Abstract
Three new cases of sponge symbiosis between species of Plakortis and Xestospongia were found in reef caves and mesophotic reef habitats of the Caribbean. Plakortis sp. 1 from the Bahamas associates exclusively with Xestospongia deweerdtae which was originally described living freely on the deep fore-reef and caves of Jamaica. In addition, we found Plakortis sp. 2 from Puerto Rico which associates with both X. deweerdtae and a different Xestospongia sp. Sponge specimens were identified using cytochrome oxidase subunit 1, 28S rRNA and 18S rRNA gene sequence fragments, spicule analysis, and histological sections with SEM. Unlike previous sponge pairs, Xestospongia spp. not only grew as a thin veneer of tissue over the Plakortis host sponge but through the mesohyl, forming inner channels (0.1–1 cm) that may provide a benefit by facilitating more efficient water transport through the dense Plakortis tissue. Symbioses with both Plakortis spp. were documented from an early recruit stage through adulthood. Spicule measurements conducted on symbiotic versus free-living X. deweerdtae revealed significantly smaller spicule sizes for symbiotic individuals, suggesting a cost in terms of silicon availability, or a benefit in terms of a lower investment in skeleton synthesis for support. This study reveals new specialized symbiotic associations between distantly related sponge genera that likely represent an alternative strategy of adaptation for life in reef caves and mesophotic reefs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptic habitats and mesophotic reefs (30–150 m depth) harbor a remarkable diversity of sponges with an increasing number of new species being discovered (Granek et al. 2009; Gerovasileiou and Voultsiadou 2012; Slattery and Lesser 2012). Like shallow coral reefs and mangrove habitats, reef cave sponges play an integral role in benthic–pelagic coupling processes (Southwell et al. 2008; Granek et al. 2009). For example, in reef caves of the Caribbean, sponges can remove from the flushing waters much of the dissolved organic matter returning nutrients and particulate matter to adjacent coral reefs (de Goeij et al. 2008; Slattery et al. 2013; de Goeij et al. 2013). On mesophotic reefs, sponge diversity exceeds that of coral and algae species making them biodiversity refuges for the more threatened shallower reef systems (Lesser et al. 2009; Kahng et al. 2010).
Reef caves can be crowded environments where sponges cope with space limitation by forming epizoic interactions with other sponge hosts without interfering with their host sponge pumping rates (Rützler 1970; Sarà 1970). In open coral reef communities, sponge epizoic interactions have been described as a strategy for surviving abiotic threats such as strong storm surges, but the cost of the interaction can also result in overgrowth and death of the host sponge (Wulff 1997, 2008b). The ability of opportunistic sponges to outgrow other sponge species may result from faster growth because of photosynthetic symbionts or by their ability to invest more energy in growing quickly rather than in producing chemical defenses (Rützler 1981; Walters and Pawlik 2005; Pawlik et al. 2008).
There have been several studies highlighting facilitation interactions between coral reef and sea grass bed sponges, where epizoism does not result in the host being smothered (Wilcox et al. 2002; Ávila et al. 2007; Wulff 2008a). For example, to avoid predation, the coral reef sponge Lissodendoryx colombiensis forms assemblages with several sea grass sponge species (Cliona caribbaea, Amphimedon erina, Clathria schoenus, and Tedania klausi) that deter the sponge-eating seastar, Oreaster reticulatus (Wulff 2008a). In turn, the sea grass species use L. colombiensis as a substrate for stable settlement. Other relationships that have persisted for longer periods of time have resulted in more specialized associations as observed between the sponge pairs A. erina and Geodia vosmaeri (Engel and Pawlik 2000; Wilcox et al. 2002; Ramsby et al. 2012). Intriguingly, A. erina that associates with L. colombiensis commonly overgrows the palatable sponge G. vosmaeri protecting it from O. reticulatus (Ramsby et al. 2012).
In the present study, we report three novel specialized sponge associations between species of Plakortis and Xestospongia found living in reef caves and on the upper mesophotic reefs (30–36 m) of the Bahamas and Puerto Rico. Photographic images of the association from these sponges have been documented by Pomponi (Twilight Zone Expedition Team 2007, NOAA-OER www.photolib.noaa.gov/htmls/reef3932.htm) at a depth of 60 m in the Cayman Islands and by Zea et al. (2009). Initial morphological analysis of associated individuals showed a similar spicule complement among all the basibiont Plakortis samples but with smaller spicule size than nearby free-living specimens of Plakortis halichondrioides, the latter previously thought to be the species in the association (Zea et al. 2009). Also, owing to its strongyle spicules and its skeletal arrangement, associated epibiont specimens were initially thought to be Xestospongia deweerdtae until free-living specimens of this species were found to have the same but larger spicules; this led Zea et al. (2009) to suggest that the epibiont Xestospongia was of a different species. In addition, while sampling the association, a clearly different species with oxea instead of strongyle spicules (hereafter named Xestospongia sp.) was found.
Frequent field observations of these interactions motivated the following three objectives of this study: (1) to carry out the taxonomic identification and classification of all species involved in the association using the cytochrome oxidase I (COI), 28S rRNA and 18S rRNA genes for bar coding in combination with classical morphological characteristics; (2) to compare structural differences between free-living versus symbiotic individuals of the epibiotic Xestospongia species where both stages have been found, to look at possible benefits or disadvantages gained from the association, and (3) to conduct surveys to determine the frequency of associated individuals. We hypothesize that this is a mutualistic relationship in which the Plakortis and the Xestospongia are receiving reciprocal benefits based on the observation that the associated Plakortis spp. and Xestospongia spp persist for long periods of time without the one species smothering the other.
Methods
Sample collection
Sponge individuals were collected from three geographic locations: Mexico in July 2012, Bahamas in July 2011 and Puerto Rico in October 2011, between a depth of 25–36 m (Supplementary Table 1). The number of individuals per species and the metadata for the samples collected are given in Table 1. Samples of free-living P. halichondrioides were also taken, as at the time it was believed that it was the basibiont species of the association (Zea et al. 2009). Sponges were cut at the base to reduce tissue damage and to avoid DNA cross-contamination between species. Whole individuals were placed in 15-l O2/CO2 permeable plastic bags Ken’s Fish (Taunton, MA) for transportation to the surface where they were placed in 200-l tanks. Careful dissections and close examinations of the association were performed in the holding tanks. The associated sponge individuals were carefully dissected away from their sponge pair to retrieve clean samples for DNA from each of the species in the association. Sponge samples were cut into 1 cm3 pieces and were frozen or fixed and preserved using different methods depending on the objective (see below).
DNA extraction and sequencing
Fresh sponge tissue cubes were fixed in RNAlater and frozen at −80 °C. DNA was extracted using Qiagen’s AllPrep DNA/RNA Mini Kit. Universal primers were used initially to amplify and sequence the cytochrome oxidase subunit I (Folmer et al. 1994), 18S rRNA gene (18SHOMOR, 18SHOMOF, from Gazave et al. 2010), and 28S rRNA gene (28SLSU5F, 28SLSU300F, 28SLSU300R, 28SLSU1200R, and 28SLSU1642R, from Redmond et al. 2011). Once initial sequences were retrieved, specific internal and pair-end primers were designed for amplification of each species-specific gene (6 primer pairs for COI, 8 primer pairs for 18S rRNA, 11 primer pairs for 28S rRNA). Primer direction and sequence were determined using Sequencher, and primer sequences were evaluated using NetPrimer (http://www.premierbiosoft.com/netprimer/index.html). Species-specific primer sequences designed for each gene of the sponges involved in the association are listed in Supplementary Tables 2 and 3.
