These findings are in accordance with previous studies and stress the importance of considering the abundance of preferred prey when assessing the actual prey availability to larvae and juveniles at size. The effect of prey size on taxonomic preferences, most clearly observed by the switch from cladocerans to Pseudocalanus spp. in 9–15-mm cod, also underlines the importance of making prey preferences calculations including effects of both prey size and taxon. When only considering the availability of preferred prey, we could illustrate an important spatio-temporal overlap between cod and their prey.
The depth distribution of the zooplankton community is strongly structured by the water-column characteristics (Arendt et al. 2010, 2011; Tang et al. 2011), resulting in large variability in the community composition over small spatial scales (e.g. Nielsen and Andersen 2002, present study). Our findings suggest that zooplankton and cod were also advected out from the innermost part of the fjord. Therefore, future climate changes, such as increased temperature and precipitation affecting the timing and magnitude of freshwater outflow to fjords, might impact the circulation patterns (Rysgaard et al. 2003; Kattsov et al. 2007; Myksvoll et al. 2011, 2013) with consequences for the distribution of preferred zooplankton taxa, such as the abundance of cladocerans (see Johns et al. 2005). Many cod populations reside in inshore areas (Robichaud and Rose 2004), and changes in physical forcing could likewise affect the advection of cod and thus the spatio-temporal match to prey. Predicted increases in stratification of the water column by freshwater outflow will also favour small-sized phytoplankton species (Ardyna et al. 2011) less efficiently ingested by the herbivorous copepods (e.g. Levinsen et al. 2000; Turner et al. 2001), which were preferred by the cod. This might reduce prey availability impacting larval and juvenile growth and their survival possibilities.
References
Andersen CM, Nielsen TG (2002) The effect of a sharp pycnocline on plankton dynamics in a freshwater influenced Norwegian fjord. Ophelia 56:135–160
Arendt KE, Nielsen TG, Rysgaard S, Tonnesson K (2010) Differences in plankton community structure along the Godthåbsfjord, from the Greenland Ice Sheet to offshore waters. Mar Ecol Prog Ser 401:49–62. doi:10.3354/meps08368
Arendt KE, Dutz J, Jonasdottir SH, Jung-Madsen S, Mortensen J, Moller EF, Nielsen TG (2011) Effects of suspended sediments on copepods feeding in a glacial influenced sub-Arctic fjord. J Plankton Res 33:1526–1537. doi:10.1093/plankt/fbr054
Buch E, Pedersen SA, Ribergaard MH (2004) Ecosystem variability in West Greenland waters. J Northwest Atl Fish Sci 34:13–28. doi:10.2960/J.v34.m479
Buskey EJ, Coulter C, Strom S (1993) Locomotory patterns of microzooplankton: potential effects on food selectivity of larval fish. Bull Mar Sci 53:29–43
Chambers RC, Leggett WC (1987) Size and age at metamorphosis in marine fishes: an analysis of laboratory-reared winter flounder (Pseudopleuronectes americanus) with a review of variation in other species. Can J Fish Aquat Sci 44:1936–1947. doi:10.1139/f87-238
Chesson J (1978) Measuring preference in selective predation. Ecology 59:211–215. doi:10.2307/1936364
Colton JB, Green JR, Byron RR, Frisella JL (1980) Bongo net retention rates as effected by towing speed and mesh size. Can J Fish Aquat Sci 37:606–623
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:249–293. doi:10.1016/s0065-2881(08)60202-3
Daase M, Eiane K, Aksnes DL, Vogedes D (2008) Vertical distribution of Calanus spp. and Metridia longa at four Arctic locations. Mar Biol Res 4:193–207. doi:10.1080/17451000801907948
Demontigny F, Ouellet P, Sirois P, Plourde S (2012) Zooplankton prey selection among three dominant ichthyoplankton species in the northwest Gulf of St Lawrence. J Plankton Res 34:221–235. doi:10.1093/plankt/fbr104
Dill L, Fraser AG (1984) Risk of predation and the feeding behavior of juvenile coho salmon (Oncorhynchus kisutch). Behav Ecol Sociobiol 16:65–71. doi:10.1007/bf00293105
