Abstract
This study used morphological, gut content analysis and carbon- and nitrogen-stable isotope analysis to investigate the trophic structure of upper sublittoral (15–30 m deep) and upper bathyal (200–300 m deep) hydrothermal vents and the adjacent non-vent upper bathyal environment off Kueishan Island. The sublittoral vents host no chemosynthetic fauna, but green and red algae, epibiotic biofilm on crustacean surfaces, and zooplankton form the base of the trophic system. Suspension-feeding sea anemones and the generalist omnivorous vent crab Xenograpsus testudinatus occupy higher trophic levels. The upper bathyal hydrothermal vent is a chemoautotrophic-based system. The vent mussel Bathymodiolus taiwanensis forms a chemosynthetic component of this trophic system. Bacterial biofilm, surface plankton, and algae form the other dietary fractions of the upper bathyal fauna. The vent hermit crab Paragiopagurus ventilatus and the vent crab X. testudinatus are generalist omnivores. The vent-endemic tonguefish Symphurus multimaculatus occupies the top level of the trophic system. The adjacent non-vent upper bathyal region contains decapod crustaceans, which function as either predators or scavengers. The assemblages of X. testudinatus from sublittoral and upper bathyal vents exhibited distinct stable isotope values, suggesting that they feed on different food sources. The upper bathyal Xenograpsus assemblages displayed large variations in their stable isotope values and exhibited an ontogenetic shift in their δ13C and δ15N stable isotope signatures. Some individuals of Xenograpsus exhibited δ15N values close to those of non-vent species, suggesting that the highly mobile Xenograpsus may transfer energy between the upper bathyal hydrothermal vents and the adjacent non-vent upper bathyal environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deep-sea is an environment that does not receive solar radiation. Energy sources in the deep-sea are principal inputs from marine snow (i.e. organic matter and plankton) sinking from the euphotic zone (Gage and Tyler 1991). However, in deep-sea hydrothermal vents, chemoautotrophic microorganisms are one of the major primary producers. These microorganisms live in various habitats and vent environments (Rau and Hedges 1979; Tunnicliffe 1991; Van Dover and Fry 1994; Karl 1995; Van Dover 2000). Many species of vent-endemic fauna (e.g. polychaetes, molluscs, and crustaceans) and chemoautotrophic bacteria (methanotrophic and thiotrophic) form symbiotic relationships in which the bacteria produce food and energy sources for their hosts (Childress and Fisher 1992; Van Dover 2000; Desbruyères et al. 2006). Carbon fixation pathways at the base of the hydrothermal vent food webs include Calvin–Benson–Bassham (CBB) and reductive tricarboxylic acid (rTCA) cycles (Campbell and Cary 2004; Hugler and Sievert 2011). Organisms situated at higher trophic levels in vent sites include non-symbiotic vent-endemic invertebrates (e.g. polychaetes, gastropods, and crustaceans), which feed on the organic matter produced from free-living chemoautotrophic bacteria or bacteria-bearing fauna (Van Dover and Fry 1989; Tunnicliffe 1991; Fisher et al. 1994; Van Dover 2002; Bergquist et al. 2007). Particulate and detrital organic matter derived from subsurface waters and from above the seabed is also important food source for protists and benthic invertebrates (Levesque et al. 2005; Govenar 2012). In some vents, there have been reports of background predators and large predators (e.g. the deep-sea octopus Graneledone boreopacifica and the spider crab Macroregonia macrochira), which are not vent-endemic but are present in high densities around vent margins and predate around the vents (Tunnicliffe and Jensen 1987; Voight 2000; Micheli et al. 2002, but see exceptions in Sweetman et al. 2013). Hydrothermal vents also exhibit a great diversity of parasites, which may function as major energy transporters (De Buron and Morand 2002; Govenar 2012). Although most hydrothermal vents are chemosynthetic-based systems, the primary production of certain vents (e.g. at the southern Mohns Ridge in the Arctic Ocean) is derived from epipelagic photosynthetic primary production, which supports part of the trophic system (Sweetman et al. 2013).
The upper sublittoral hydrothermal vent biota was described at a similar time as biota from deep-sea hydrothermal vents (Vidal et al. 1978). Unlike their deep-sea counterparts, sublittoral vents receive solar radiation, and consequently, photosynthetic organisms (e.g. algae and phytoplankton) can act as primary producers. Some upper sublittoral vents have a mixed photosynthetic-chemosynthetic system (Comeault et al. 2010), allowing energy to be transferred among fauna. For example, the upper sublittoral vents in Kagoshima Bay, Japan (82 m deep), host the symbiotic vestimentiferan Lamellibrachia satsuma, which depends on chemosynthetic bacteria as its primary energy source (Hashimoto et al. 1993; Tarasov et al. 2005). In general, vent-endemic species rarely appear in upper sublittoral vents (Dando et al. 1995; Tarasov et al. 2005; Desbruyères et al. 2006). However, at upper bathyal depths, there are also vents that do not host vent-endemic species (see Sweetman et al. 2013).