Preliminary optimization runs were performed using a 15 °C gradient, and specific annealing temperatures for each primer pair were selected for each species of the association. The highest annealing temperature showing a strong PCR product from the optimization gradient was selected for highest primer specificity. For each reaction, DNA from both sponges was used as a control to make sure that the PCR product was from the species of interest. Polymerase chain reactions were carried out in 50 µl total volume including the following: 39.3 µL of H2O, 1 µl of each primer (10 mM), 1 µl dNTPs (10 mM), 5 µl 10× Buffer, 1.5 µl of MgCl2 (50 mM), 0.2 µl of Platinum Taq polymerase, 1 µl of template DNA (30 µM). The PCR program consisted of an initial denaturation at 94 °C for 2 min followed by 35 cycles of 94 °C for 30 s; annealing at 50 °C (PLRV2/PLFWD3 P. halichondrioides), 63 °C (PLRV2/PLFWD3 other Plakortis spp.), and 68 °C (XDRV6/XDFWD6 associated Xestospongia spp.) for COI; 60.9 °C (PLRV1/PLFWD1 P. halichondrioides), 63 °C (PLRV2/PLFWD3 Plakortis spp.), and 63 °C (XDRV1/XDFWD1 Xestospongia spp.) for 18S rRNA gene; 54.6 °C (PLRV1/PLFWD1 P. halichondrioides), 63 °C (XDRV1/XDFWD1 X. deweerdtae) for 28S rRNA gene; and an extension at 72 °C for 5 min and a final extension of 72 °C for 5 min. PCR products were ran in 1 % agarose gel and purified using the QIAquick Gel Extraction Kit (Qiagen). Sequencing reactions were performed using the BigDye TM terminator v. 3.1 with species-specific PCR primers. Sequencing was done with an ABI Prism 2100 automated sequencer.
Phylogenetic analysis
Sequences were assembled and edited using Sequencher. Double coverage reads allowed for accurate editing of sequences. Complete consensus sequences for each gene fragment were exported into a single fasta file format from Sequencher and uploaded with MEGA 5 (Tamura et al. 2011). Sequences were then aligned using the ClustalW function with default parameters. The closest relative to our sequences was searched using the BLAST function from GenBank. These sequences were used as reference sequences for alignment and tree generating purposes. Aligned sequences for each gene (COI/18SrRNA/28SrRNA) were joined. MEGA 5 was used to construct a concatenated tree of the three genes using a neighbor-joining (NJ), maximum parsimony (MP), and maximum likelihood analysis. We ran the NJ analysis using a maximum composite likelihood model of nucleotide substitution with substitutions to include Transitions + Transversions, and data were re-sampled using 1,000 bootstrap replicates. The MP analysis consisted of a heuristic search with a subtree pruning regrafting method using 10 random addition replicates. A Close-Neighbor-Interchange branch-swapping algorithm was used, and data resampled using 1,000 bootstrap replicates. The ML analysis was run using the Tamura Nei model with uniform rates among sites (Tamura and Nei 1993). A nearest-neighbor interchange ML heuristic method was used, and data were resampled using 1,000 bootstrap replicates. All sequences were deposited in GenBank.
Spicule morphology and size
Sponge spicules were prepared by immersing 1 mm3 pieces of previously frozen tissue into 1 ml of 65 % nitric acid. Samples were hand-stirred and incubated at room temperature for 3 h. Samples were centrifuged and washed with distilled water, and a few drops of spicules suspensions were mounted fresh on a microscope slide; spicules were characterized and measured under a light microscope. Fifty spicules were measured for each sample of free-living and associated Xestospongia spp. One hundred diod spicules and 10 triod spicules were measured for each sample of Plakortis spp. A nested analysis of variance (ANOVA) for X. deweerdtae spicule dimensions for the Bahamas was carried out using association as a factor, and individuals as a nested factor (there being 50 spicule measurements per individual), separately for length and width. The same was carried out to compare associated Plakortis sp. 1 with P. halichondrioides from the Bahamas with the factor being species, and individuals as nested factor. A third ANOVA was carried to compare spicule sizes between the three Plakortis spp. in Puerto Rico with the factor being species, and individuals as nested factor. Tukey’s post hoc test was run following significant (P < 0.05) ANOVA outcomes. Log10 or square root transformations were employed when necessary to adjust for normality of residuals and homogeneity of variance among levels of the main factor. Statistical analyses were performed using the software program Statgraphics Plus 5.1.
Sectioning and scanning electron microscope imaging
To observe tissue arrangements between Plakortis and Xestospongia sponge pairs, sponge pieces (0.25 cm3) were prepared. Fresh sponge tissue cubes were fixed in 9 ml of 0.1 M sodium cacodylate prepared in artificial seawater (ASW) with 1 ml of 2.5 % glutaraldehyde at 4 °C for 24 h. Pieces were transferred to 10 ml of sodium cacodylate buffer in ASW and held at 4 °C until further processing. Fixed sponge tissues were then placed in 1 % (w/v) osmium tetroxide solution (prepared in 0.2 M potassium phosphate buffer, pH 7.4) for 3.5 h and subsequently dehydrated in a graded ethanol (EtOH) series (15, 35, 55, 75, 85, and 95 % (v/v) ethanol). Gold-coated sponge pieces (0.25 cm3) were mounted on lead stubs and gold-coated in a SeeVac Auto conductavac IV sputter coater. Samples were visualized under a JEOL JCM-5700 Scanning Electron Microscope.
Hematoxylin and eosin staining
Sponge samples preserved in 4 % paraformaldehyde were embedded in paraffin. Tissue was cut in 7–60-µm-thick sections with a LKB 2128 Ultrotome IV. Paraffin sections were placed on slides, immersed in xylene, and dehydrated in a graded EtOH series (70, 85, 95 %). Sections were immersed in PBS buffer for two minutes and then washed with deionized water (DI). Sections were stained with Harris hematoxylin solution for 20 min followed by a 5-min rinse with running DI. Sections were then destained in 1 % acid EtOH for 30 s and subsequently washed with DI for 1 min. Sections were exposed to bluing in 0.2 % ammonia water for 1 min and washed in DI for 5 min. Sections were dehydrated in a graded EtOH series (25, 50, 70, 95 %), counterstained in eosin–phloxine solution for 1 min, and washed in 95 % EtOH before mounting with xylene-based mounting medium.
Reef surveys
Candidate survey sites were scouted and selected based on the presence of steep drop-offs, overhangs, and caves, all habitats likely to host associated individuals. GPS coordinates and full survey site names can be found in Supplementary Table 1. Surveys using 100-m transect tape between 20 and 30 m depth were conducted along the Yucatan coast (N = 4) in July 2012, in Puerto Rico in June 2011 (N = 6), and in the Bahamas (N = 9) in July 2011 and July 2013. The tape was loosely laid or hanged on the reef wall, and all caves and crevices found in about 1 m on each side of the tape were examined. Since Plakortis sp. 1 and Plakortis sp. 2 individuals are difficult to distinguish in the field, we refrained from separating them as two different species in our analysis. There are obvious morphological differences between X. deweerdtae and Xestospongia sp. that made it easy for us to indicate which species of Xestospongia was spotted. For our surveys, we recorded the presence of Plakortis/Xestospongia associated pairs and of free-living X. deweerdtae and observations on their association status were noted (associated/free-living). Underwater digital photographs of different sponge morphologies were taken for associated and free-living individuals. In Puerto Rico, five sponge associates were tagged (Plakortis sp. 2/Xestospongia sp. (N = 4) and Plakortis sp. 2/X. deweerdtae (N = 1)), photographed, and monitored for growth for 8 months.