Fortier L, Leggett WC (1983) Vertical migrations and transport of larval fish in a partially mixed estuary. Can J Fish Aquat Sci 40:1543–1555. doi:10.1139/f83-179
Goulden CE, Place AR (1993) Lipid accumulation and allocation in Daphniid Cladocera. Bull Mar Sci 53:106–114
Goulden CE, Henry LL, Tessier AJ (1982) Body size, energy reserves, and competitive ability in three species of cladocera. Ecology 63:1780–1789. doi:10.2307/1940120
Hansen PM (1949) Studies on the biology of the cod in Greenland waters. Matematisk-Naturvidenskabeligt Fakultet, København
Harris RK, Nishiyama T, Paul AJ (1986) Carbon, nitrogen and caloric content of eggs, larvae, and juveniles of the walleye pollock, Theragra chalcogramma. J Fish Biol 29:87–98. doi:10.1111/j.1095-8649.1986.tb04928.x
Hays GC (1995) Ontogenetic and seasonal variation in the diel vertical migration of the copepods Metridia lucens and Metridia longa. Limnol Oceanogr 40:1461–1465
Heath MR (1993) The role of escape reactions in determining the size distribution of prey captured by herring larvae. Environ Biol Fishes 38:331–344. doi:10.1007/bf00007527
Heath MR, Lough RG (2007) A synthesis of large-scale patterns in the planktonic prey of larval and juvenile cod (Gadus morhua). Fish Oceanogr 16:169–185. doi:10.1111/j.1365-2419.2006.00423.x
Hirche HJ, Mumm N (1992) Distribution of dominant copepods in the Nansen Basin, Arctic-Ocean, in summer. Deep Sea Res Part 1 Oceanogr Res Pap 39:485–505
Hjort J (1914) Fluctuations in the great fisheries of Northern Europe. Rapp P V Réun Cons Int Explor Mer 20:1–228
Hossain MAR, Tanaka M, Masuda R (2002) Predator–prey interaction between hatchery-reared Japanese flounder juvenile, Paralichthys olivaceus, and sandy shore crab, Matuta lunaris: daily rhythms, anti-predator conditioning and starvation. J Exp Mar Biol Ecol 267:1–14. doi:10.1016/S0022-0981(01)00340-9
Houde ED (1987) Early life dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Houde ED (2008) Emerging from Hjort’s shadow. J Northwest Atl Fish Sci 41:53–70
Hwang J-S, Turner JT (1995) Behaviour of cyclopoid, harpacticoid, and calanoid copepods from coastal waters of Taiwan. Mar Ecol 16:207–216. doi:10.1111/j.1439-0485.1995.tb00406.x
Hygum BH, Rey C, Hansen BW (2000) Growth and development rates of Calanus finmarchicus nauplii during a diatom spring bloom. Mar Biol 136:1075–1085
ICES (2013) Report of the North Western Working Group (NWWG). ICES Headquarters, Copenhagen
Johns DG, Edwards M, Greve W, Sjohn AWG (2005) Increasing prevalence of the marine cladoceran Penilia avirostris (Dana, 1852) in the North Sea. Helgol Mar Res 59:214–218. doi:10.1007/s10152-005-0221-y
Jung-Madsen S, Nielsen TG, Grønkjær P, Hansen BW, Møller EF (2013) Early development of Calanus hyperboreus nauplii: response to a changing ocean. Limnol Oceanogr 58:2109–2121. doi:10.4319/lo.2013.58.6.2109
Kane J (1984) The feeding habits of co-occurring cod and haddock larvae from Georges Bank. Mar Ecol Prog Ser 16:9–20. doi:10.3354/meps016009
Kattsov VM, Walsh JE, Chapman WL, Govorkova VA, Pavlova TV, Zhang X (2007) Simulation and projection of Arctic freshwater budget components by the IPCC AR4 global climate models. J Hydrometeorol 8:19
Koski M, Swalethorp R, Kjellerup S, Nielsen TG (2013) The mystery of Microsetella: combination of sac- and broadcast-spawning in an Arctic fjord. J Plankton Res 36:259–264. doi:10.1093/plankt/fbt117
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306. doi:10.3354/meps307273
Leggett WC, Deblois E (1994) Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Neth J Sea Res 32:119–134. doi:10.1016/0077-7579(94)90036-1
Levinsen H, Turner JT, Nielsen TG, Hansen BW (2000) On the trophic coupling between protists and copepods in arctic marine ecosystems. Mar Ecol Prog Ser 204:65–77
Lynch M (1980) The evolution of cladoceran life histories. Q Rev Biol 55:23–42. doi:10.1086/411614
Madsen SD, Nielsen TG, Hansen BW (2001) Annual population development and production by Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland. Mar Biol 139:75–93
Mayer CM, Wahl DH (1997) The relationship between prey selectivity and growth and survival in a larval fish. Can J Fish Aquat Sci 54:1504–1512. doi:10.1139/f97-056