Most trophic studies on hydrothermal vents have been conducted in the lower bathyal zones, at depths of more than 1,000 m (e.g. Galapagos Rifts, Mid-Atlantic Ridges, and East Scotia Ridge; Fisher et al. 1994; Colaço et al. 2002; Reid et al. 2013). Compared with studies on lower bathyal vents, relatively fewer studies have investigated the trophic ecology of upper sublittoral vents (10–30 m deep) and upper bathyal vents in the upper bathyal zone (200–500 m deep) (but see upper bathyal vents in Colaço et al. 2002; Sweetman et al. 2013). The communities formed in sublittoral to upper bathyal hydrothermal vents can differ from the patterns observed from lower bathyal vents. The Menez Gwen vents (800 m deep) are a chemosynthetic system containing many vent-endemic fauna (Colaço et al. 2002). However, the upper bathyal vents (600–700 m deep) in the southern Mohns Ridge (Arctic Ocean) only contain the vent- and seep-associated gastropod Pseudosetia griegi (Pedersen et al. 2010), and photosynthetic organic matter is one of the food sources (Sweetman et al. 2013). Thus, the height of the food chain of these shallow and upper bathyal vents, as well as the pathway of energy transfer between vent and non-vent ecosystem, is likely to differ from the patterns observed in vents at deep bathyal depths.
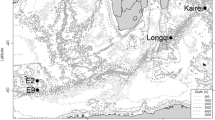
Kueishan Island (also called Gueishandao or Turtle Mountain Island), located in northeastern Taiwan, is known worldwide for its relatively shallow (upper sublittoral to upper bathyal) hydrothermal vents, which are located off the eastern side of the island at depths of 10–400 m (Jeng et al. 2004; Wang et al. 2013; Fig. 1a, b). The temperature of the venting water can reach between 65 and 112 °C, and it is highly acidic (pH 1.75–4.60) and contains high-purity elemental sulphur (Jeng et al. 2004). Bubbles from the gas discharge consist of carbon dioxide, nitrogen, oxygen, sulphur dioxide, and hydrogen sulphide (Kuo 2001). In upper sublittoral vents off the northern part of Kueishan Island, all waters are coloured white and smell strongly of sulphur (Fig. 1b). This indicates that strong surface currents (including the Kuroshio Current) enhance the export of chemosynthetic carbon from upper sublittoral vents and may provide an alternative food source for consumers in the ecosystem. Several upper bathyal vent sites have been identified in the waters around the Kueishan Island (Yang et al. 2005), and the gases emitted from these vents include high concentrations of carbon dioxide and hydrogen sulphide (Yang et al. 2005). Sulphur blocks were collected from trawl samples conducted in these vent sites (unpublished data), supporting the presence of the hydrothermal vents at the upper bathyal depths. We have previously examined upper bathyal vents and their associated fauna from a depth of 200–400 m in the waters around Kueishan Island (Wang et al. 2013). Eight upper bathyal vent-endemic species have been reported near Kueishan Island, including five species of decapod crustaceans (Xenograpsus testudinatus, Paragiopagurus ventilatus, Nihonotrypaea thermophila, Alvinocaris chelys, and Alvinocaridinides formosa; see Ng et al. 2000; Lemaitre 2004; Lin et al. 2007; Komai and Chan 2010) and three species of bivalves (B. taiwanensis, Lucinoma taiwanensis, and Meganodontia acetabulum; see Bouchet and Cosel 2004; Cosel and Bouchet 2008; Cosel 2008). The vent crab X. testudinatus is abundant at both upper sublittoral (Jeng et al. 2004) and upper bathyal vents (Komai and Chan 2010). This species is probably highly mobile (Lin 2011) and occasionally appears in upper sublittoral non-vent habitats (Ng et al. 2000). X. testudinatus in the upper sublittoral vents feed mainly on the zooplankton killed by the vent plumes and are highly mobile (Jeng et al. 2004).