Results
Phylogenetic analysis
The concatenated phylogenetic tree generated from partial sequences of the COI (463 bp), the 18S rRNA (1,300 bp), and the 28S rRNA (750 bp) gene is shown in Fig. 1. The closest concatenated sequences to the COI/18S rRNA/28S rRNA genes of Plakortis sp. 2 were those from Plakortis simplex (91.9 % identity, 100 % coverage), P. halichondrioides (91.2 % identity, 100 % coverage), Plakina jani (91.1 % identity, 100 % coverage), and Plakinastrella cf. onkodes (90.9 % identity, 100 % coverage). All Plakortis sp. 2 individuals shared identical sequence irrespective of whether they were associated with X. deweerdtae or Xestospongia sp. and were supported by bootstrap values of 89/100/100. P. halichondrioides from Puerto Rico and the Bahamas were 100 % identical and showed 0.48 % divergence (5 bp) with P. halichondrioides (GenBank accession no. HM118543). Identical COI/18S rRNA/28S rRNA gene sequences for P. halichondrioides samples were obtained from Puerto Rico and the Bahamas. Samples of P. halichondrioides collected in this study showed 1.29 % (29 bp) divergence to P. halichondrioides with 100 % coverage supported by bootstrap values of 88/100/100.
Concatenated phylogenetic tree generated from an alignment of 463 bp of the cytochrome subunit 1, 1300 bp of the 18S rRNA gene, and 750 bp of the D1-D2 regions of the 28S rRNA gene of sponges sequenced in this study (bold). Reference sequences downloaded from GenBank are indicated by species name in italics. Coding preceding sample name refers to collection location as referenced in Table 1. Following the location is the association status of the individual (A—associated; FL—free-living). The tree topology was obtained from neighbor-joining (NJ) analysis. The bootstrap values at each node were generated from (NJ), maximum parsimony (MP), and maximum likelihood (ML) analysis, respectively
All X. deweerdtae individuals formed a monophyletic group supported by bootstrap values of 99/100/90. However, there were some subtle base pair differences between individuals. For example, X. deweerdtae individuals (PKBHFL, LPPRA, DCPRA) were all 100 % identical, but were 0.13 % (3 bp) divergent to X. deweerdtae (DCPRFL, COMXFL). In addition, X. deweerdtae individuals (LSBHA, MPVBHA) were 0.42 % (10 bp) divergent to X. deweerdtae individuals (PKBHFL, LPPRA, DCPRA). Amplification of the 28S rRNA gene of Xestospongia sp. and Plakortis sp. 1 was attempted with eight different primer combinations listed in Supplementary Table 2 without successful amplification. This failure suggests the possible presence of mutations in the primer annealing sites.
A second concatenated tree from partial sequences (463 bp) of the COI and (1,300 bp) of the 18S rRNA was generated to include sponges Xestospongia sp. and Plakortis sp. 1 (Fig. 2). The Xestospongia sp. and Plakortis sp. could not be included in the concatenated tree shown in Fig. 1 because we were unable to amplify the 28S rRNA genes from these sponges. The closest concatenated sequence to the COI/18S rRNA genes of Plakortis sp. 1 is that of Plakortis sp. 2 (96.6 % identity, 100 % coverage) and together formed a clade supported by bootstrap values of 97/97/99. Xestospongia sp. shared 89.9 % identity (100 % coverage) with X. deweerdtae and 91.5 % identity (97 % coverage) with Xestospongia muta. The closest 18S rRNA gene sequence to Xestospongia sp. was Oceanapia sp. (GenBank accession no. DQ927317), Gelliodes callista (GenBank accession no. KC902338), and Dasychalina fragilis (GenBank accession no. DQ927316) all showing 91 % identity (100 % coverage) (Supplementary Fig. 1). However, the closest COI gene sequence to Xestospongia sp. LPPRA was Petrosia sp. (GenBank accession no. JN242216) showing 94 % identity (100 % coverage) (Supplementary Fig. 2).
Concatenated phylogenetic tree generated from an alignment of 463 bp of the cytochrome subunit 1 and 1300 bp of the 18S rRNA gene of sponges sequenced in this study (bold). Reference sequences downloaded from GenBank are indicated by species name in italics. Coding preceding sample name refers to collection location as referenced in Table 1. Following the location is the association status of the individual (A—associated; FL—free-living). The tree topology was obtained from neighbor-joining (NJ) analysis. The bootstrap values at each node were generated from (NJ), maximum parsimony (MP), and maximum likelihood (ML) analysis, respectively
Tree topologies supported by bootstrap values from NJ, ML, and MP analysis were consistent in separating haplosclerid from homoscleromorph sponges for the three genes; X. deweerdtae individuals and associated Plakortis spp. were each also monophyletic for the three genes used in this study (Figs. 1 and 2). Basibiont Plakortis spp. was definitely not P. halichondrioides or any of the other included species of Homoscleromorpha. Regardless of origin (free-living vs. associated), all samples of strongyle-bearing Xestospongia were >99 % identical for the three genes. This led us to identify them as X. deweeerdtae and to hypothesize that the association is responsible for the differences in spicule size (see below). Oxea-bearing epibionts were genetically very far from X. deweerdtae in the two genes that could be amplified, confirming them to be a different species. We have tentatively named this species as Xestospongia sp. owing to its spicule size and skeletal architecture; however, judging from the polyphyletic nature of this genus (Supplementary Figs. 1 and 2), and the genetic proximity to some Haliclona-like species, further analyses are needed to clarify its taxonomic status.
Distribution and morphological characters of sponge pairs
Plakortis sp. 1 was found in the Bahamas, and individuals were thickly encrusting to massive with lobate morphology. Individuals of Plakortis sp. 1 measured 3 × 30 cm by 1–8 cm thick with oscules 0.2–0.9 cm in diameter. Plakortis sp. 1 individuals were dark brown in vivo and exuded a brown pigment when preserved in ethanol. Individuals had a soft compressible consistency and exhibited an irregular shape of the outer surface with grooves along the surface of the sponge body. The ectosome (40–60 µm) in transversal sections was poorly differentiated but distinguishable, being denser than the choanosome. A confused choanosomal skeleton with spherical and irregular canals (100–300 µm) was visible. Both adults and sponge recruits of Plakortis sp. 1 were exclusively associated with X. deweerdtae in cryptic habitats but were also found settling on the bedrock at a depth of 36 m. Since we initially assumed free-living individuals of this association were P. halichondrioides, we did not purposely search for other possible species. We observed the association from an early recruitment stage (Fig. 3a) where X. deweerdtae is growing as small translucent colonies through the Plakortis sp. 1 tissue. In adult individuals, X. deweerdtae can grow as patches over the Plakortis sp. 1 body (Fig. 3b) or completely cover Plakortis sp. 1 as a pink encrusting tissue without overgrowing the oscula (Fig. 3c). Diod spicules were observed for Plakortis sp. 1 where diod lengths ranged between 21 and 223 μm, individual means ranging from 103 to 124 μm (Table 2). Although rare, small diods (21–40 μm) were observed in all individuals, large diods in Plakortis sp. 1 had mixed morphologies between straight and irregular with acerate endings. Diod centers were thick and occasionally S-bent (Supplementary Fig. 3A).
Growth stages of Plakortis/Xestospongia associations. a Plakortis sp. 1 (brown) associated with X. deweerdtae (translucent spots) at a recruit stage. b, c) Plakortis sp. 1 adult growing massively with X. deweerdtae (pink). d Plakortis sp. 2 recruit associated with X. deweerdtae (translucent spots). e Plakortis sp. 2 adult associated with X. deweerdtae. f Free-living morphotype of X. deweerdtae. g–i Plakortis sp. 2 associated with Xestospongia sp. (blue) growing with encrusting and papillate morphology. Scale bar (a) 1 cm, (b–d), (g–i) 5 cm, (e–i) 2 cm
In the Puerto Rican archipelago, Plakortis sp. 2 can be found in reefs off the south and west continental shelf (30–36 m) as well as shallow reef caves (12 m) in Mona and Desecheo Island (personal observations of JV). Only associated individuals of Plakortis sp. 2 were found. Plakortis sp. 2 individuals were more commonly found thinly encrusting, measuring between 3 and 10 cm of maximum diameter by 1–3 cm thick, with oscules 0.3–1.7 cm in diameter. Plakortis sp. 2 individuals were dark brown to dark green in vivo and exuded a brown pigment when preserved in ethanol. Individuals had a soft compressible consistency and exhibited a smooth outer surface with occasional grooves along the surface of the sponge body. Like Plakortis sp. 1, the ectosome (30–50 µm) in transversal sections was poorly differentiated. Irregular shaped canals (100–300 µm) were surrounded by a confused choanosomal skeleton. Diod length in Plakortis sp. 2 ranged between 43 and 160 μm, individual means ranging from 115 and 121 μm when associated with X. deweerdtae and 107 to 114 μm when associated with Xestospongia sp. (Table 2). Like Plakortis sp. 1, the Plakortis sp. 2/X. deweerdtae association was found at a recruit stage and an adult phase (Fig. 3d, e). However, overgrowth by X. deweerdtae throughout the Plakortis sp. 2 body is limited to grooves along the sponge surface as well as the mesohyl, but never overgrows individuals completely, as observed in Plakortis sp. 1 (Fig. 3e).