Munk P (1992) Foraging behavior and prey size spectra of larval herring Clupea harengus. Mar Ecol Prog Ser 80:149–158. doi:10.3354/meps080149
Munk P (1995) Foraging behaviour of larval cod (Gadus morhua) influenced by prey density and hunger. Mar Biol 122:205–212
Munk P (1997) Prey size spectra and prey availability of larval and small juvenile cod. J Fish Biol 51:340–351
Munk P, Nielsen TG (1994) Trophodynamics of the plankton community at Dogger Bank: predatory impact by larval fish. J Plankton Res 16:1225–1245. doi:10.1093/plankt/16.9.1225
Munk P, Hansen BW, Nielsen TG, Thomsen HA (2003) Changes in plankton and fish larvae communities across hydrographic fronts off West Greenland. J Plankton Res 25:815–830. doi:10.1093/plankt/25.7.815
Myksvoll MS, Sundby S, Ådlandsvik B, Vikebø FB (2011) Retention of coastal cod eggs in a fjord caused by interactions between egg buoyancy and circulation pattern. Mar Coast Fish 3:279–294. doi:10.1080/19425120.2011.595258
Myksvoll MS, Sandvik AD, Asplin L, Sundby S (2013) Effects of river regulations on fjord dynamics and retention of coastal cod eggs. ICES J Mar Sci: Journal du Conseil. doi:10.1093/icesjms/fst113
Nielsen TG (1991) Contribution of zooplankton grazing to the decline of a Ceratium bloom. Limnol Oceanogr 36:1091–1106
Nielsen TG, Andersen CM (2002) Plankton community structure and production along a freshwater-influenced Norwegian fjord system. Mar Biol 141:707–724. doi:10.1007/s00227-002-0868-8
Onbe T, Ikeda T (1995) Marine cladocerans in Toyama Bay, southern Japan Sea: seasonal occurrence and day-night vertical distributions. J Plankton Res 17:595–609. doi:10.1093/plankt/17.3.595
Pagano M, Gaudy R, Thibault D, Lochet F (1993) Vertical migrations and feeding rhythms of mesozooplanktonic organisms in the Rhone River plume area (North-west Mediterranean Sea). Estuar Coast Shelf Sci 37:251–269. doi:10.1006/ecss.1993.1055
Pedersen SA, Rice JC (2002) Dynamics of fish larvae, zooplankton, and hydrographical characteristics in the West Greenland large marine ecosystem 1950–1984. In: Kenneth S, Hein Rune S (eds) Large Marine Ecosystems. Elsevier, pp 151–193
Pepin P, Penney RW (1997) Patterns of prey size and taxonomic composition in larval fish: are there general size-dependent models? J Fish Biol 51:84–100. doi:10.1111/j.1095-8649.1997.tb06094.x
Petrik CM, Kristiansen T, Lough RG, Davis CS (2009) Prey selection by larval haddock and cod on copepods with species-specific behavior: an individual-based model analysis. Mar Ecol Prog Ser 396:123–143. doi:10.3354/meps08268
Platt T, Fuentes-Yaco C, Frank KT (2003) Spring algal bloom and larval fish survival. Nature 423:398–399. doi:10.1038/423398b
Riisgaard K, Swalethorp R, Kjellerup S, Juul-Pedersen T, Nielsen TG (2014) Trophic role and top-down control of a subarctic protozooplankton community. Mar Ecol Prog Ser 500:67–82. doi:10.3354/meps10706
Robert D, Castonguay M, Fortier L (2008) Effects of intra- and inter-annual variability in prey field on the feeding selectivity of larval Atlantic mackerel (Scomber scombrus). J Plankton Res 30:673–688. doi:10.1093/plankt/fbn030
Robert D, Levesque K, Gagne JA, Fortier L (2011) Change in prey selectivity during the larval life of Atlantic cod in the southern Gulf of St Lawrence. J Plankton Res 33:195–200. doi:10.1093/plankt/fbq095
Robert D, Murphy HM, Jenkins GP, Fortier L (2013) Poor taxonomical knowledge of larval fish prey preference is impeding our ability to assess the existence of a “critical period” driving year-class strength. ICES J Mar Sci Journal du Conseil. doi 10.1093/icesjms/fst198
Robichaud D, Rose GA (2004) Migratory behaviour and range in Atlantic cod: inference from a century of tagging. Fish Fish 5:185–214. doi:10.1111/j.1467-2679.2004.00141.x
Rowlands WL, Dickey-Collas M, Geffen AJ, Nash RDM (2008) Diet overlap and prey selection through metamorphosis in Irish Sea cod (Gadus morhua), haddock (Melanogrammus aeglefinus), and whiting (Merlangius merlangus). Can J Fish Aquat Sci 65:1297–1306. doi:10.1139/f08-041