Although rare, both Plakortis sp. 1 and Plakortis sp. 2 had Y-shaped triods with sharp endings (Supplementary Fig. 3A, B). Triods in Plakortis sp. 1 measured between 40 and 107 μm (longest span); triods in Plakortis sp. 2 measured between 38 and 108 μm averaging 67 to 78 μm when associated with X. deweerdtae and 57 to 64 μm when associated with Xestospongia sp. (Table 2).
Following up on the original idea that associated Plakortis were P. halichondrioides, spicule size of Plakortis sp. 1 and 2 was compared with those of P. halichondrioides individuals from the Bahamas and Puerto Rico (Supplementary Fig. 3C). Diod length and width of P. halichondrioides from the Bahamas were significantly larger than Plakortis sp. 1 (ANOVA, between species P < 0.001), but widths were not (P > 0.05). Mean sizes (±1 standard deviation, length × width) for P. halichondrioides individuals from the Bahamas were 166 ± 20 by 4.0 ± 1.0 μm in width (N individuals = 3), while those of Plakortis sp. 1 were 113 ± 29.6 by 3.4 ± 0.9 μm (N individuals = 3). A subsequent ANOVA and Tukey’s post hoc analyses comparing diod mean length and width (±1 standard deviation, length × width) between Plakortis sp. 1, Plakortis sp. 2, and P. halichondrioides from Puerto Rico revealed significantly larger diod lengths (P < 0.001) but not widths (P > 0.05) for P. halichondrioides than for Plakortis sp. 1, which were in turn longer than for Plakortis sp. 2 (Fig. 4). Mean sizes (±1 standard deviation, length × width) for P. halichondrioides individuals from Puerto Rico were higher in length [162 ± 14 by 4 ± 0.8 μm in width (N individuals = 3)], followed by Plakortis sp. 2 associated with X. deweerdtae [117 ± 16 by 3.7 ± 0.9 μm (N individuals = 3)], Plakortis sp. 1 [113 ± 30 by 3.4 ± 0.9 μm (N individuals = 3)], and with smallest diod size Plakortis sp. 2 associated with Xestospongia sp. [110 ± 16 by 3.6 ± 0.9 μm (N individuals = 3)].
Diod spicule length and width (mean ± 1 standard deviation) of diods from P. halichondrioides (circles), Plakortis sp. 1 associated with X. deweerdtae (squares), Plakortis sp. 2 associated with X. deweerdtae (triangles) and with Xestospongia sp. (diamonds). Open shapes represent samples from the Bahamas and closed shapes from Puerto Rico. Light microscope photographs of the spicules of each species at relative size are shown for comparisons
Free-living individuals of X. deweerdtae grew as thickly (1–2 cm) encrusting circular patches (6–12 cm) with 5–10 oscules per individual measuring up to 5 cm in height and 1 cm in diameter (Fig. 3f). Color between X. deweerdtae individuals varied between purple, bright pink, and white. Sponges had a smooth surface and were slightly compressible, although dried specimens were hard. Associated individuals grew as a thin veneer of tissue through the mesohyl and surface of Plakortis spp.
Associated and free-living forms of X. deweerdtae share a unispicular isotropic reticulation of strongyle spicules. Strongyle length and width of free-living individuals of X. deweerdtae from the Bahamas were significantly larger than associated individuals of X. deweerdtae (ANOVA, between associations P < 0.001) (Supplementary Fig. 3D, E). Indeed, mean sizes (±1 standard deviation, length × width) for associated individuals were lower [218 ± 17 μm in length by 8.2 ± 1.7 μm in width (N individuals = 4)] than those of free-living individuals [340 ± 22 by 11.2 ± 2.2 μm (N individuals = 2)]. The same situation was found in Puerto Rico, although statistical comparisons could not be carried out owing to lack of replication, and associated X. deweerdtae individuals averaged 236 ± 21 by 9.7 ± 1.9 μm (N individuals = 4) compared to a single free-living individual which averaged 327 ± 15 by 13.3 ± 2.1 μm. In Mexico, only free-living individuals were observed and measured 373 ± 27.9 by 13.5 ± 2.6 μm (N individuals = 5) (Fig. 5).
Xestospongia sp. grew as encrusting or papillate morphology with a light blue to white veneer of tissue that also penetrated the Plakortis sp. 2 mesohyl (Fig. 3g–i). The association between Plakortis sp. 2 and Xestospongia sp. has currently been observed only on the south continental shelf reef habitats of La Parguera, Puerto Rico. The skeleton arrangement of Xestospongia sp. consisted of a unispicular isodictyal reticulation of oxeas (Supplementary Fig. 3F). Oxea size ranged between 199 and 277 μm individual means ranging from 229 to 230 μm.
Morphology of the association
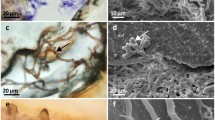
Images from dissected specimens revealed that the epibiont X. deweerdtae penetrated through the Plakortis body forming inner channels that measured 0.1–1 cm in diameter (Fig. 6a–c). SEM images of the association between Plakortis sp. 1, 2 and X. deweerdtae sponge pairs showed detailed spicule arrangement in the mesohyl (Fig. 6a, b). Despite boring into the Plakortis body, a well-defined barrier between the two species was observed in the mesohyl (Fig. 7). The choanosome of Plakortis spp. and Xestospongia spp. is separated in the sponge mesohyl (Fig. 7a, c, e). Diods and triods from Plakortis spp. never crossed the surface of the interface, and it was the strongyles from X. deweerdtae and oxeas from Xestopongia sp. that slightly penetrated the Plakortis sp. 1 and 2 tissue instead (Fig. 7b, d, f).
Transversal dissection of a Plakortis sp. 1 adult with X. deweerdtae (epibiont) boring from the Plakortis outer surface into the mesohyl. b, c Inner channels formed by X. deweerdtae measuring from 1 mm up to 1 cm in diameter. d, e Handmade transverse section of the mesohyl of Plakortis sp. 1 and 2, respectively, associated with X. deweerdtae (SEM). Scale bar (d, e) 200 µm
Hemocytoxylin and eosin-stained transverse section of the mesohyl at the interface of Plakortis sp. 1 and X. deweerdtae (a, b), Plakortis sp. 2 and X. deweerdtae (c, d), Plakortis sp. 2 and Xestospongia sp. (e, f). Scale bar (a, c, e) 200 µm (B, D, F) 100 µm. Section thickness (a, b) 30 µm, (c–f) 10 µm
Reef surveys
The highest number of free-living individuals of X. deweerdtae was found in the Bahamas with an average of 4.1 individuals per site (LIBH, N = 4; AIBH, N = 3; PKBH, N = 1; GIBH, N = 1; MIBH, N = 1) from nine sites that were surveyed, followed by Mexico with 0.75 individuals per site (CB2COMX, N = 1; CBCOMX, N = 1, CACOMX, N = 1) from five sites that were surveyed and Puerto Rico with 0.33 individuals per site (DCPR, N = 2) from four sites that were surveyed. Associated individuals of X. deweerdtae were confined to the Bahamas sites (LIBH, N = 8; MIBH, N = 7; SSBH, N = 7; HRBH, N = 4; GIBH, N = 3; PKBH, N = 2; MVPBH, N = 1) and in Puerto Rico (IMMOPR, N = 13, PCMOPR; N = 11, DCPR, N = 9; CNMOPR, N = 6; CECMOPR, N = 5; LPPR, N = 2) (Fig. 8; Supplementary Table 1). Individuals of Xestospongia sp. were only found associated with Plakortis in the southern continental shelf reef habitats of Puerto Rico (LPPR, N = 6) with an average of 1.2 individuals. Associated individuals of Plakortis spp. were absent in all transects done in Mexico. From the six associated individuals that were tagged and photographically monitored for an 8-month period, none of the sponge pairs’ growth morphologies showed signs of smothering over the other (Supplementary Fig. 4). Zooanthids were observed growing exclusively on individuals of X. deweerdtae associated with both Plakortis spp. No free-living individuals of X. deweerdtae intercepted in our transects were observed with zooanthids.
Average number of free-living (solid bars) and associated (diagonal striped bars) individuals of X. deweerdtae in Mexico (N sites = 4), Puerto Rico (N sites = 5), and the Bahamas (N sites = 9) along 100-m transects; the number of associated Xestospongia sp. (horizontal striped bars); location names for each acronym and number of individuals spotted for each species are listed in Supplementary Table 1
Discussion
Mutualistic epizoic interactions have long been documented as a facilitation strategy by sponges to deal with competition in highly diverse and densely packed sponge communities of reef cave habitats (Rützler 1970). Likely to represent a facilitation strategy, three new specialized associations between Homoscleromorph and Haploscerid sponges are reported in this study. We found Plakortis sp. 1 and 2 individuals always living beneath the thin veneer of tissue of X. deweerdtae which was originally described living freely on the deep fore-reef (76–82 m) of Jamaica (Lehnert and van Soest 1999), and later in shallow reef caves of Curaçao (10–12 m) (Soest and Weerdt 2001) and in the Bahamas (Zea et al. 2009). We also found a second species of Xestospongia sp. associated with Plakortis sp. 2 in the southern continental shelf of Puerto Rico. The tissues of both Xestospongia spp. grow within the mesohyl and part of the surface of Plakortis spp., unlike the basibiont–epibiont associations documented for other sponge pairs (Figs. 6 and 7). For example, in previously reported sponge pairs, the epibiont is found living on top of the basibiont where there is a clear barrier between both sponge tissues and mixed sponge tissues are never found in the mesohyl (Wulff 2008a, Ramsby et al. 2012). Mixed tissues in the mesohyl are still differentiated, where the choanosome of Plakortis spp. is enclosed from Xestospongia spp. (Figs. 6b, 7a, d, f). A similar system was observed by Rützler (1970) in the Adriatic Sea where Aplysilla sulfurea was fully overgrown by the basobiont Gellius fibulatus but remained living in its mesohyl. Through extensive survey data, we observed the association from an early recruitment stage for both Plakortis spp. (Fig. 6a, d) where X. deweerdtae is observed growing as small translucent colonies within the Plakortis spp. tissues. These are the first observations of sponge pairs ever reported from a recruiting stage that could be representative of a highly specialized mutualistic relationship.
Associated specimens of X. deweerdtae were previously thought by Zea et al. (2009) to be a different species, to be compared with Haliclona strongylophora (Lehnert and Van Soest 1996), the only other sponge species with a unispicular skeleton of 150- to 200-μm strongyles. However, our work with partial sequences of the 18S rRNA, 28S rRNA, and COI genes of associated individuals compared with free-living individuals of X. deweerdtae collected from three different geographic locations revealed that associated individuals were all >99 % similar to free-living X. deweerdtae (Figs. 1, 2). All X. deweerdtae individuals were monophyletic and supported by a single clade across the three genes. However, the sequences most closely related to the X. deweerdtae clade were those of Geliodes gallista and Dasychalina fragilis. The sponges G. gallista and D. fragilis both belong to sponges of the family Niphatidae but in our analysis are grouped with X. deweerdtae which is in the family Petrosidae (Supplementary Figs. 1, 2). Similar groupings were observed by Redmond et al. (2011) and are characteristic of the polyphyletic nature of the Haplosclerida.
Molecular confirmation of the conspecificity of associated and free-living specimens of X. deweerdtae suggests that differences in spicule size are due to its association with Plakortis spp. and not due to differences in silica concentrations from different geographic locations (Bavestrello et al. 1993). This would imply a cost in terms of silicon availability, or a benefit in terms of a lower investment in skeleton for support and defense (Hill et al. 2005). Assuming that sponges can be severely limited for silica (Maldonado et al. 2012a) and that there is a need for larger spicules for support or defense, these results suggest that the association could be beneficial for X. deweerdtae, allowing it to invest less energy in spicule synthesis. A similar observation was made for the association between G. vosmaeri and A. erina, in which the cortex of G. vosmaeri was significantly reduced when associated with A. erina in comparison with the free-living form (Ramsby et al. 2012).
Spicule composition of Plakortis sp. 1 and 2 consisted of diods and triods, but lacked microrhabds or quasiamphiasters, which drew our attention to consider Plakortis simplex and P. halichondrioides as closely related species. The concatenated COI/18SrRNA gene sequences of Plakortis sp. 1 and 2 showed only 91.9 % identity, 100 % coverage with Plakortis simplex and 91.2 % identity, 100 % coverage with P. halichondrioides (Fig. 2), making it unlikely that Plakortis sp. 1 and 2 are closely related to either P. simplex or P. halichondrioides. Plakortis sp. 1 showed microsclere diods which has only been observed in Plakortis berquistae (Muricy 2011), Plakortis galapagensis (Desqueyroux-Faundez 1981), and the recently described Plakortis dariae and Plakortis edwardsi (Ereskovsky et al. 2013). Unfortunately, we did not have access to individuals of P. dariae and P. edwardsi to compare molecular sequences. However, morphological differences consisted of Plakortis sp. 1 only having Y-shaped triods and individual measuring up 3–30 cm by 1–8 cm thick with oscules 0.2–0.9 cm in diameter which are significantly larger than those of P. dariae. and P. edwardsi individuals. Spicule arrangements in the ectosome of both Plakortis sp. 1 and 2 never crossed the surface, whereas spicules of Plakortis dariae do. Plakortis sp. 1 and 2 also exhibit an irregular shape of the outer surface resulting in grooves along the surface of the sponge body where X. deweerdtae tissue occurs (Fig. 3b, e), whereas the surface of P. edwardsi and of Plakortis dariae is smooth (Ereskovsky et al. 2013). In addition, P. dariae and P. edwardsi are found in the same deep reef habitats (25–28 m) of Jamaica where X. deweerdtae was originally documented (Lehnert and van Soest 1999), but neither of these species has been reported to be associated with X. deweerdtae. If, in fact, P. dariae and P. edwardsi were to be either of the Plakortis species reported here, they would likely be found associated with X. deweerdtae which at this point remains an exclusive characteristic of Plakortis sp. 1 and 2. We therefore conclude that Plakortis sp. 1 and 2 are likely undescribed species.