Rysgaard S, Vang T, Stjernholm M, Rasmussen B, Windelin A, Kiilsholm S (2003) Physical conditions, carbon transport, and climate change impacts in a Northeast Greenland Fjord. Arct Antarct Alp Res 35:301–312. doi:10.1657/1523-0430(2003)035[0301:PCCTAC]2.0.CO;2
Saito H, Hattori H (2000) Diel vertical migration of the marine cladoceran Podon Leuckarti: variations with reproductive stage. J Oceanogr 56:153–160. doi:10.1023/a:1011131012171
Simonsen CS, Munk P, Folkvord A, Pedersen SA (2006) Feeding ecology of Greenland halibut and sand eel larvae off West Greenland. Mar Biol 149:937–952. doi:10.1007/s00227-005-0172-5
Smidt ELB (1979) Annual cycles of primary production and of zooplankton at Southwest Greenland. Greenland Biosci 1
Stein M, Borovkov VA (2004) Greenland cod (Gadus morhua): modeling recruitment variation during the second half of the 20th century. Fish Oceanogr 13:111–120. doi:10.1046/j.1365-2419.2003.00280.x
Swalethorp R, Kjellerup S, Dünweber M, Nielsen TG, Møller E, Rysgaard S, Hansen BW (2011) Grazing, egg production, and biochemical evidence of differences in the life strategies of Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland. Mar Ecol Prog Ser 429:125–144. doi:10.3354/meps09065
Takasuka A, Aoki I, Mitani I (2003) Evidence of growth-selective predation on larval Japanese anchovy Engraulis japonicus in Sagami Bay. Mar Ecol Prog Ser 252:223–238
Tang KW, Nielsen TG, Munk P, Mortensen J, Moller EF, Arendt KE, Tonnesson K, Juul-Pedersen T (2011) Metazooplankton community structure, feeding rate estimates, and hydrography in a meltwater-influenced Greenlandic fjord. Mar Ecol Prog Ser 434:77–90. doi:10.3354/meps09188
Theilacker GH (1980) Changes in body measurements of larval northern anchovy, Engraulis mordax, and other fishes due to handling and preservation. Fish Bull 78:685–692
Titelman J, Kiørboe T (2003a) Motility of copepod nauplii and implications for food encounter. Mar Ecol Prog Ser 247:123–135. doi:10.3354/meps247123
Titelman J, Kiørboe T (2003b) Predator avoidance by nauplii. Mar Ecol Prog Ser 247:137–149. doi:10.3354/meps247137
Turner JT, Levinsen H, Nielsen TG, Hansen BW (2001) Zooplankton feeding ecology: grazing on phytoplankton and predation on protozoans by copepod and barnacle nauplii in Disko Bay, West Greenland. Mar Ecol Prog Ser 221:209–219. doi:10.3354/meps221209
Verity PG, Smetacek V (1996) Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar Ecol Prog Ser 130:277–293. doi:10.3354/meps130277
Vollset KW, Folkvord A, Browman HI (2011) Foraging behaviour of larval cod (Gadus morhua) at low light intensities. Mar Biol 158:1125–1133. doi:10.1007/s00227-011-1635-5
Voss R, Köster FW, Dickmann M (2003) Comparing the feeding habits of co-occurring sprat (Sprattus sprattus) and cod (Gadus morhua) larvae in the Bornholm Basin, Baltic Sea. Fish Res 63:97–111. doi:10.1016/S0165-7836(02)00282-5
Zamora-Terol S, Kjellerup S, Swalethorp R, Saiz E, Nielsen TG (2014) Phenology of the small copepod Oithona similis in a subarctic West Greenlandic fjord. Polar Biol. doi:10.1007/s00300-014-1493-y
Zaret TM, Kerfoot WC (1975) Fish predation on Bosmina longirostris: body size selection versus visibility selection. Ecology 56:232–237. doi:10.2307/1935317
Acknowledgments
This research project was funded by the Greenland Climate Research Centre (Project 6505). We also thank the Brazilian CsF/CNPq program (Grant no. 201086/2012-3) for its contribution. We wish to thank the captains and crew on Lille Masik and RV Dana for their help during sampling. We would also like to thank Karen Riisgaard, Sara Zamora-Terol, Birgit Søborg, Thomas Krog, Knud Kreutzmann, Henrik Philipsen and John Mortensen for help with logistics and equipment. Furthermore, we would like to thank Kerstin Geitner for graphical support and Julie Dinasquet for her comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swalethorp, R., Kjellerup, S., Malanski, E. et al. Feeding opportunities of larval and juvenile cod (Gadus morhua) in a Greenlandic fjord: temporal and spatial linkages between cod and their preferred prey. Mar Biol 161, 2831–2846 (2014). https://doi.org/10.1007/s00227-014-2549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2549-9