Although smaller diod size classes are characteristic of P. dariae, and P. edwardsi, we believe that the smaller spicule size for the associated Plakortis individuals is a benefit for Plakortis spp. By producing smaller spicules, these sponges are possibly investing less energy in skeleton synthesis. One additional potential benefit for Plakortis spp. is that the inner channels formed from the less dense Xestosgpongia spp. tissue would facilitate more efficient water transport through the denser Plakortis spp. tissue. This builds on the hypothesis from Vogel (1978) proposing that denser sponge tissues tend to have a greater abundance of small water channels that require more energy to pump water than less dense sponge tissues. Images of dissected Plakortis spp. associated with Xestospongia spp. have been observed to have a less dense arrangement of spicules forming channels (0.1–1 cm) with wider meshes (200–500 μm) throughout the Plakortis spp. body (Figs. 6, 7). Since both associated cases of Xestospongia spp. are thin walled sponges in comparison with the Plakortis spp., the Xestospongia spp. are also more likely to display current induced flow than their basibiont (Leys et al. 2011).
Survey data also revealed a higher abundance of associated X. deweerdtae individuals over free-living individuals only when either Plakortis sp. 1 or 2 were present indicating that recruitment might be enhanced in the presence of either Plakortis sp. (Fig. 8). However, the apparent obligate symbiosis between Plakortis spp. and Xestospongia spp. could also be due to our inability to exhaustively survey all possible habitats where these sponges may be found in free-living form. Interestingly, Plakortis sp. 2 was found associated with Xestospongia sp. only in La Parguera, Puerto Rico (Fig. 8), suggesting possible host competition between different Haplosclerid species for Plakortis sp. 2. Associated individuals of X. deweerdtae were sometimes exposed on the wall of the deep fore-reef or on the bedrock at >30 m. Free-living X. deweerdtae were only found in entirely shaded areas and never exposed to light. Perhaps, Plakortis spp. allows X. deweerdtae to survive in more exposed habitats with higher predator pressure, either by providing the sponge with chemical defenses that it lacks or by providing UV protecting pigments to its tissue (Makarchenko and Utkina 2006). The chemical defense hypothesis is a likely possibility as sponges belonging to the genus Plakortis are well known to produce a wide range variety of biologically active cyclic peroxides (del Sol Jiménez et al. 2003). More specifically, bioactive polyketide endoperoxides and peroxide lactones have been isolated from Plakortis sp. 1 and 2 (Dalisay et al. 2010; Jiménez-Romero et al. 2010). Functional roles of cyclic peroxides from P. halichondrioides have been speculated to be involved in causing bleaching and death of 14 different coral species (Porter and Targett 1988). Chemical extracts from tissue of several Homoscleromorph species have also proven to provide chemical defense in feeding assays with the bluehead (Thalassoma bifasciatum) (Pawlik et al. 1995). Despite the overwhelming evidence of Plakortis spp. being heavily defended species, it could be that the Xestospongia spp. sponge pairs are chemically defended as well, therefore invalidating the chemical defense hypothesis as an ecological trade-off. Regardless, we are uncertain of possible predation pressures from spongivorous organisms in low-light habitats like reef caves and mesophotic reefs, and it is not known whether spongivory is similar to that found in shallow coral reef habitats or sea grass beds. We suspect that cave habitats have few spongivorous fish species, but cryptic mollusks, echinoderms, and crustaceans could be a threat to undefended sponges. Potential spongivores in these habitats may include cryptic starfish, sea urchins, nudibranchs, limpets, or cowries (Faulkner and Ghiselin 1983; McLean and Harasewych 1995; Wilson and Clarkson 2004). Unfortunately, there are limited studies on spongivory in reef cave habitats of the Caribbean.
Despite the evidence suggesting that the symbioses between Plakortis and Xestospongia are mutualistic, it is important to discuss any potential negative effects of the association. For example, individuals of Plakortis sp. 1 can be completely overgrown by X. deweerdtae leaving only the oscula of Plakortis sp. 1 free of tissue (Fig. 3c). We believe that when completely overgrown by X. deweerdtae, water is pumped through the X. deweerdtae ostia and enters the Plakortis spp. mesohyl through the inner channels (Fig. 6b) that interact with the Plakortis spp. body. X. deweerdtae could be at an advantage over Plakortis sp. 1 as it is the first to access any particulates in the water column. Although unlikely, we are uncertain whether X. deweerdtae could completely outgrow and eliminate Plakortis sp. 1. We have photographically monitored several individuals of Plakortis sp. 2 with X. deweerdtae and Xestospongia sp. which showed even distribution and no evidence of smothering by either species throughout an 8-month period (Supplementary Fig. 4). Stable growth morphologies of both species support a mutualistic relationship. In addition, individuals of both Plakortis sp. 1 and 2 are associated with X. deweerdtae from a recruiting stage (5 × 2 cm) (Fig. 6a, d), suggesting that these associations begin early in their life cycle. We also noticed that associated X. deweerdtae individuals were commonly colonized by zoanthids (personal observations by JV) (Fig. 3b, c) which are known to reduce pumping rates of host sponges (Lewis 1982). Intriguingly, zoanthids only colonized the X. deweerdtae tissue but not the Plakortis sp. 1 or 2 tissue. None of the free-living individuals of X. deweerdtae encountered in our surveys showed the presence of zoanthids although they have been observed in individuals collected in Curacao (Soest and Weerdt 2001). The presence of zoanthids could therefore affect the pumping rates and be a disadvantage for the Plakortis host that, without the presence of X. deweerdtae, would possibly avoid fouling as it is uncommon for Homoscleromorphs to host zoanthids (Swain and Wulff 2007).
We have discussed both mutualistic and commensalism scenarios for the associations between Plakortis and Xestospongia, and we conclude that these are likely of mutualistic nature. There are several hypothetical possibilities on how these associations originated. Based on biomass proportions of each associated sponge species, it seems as though at one point X. deweerdtae larvae likely settled on the Plakortis spp. becoming the epibiont. X. deweerdtae could have been resistant to antifouling allelochemicals from Plakortis species and overtime managed to grow from the Plakortis pinacoderm through the mesohyl. Persistence of these associations overtime presumably allowed for reproductive synchronization between both species. A reef cave habitat rather than the bedrock of the upper mesophotic reef is the best hypothetical setting where these associations first occurred, where larval dispersal from different sponge species is confined and more susceptible to interact with each other (Harmelin et al. 1985). Similar to the genetic isolation observed in Mediterranean caves that allowed for speciation of four different cryptic species of Plakina trilopha (Muricy et al. 1996), a similar process may have resulted in speciation of Plakortis sp. 1 and 2. These are all hypothetical scenarios that need to be tested in future studies. Long-term monitoring studies of tagged Plakortis sp. 1 and 2 recruits in combination with histological sections highlighting reproductive strategies of both sponges would help determine if in fact reproduction between both sponges is synchronous.
Another interesting topic for future study is to determine the allorecognition mechanism between free-living individuals of X. deweerdtae and Plakortis spp., to determine whether the interaction results in recognition or rejection of the heterospecific tissues (Connes et al. 1974; Mukai and Shimoda 1986). Parabiosis experiments using free-living individuals of X. deweerdtae with Plakortis sp. 1 and 2 would help determine how these sponges interact with one another at the interphase (Gaino et al. 1999). Characterization of the aggregation factors involved in cellular adhesion of both heterospecifics could also aid in understanding self- to non-self-recognition mechanisms of these sponges (Haseley et al. 2001).
Resolving the genetic structure of associated X. deweerdtae in cave habitats where free-living individuals co-occur would reveal whether there is genetic isolation between associated and the free-living X. deweerdtae individuals (López-Legentil and Pawlik 2009). Plakortis species are known to be a “high microbial abundance” (HMA) sponge, while Xestospongia species belong to the order Haplosclerida which have many species that are “low microbial abundance” (LMA) sponges (Maldonado et al. 2012b). It would be interesting to test the hypothesis that tissue densities between both species correlate with bacterial abundance and whether the more bacteria-rich Plakortis tissue serves as a source for key symbionts with important metabolic pathways that Xestospongia spp. would not be able to otherwise access. In this regard, it would also be interesting to look at the proportions of choanocyte chambers for both species and see how these are distributed and fit with the HMA/LMA hypothesis suggested by Poppell et al. (2013) where HMA sponges have a lower amount of choanocyte chambers and rely more on a heterotrophic lifestyle than LMA sponges.
Conclusions
Our study reveals two potentially new Plakortis species from reef caves and mesophotic reefs of the Caribbean that are found exclusively in association with either X. deweerdtae or Xestospongia sp. resulting in three new sponge–sponge symbiotic associations. Phylogenetic analysis using partial 28S rRNA, 18S rRNA, and COI gene sequences of associated and free-living individuals of X. deweerdtae revealed that these were all >99 % identical, thus being of the same species. Spicule sizes for associated X. deweerdtae individuals were significantly smaller than those of free-living individuals, suggesting a possible benefit in terms of costs by investing less energy in synthesizing smaller spicules. Symbiotic individuals of Plakortis spp. are thought to benefit from the association by receiving more efficient water flow through its mesohyl by a complex system of inner channels created by Xestospongia spp. Other possible roles of these associations are under investigation. This study reflects the unusual adaptations of marine sponges in light-limited habitats where they are likely subjected to different space limitations and a different suite of predators than sponges in open reef environments.
References
Ávila E, Carballo JL, Cruz-Barraza JA (2007) Symbiotic relationships between sponges and other organisms from the Sea of Cortes (Mexican Pacific coast): same problems, same solutions. In: Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G (eds) Porifera research: biodiversity. Innovation and sustainability, Série Livros 28. Museu Nacional, Rio de Janeiro, pp 147–156
Bavestrello G, Bonito M, Sarà M (1993) Silica content and spicular size variation during an annual cycle in Chondrilla nucula Schmidt (Porifera, Demospongiae) in the Ligurian Sea. Sci Mar (Barcelona) 57:421–425
Connes R, Diaz J, Negre G, Paris J (1974) Étude morphologique, cytologique et sérologique de deux formes de Suberites massa de l’étang de Thau. Vie Milieu 24:213–224
Dalisay DS, Quach T, Molinski TF (2010) Liposomal circular dichroism. Assignment of remote stereocenters in plakinic acids K and L from a Plakortis–Xestospongia Sponge Association. Org Lett 12:1524–1527
de Goeij JM, van den Berg H, van Oostveen MM, Epping EH, Van Duyl FC (2008) Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar Ecol Prog Ser 357:139
de Goeij JM, van Oevelen D, Vermeij MJ, Osinga R, Middelburg JJ, de Goeij AF, Admiraal W (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110
del Sol Jiménez M, Garzón SP, Rodríguez AD (2003) Plakortides M and N, bioactive polyketide endoperoxides from the Caribbean marine sponge Plakortis halichondrioides. J Nat Prod 66:655–661
Desqueyroux-Faundez R (1981) Révision de la collection d’éponges d’Amboine (Moluques, Indonésie) constituée par Bedot et Pictet et conservée au Muséum d’histoire naturelle de Genève. Rev Suisse Zool 88:723–764
Engel S, Pawlik J (2000) Allelopathic activities of sponge extracts. Mar Ecol Prog Ser 207:273–281
Ereskovsky AV, Lavrov DV, Willenz P (2013) Five new species of Homoscleromorpha (Porifera) from the Caribbean Sea and re-description of Plakina jamaicensis. J Mar Biol Assoc UK 1–23
Faulkner D, Ghiselin MT (1983) Chemical defense and evolutionary ecology of dorid nudibranchs and some other opisthobranch gastropods. Mar Ecol Prog Ser 13:295–301
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294
Gaino E, Bavestrello G, Magnino G (1999) Self/non-self recognition in sponges. Ital J Zool 66:299–315
Gazave E, Lapébie P, Renard E, Vacelet J, Rocher C, Ereskovsky AV, Lavrov DV, Borchiellini C (2010) Molecular phylogeny restores the supra-generic subdivision of homoscleromorph sponges (Porifera, Homoscleromorpha). PLoS ONE 5:e14290
Gerovasileiou V, Voultsiadou E (2012) Marine caves of the Mediterranean Sea: a sponge biodiversity reservoir within a biodiversity hotspot. PLoS ONE 7:e39873
Granek EF, Compton JE, Phillips DL (2009) Mangrove-exported nutrient incorporation by sessile coral reef invertebrates. Ecosystems 12:462–472
Harmelin JG, Vacelet J, Vasseur P (1985) Les grottes sous-marines obscures: un milieu extrême et un remarquable biotope refuge. Téthys 1:214–229
Haseley SR, Vermeer HJ, Kamerling JP, Vliegenthart JF (2001) Carbohydrate self-recognition mediates marine sponge cellular adhesion. Proc Natl Acad Sci U S A 98:9419–9424
Hill MS, Lopez NA, Young KA (2005) Anti-predator defenses in western North Atlantic sponges with evidence of enhanced defense through interactions between spicules and chemicals. Mar Ecol Prog Ser 291:93–102
Jiménez-Romero C, Ortiz I, Vicente J, Vera B, Rodríguez AD, Nam S, Jove R (2010) Bioactive Cycloperoxides isolated from the Puerto Rican sponge Plakortis halichondrioides. J Nat Prod 73:1694–1700
Kahng S, Garcia-Sais J, Spalding H, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen R (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275
Lehnert H, Van Soest R (1996) North Jamaican deep fore-reef sponges. Beaufortia 46:53–81
Lehnert H, van Soest RWM (1999) More North Jamaican deep fore-reef sponges. Beaufortia 49:142–169
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375:1–8
Lewis S (1982) Sponge-Zoanthid associations: functional interactions. In: Rutzler K, Macintyre IG (eds) The Atlantic Barrier Reef ecosystem at Carrie Bow Cay, Belize, I: structure and communities. Smithsonian Institution Press, Washington, pp 465–474
Leys SP, Yahel G, Reidenbach MA, Tunnicliffe V, Shavit U, Reiswig HM (2011) The sponge pump: the role of current induced flow in the design of the sponge body plan. PLoS ONE 6:e27787
López-Legentil S, Pawlik J (2009) Genetic structure of the Caribbean giant barrel sponge Xestospongia muta using the I3-M11 partition of COI. Coral Reefs 28:157–165
Makarchenko A, Utkina N (2006) UV-stability and UV-protective activity of alkaloids from the marine sponge Zyzzya fuliginosa. Chem Nat Compd 42:78–81
Maldonado M, Cao H, Cao X, Song Y, Qu Y, Zhang W (2012a) Experimental silicon demand by the sponge Hymeniacidon perlevis reveals chronic limitation in field populations. Hydrobiologia 687:251–257
Maldonado M, Ribes M, van Duyl FC (2012b) 3 Nutrient fluxes through sponges: biology, budgets, and ecological implications. Adv Mar Biol 62:113
McLean JH, Harasewych M (1995) Review of western Atlantic species of cocculinid and pseudococculinid limpets, with descriptions of new species (Gastropoda: Cocculiniformia). Allen Press, Inc., Lawrence
Mukai H, Shimoda H (1986) Studies on histocompatibility in natural populations of freshwater sponges. J Exp Zool 237:241–255
Muricy G (2011) Diversity of Indo-Australian Plakortis (Demospongiae: Plakinidae), with description of four new species. J Mar Biol Assoc U K 91:303–319
Muricy G, Solé-Cava AM, Thorpe JP, Boury-Esnault N (1996) Genetic evidence for extensive cryptic speciation in the subtidal sponge Plakina trilopha (Porifera: Demospongiae: Homoscleromorpha) from the Western Mediterranean. Mar Ecol Prog Ser 138:181–187
Pawlik J, Chanas B, Toonen R, Fenical W (1995) Defenses of Caribbean sponges against predatory reef fish. I. Chemical deterrency. Mar Ecol Prog Ser 127:183–194
Pawlik JR, Henkel TP, McMurray SE, López-Legentil S, Loh T-L, Rohde S (2008) Patterns of sponge recruitment and growth on a shipwreck corroborate chemical defense resource trade-off. Mar Ecol Prog Ser 368:137–143
Poppell E, Weisz J, Spicer L, Massaro A, Hill A, Hill M (2013) Sponge heterotrophic capacity and bacterial community structure in high-and low-microbial abundance sponges. Mar Ecol. doi:10.1111/maec.12098
Porter JW, Targett NM (1988) Allelochemical interactions between sponges and coral. Biol Bull 175:230–239
Ramsby B, Massaro A, Marshall E, Wilcox T, Hill M (2012) Epibiont–basibiont interactions: examination of ecological factors that influence specialization in a two-sponge association between Geodia vosmaeri (Sollas, 1886) and Amphimedon erina (de Laubenfels, 1936). Hydrobiologia 687:331–340
Redmond NE, Raleigh J, van Soest RW, Kelly M, Travers SA, Bradshaw B, Vartia S, Stephens KM, McCormack GP (2011) Phylogenetic relationships of the marine Haplosclerida (Phylum Porifera) employing ribosomal (28S rRNA) and mitochondrial (cox1, nad1) gene sequence data. PLoS ONE 6:e24344
Rützler K (1970) Spatial competition among Porifera: solution by epizoism. Oecologia 5:85–95
Rützler K (1981) An unusual bluegreen alga symbiotic with two new species of Ulosa (Porifera: Hymeniacidonidae) from Carrie Bow Cay, Belize*. Mar Ecol 2:35–50
Sarà M (1970) Competition and cooperation in sponge populations. Symp Zool Soc Lond 25:273–284
Slattery M, Lesser M (2012) Mesophotic coral reefs: a global model of community structure and function Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia
Slattery M, Gochfeld DJ, Easson CG, O’Donahue LR (2013) Facilitation of coral reef biodiversity and health by cave sponge communities. Mar Ecol Prog Ser 476:71–86
Southwell MW, Weisz JB, Martens CS, Lindquist N (2008) In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol Oceanogr 53:986
Swain TD, Wulff JL (2007) Diversity and specificity of Caribbean sponge–zoanthid symbioses: a foundation for understanding the adaptive significance of symbioses and generating hypotheses about higher-order systematics. Biol J Linn Soc 92:695–711
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
van Soest RWM, de Weerdt W (2001) New records of Xestospongia species (Haplosclerida: Petrosiidae) from the Curacao reefs, with a description of a new species. Beaufortia 51:109–117
Vogel S (1978) Evidence for one-way valves in the water-flow system of sponges. J Exp Biol 76:137–148
Walters KD, Pawlik JR (2005) Is there a trade-off between wound-healing and chemical defenses among Caribbean reef sponges? Integr Comp Biol 45:352–358
Wilcox T, Hill M, DeMeo K (2002) Observations on a new two-sponge symbiosis from the Florida keys. Coral Reefs 21:198–204
Wilson B, Clarkson P (2004) Australia’s spectacular cowries: a review and field study of two endemic genera: Zoila and Umbilia. Odyssey Publishing, El Cajon California
Wulff JL (1997) Mutualisms among species of coral reef sponges. Ecology 78:146–159
Wulff JL (2008a) Collaboration among sponge species increases sponge diversity and abundance in a seagrass meadow. Mar Ecol 29:193–204
Wulff JL (2008b) Life-history differences among coral reef sponges promote mutualism or exploitation of mutualism by influencing partner fidelity feedback. Am Nat 171:597–609
Zea S, Henkel T, Pawlik J (2009) The sponge guide: a picture guide to Caribbean sponges. Available online at www.spongeguide.org. Accessed on: 2014-02-06
Acknowledgments
Funding for this project was supported by NOAA’s Living Marine Resource Cooperative Science Center award number NA11SEC4810002. JV received additional support by the American Museum of National History Lerner-Gray Fund for Marine Research for the collection of sponges in Puerto Rico. JV’s stipend was provided by NOAA’s Nancy Foster Scholarship award NA12NOS4290142. Support for sequencing of sponge samples was provided by the NSF BIO/IOS Program (IOS-0919728) Grant to RTH. We would like to thank the government of the Bahamas for letting us collect samples provided by an unnumbered scientific permit for the operation of the R/V Walton Smith in their territorial waters; the government of Mexico for providing the CONAPESCA permit DAPA/2/06504110612/1608; and the government of Puerto Rico for providing permit 2010-IC-043 (R-VS-PVS15-SJ-00190-08062010). All sponge collections from the Bahamas and Mexico were supported by NSF award OCE-1029515 to JRP. Scanning electron microscope images were provided by the University of Maryland Baltimore County’s NanoImaging facility with the assistance of Dr. Laszlo Takacs. Paul Jensen and Laura Grice are thanked for providing key points to the discussion of this manuscript. Micah Marty is thanked for his assistance in performing dissections and photographing sponge individuals during cruises to the Bahamas. JV is indebted to Milton Carlo at the University of Puerto Rico at Mayaguez for his dive assistance with the collection of sponge individuals from Puerto Rico. Fred Lentz and Jose A. Rivera are also thanked for providing boat access for the collection of sponges in Desecheo, Puerto Rico. Niamh E. Redmond is thanked for her advice in suggesting primers for this study. We thank Leah Blasiak for her assistance in troubleshooting phylogenetic tree analyses. Nilli Zmora is thanked for providing histological equipment to perform hematoxylin and eosin staining for light microscopy images. SZ’s work is contribution of the Centro de Estudios en Ciencias del Mar – CECIMAR, Universidad Nacional de Colombia, Sede Caribe. We thank two anonymous reviewers for very helpful comments. This is IMET contribution no. 14–136 and UMCES contribution no. 4960.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. G. Chapman.
Electronic supplementary material
Below is the link to the electronic supplementary material.
227_2014_2546_MOESM2_ESM.eps
Supplementary material Phylogenetic tree generated from 1,300 bp of the 18S rRNA gene of sponges sequenced in this study (bold). Reference sequences downloaded from GenBank are indicated by species name and accession numbers. The tree topology was obtained from neighbor-joining (NJ) analysis. The bootstrap values at each node were generated from (NJ), maximum parsimony (MP), and maximum likelihood (ML) analysis, respectively (EPS 672 kb)
227_2014_2546_MOESM3_ESM.eps
Supplementary material Phylogenetic tree generated from 463 bp of the cytochrome oxidase subunit I of sponges sequenced in this study (bold). Reference sequences downloaded from GenBank are indicated by species name and accession numbers. The tree topology was obtained from neighbor-joining (NJ) analysis. The bootstrap values at each node were generated from (NJ), maximum parsimony (MP), and maximum likelihood (ML) analysis, respectively (EPS 668 kb)
227_2014_2546_MOESM4_ESM.eps
Supplementary material SEM images of (A) diods and triods from Plakortis sp. 1, (B) diods and triods from Plakortis sp. 2, (C) diods from Plakortis halichondrioides, (D) strongyles from free-living X. deweerdtae, (E) strongyles from associated X. deweerdtae, (F) isodictyal reticulation of oxeas from Xestospongia sp. Scale bar for all images 100 µm (EPS 22754 kb)
227_2014_2546_MOESM5_ESM.eps
Supplementary material Field marked specimens of Plakortis sp. 2/Xestospngia sp. (20–23) and Plakortis sp. 2/X. deweerdtae (24) monitored for growth over eight months. Scale bar for sponges photographed in August 2012 (20) 2 cm, (21) 3 cm, (22) 1 cm, (23) 2 cm, (24) 3 cm (EPS 204317 kb)
Rights and permissions
About this article
Cite this article
Vicente, J., Zea, S., Powell, R.J. et al. New epizooic symbioses between sponges of the genera Plakortis and Xestospongia in cryptic habitats of the Caribbean. Mar Biol 161, 2803–2818 (2014). https://doi.org/10.1007/s00227-014-2546-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2546-z