a Location of the sampling sites in Kueishan Island waters, where black arrow indicate the location of Kueishan Island in Taiwan. Square represents sampling stations of upper sublittoral vents, triangle represents sampling stations in upper bathyal hydrothermal vents, and circle represents sampling stations in non-vent fishing ground. b Waters around the upper sublittoral vent region in Kueishan Island; note large area of the sea surface becomes white because of the sulphurous particles produced by the vents. c An undescribed red sea anemone present in high abundance in the upper sublittoral vent region. Note red algae are also present in the spaces among the red sea anemones. d The vent crab Xenograpsus testudinatus in the upper sublittoral vent. Note the surface of the vent crabs is covered by a thin white biofilm. e Seafloor topography of vent sites revealed from underwater sonar screening, showing the upwelled topography and the gas bubbles in the water column (indicated by white arrow). f Underwater sonar screening reveals seafloor topography of non-vent sites are flat and without any upwelled topography
Most biological studies of the hydrothermal vents off Kueishan Island are taxonomically based, and no study has examined the trophic structure of the upper sublittoral and upper bathyal vent fauna. Whether energy and nutrients are transferred between the upper bathyal vents and the adjacent environment remains unclear. Because vent crabs are abundant in upper bathyal vents and are highly mobile in the Kueishan Island waters, they may contribute to the energy transfer between the vents and the adjacent non-vent environment. We investigated the trophic structure of the upper sublittoral and upper bathyal vents off Kueishan Island using a combined morphological, gut content analysis and stable isotope approach. Specifically, the aims were to: (1) investigate whether the upper sublittoral and upper bathyal hydrothermal vents rely on chemosynthetic, photosynthetic or both energetic pathways, (2) examine evidence for ontogenetic dietary variation in the vent crab X. testudinatus from sublittoral and upper bathyal vents, and (3) investigate whether the vent crabs aid exportation of chemosynthetic energy from the upper bathyal vents into non-vent systems.
Materials and methods
Study sites, timing, and assemblage structures
Faunal sampling was carried out at the upper sublittoral vents, upper bathyal vents and adjacent non-vent upper bathyal regions off Kueishan Island in 2008 and 2010 (Table 2). Figure 1a shows the locations of the vent and non-vent sampling stations, and certain fauna from upper sublittoral vents (Fig. 1c, d). The upper sublittoral vent stations were located at a depth of 15–30 m off the eastern side of Kueishan Island (Fig. 1a, b). Upper bathyal vents were mainly located at the eastern and northeastern sides of Kueishan Island, at depths of 206–323 m (Fig. 1a). Non-vent stations were located at least one nautical mile from the vent sites in traditional upper bathyal fishing grounds (201–417 m deep) (Fig. 1a; also see Wang et al. 2013).
The red algae Gelidiopsis sp. and green algae Cladophora catenata are common on rock surfaces in upper sublittoral vents. The vent crab X. testudinatus is the dominant species in the upper sublittoral vents, in which the crab surface is fouled by a thin biofilm (Fig. 1c). Two undescribed sea anemones were also observed at the upper sublittoral vents (hereafter called red and black anemones; Fig. 1d).
In the upper bathyal vents, common fauna include the bivalve B. taiwanensis (which we suggest harbour chemosynthetic bacteria in their gills), the callianassid shrimp N. thermophila, the shrimp A. chelys, and the hermit crab P. ventilatus. The vent crab, X. testudinatus, is also common in upper bathyal vents. In addition to bivalves and decapod crustaceans, the tonguefish Symphurus multimaculatus is observed regularly from upper bathyal vent habitats. The nearby non-vent upper bathyal region supports a high diversity of crustaceans with various feeding modes, including predators, scavengers, plankton feeders, and deposit feeders (for details of the nearby non-vent assemblage, see Wang et al. 2013).
Sampling methods
Faunal assemblages in upper sublittoral vents (10–30 m deep) were collected from several SCUBA diving trips in October 2010. More than 30 specimens of the vent crab X. testudinatus and more than ten specimens of each of the two undescribed sea anemone species were collected in the active vent sites. The red algae Gelidiopsis sp. and the green algae C. catenata (potential food sources for grazers) were also sampled from rock surfaces in the vent region.
In addition to benthic communities, zooplankton samples (potential food sources for suspension feeders or vent crabs) were collected in the pelagic zone above the upper sublittoral and upper bathyal hydrothermal vents and adjacent upper bathyal regions. At each of the sampling sites (Fig. 1a), five trawls were conducted using a plankton net with a mouth diameter of 45 cm (mesh size, 200 µm) at a depth of 15–20 m for 5 min at a speed of 1 knot. Zooplankton samples were frozen immediately after they were collected.
Upper bathyal hydrothermal vent fauna was collected off the coast of Kueishan Island from 2008 to 2010, using commercial trawlers that departed from the Dasi fishing port (Fig. 1a). Upper bathyal vent sites were identified using high-frequency underwater sonar (Fig. 1e, f), which can detect the upwelled seafloor topography of vent sites and the gas bubbles produced by the vents (Fig. 1f). Successful detection of hydrothermal vents using high-frequency underwater sonar has been demonstrated in Hwang and Lee (2003). For the non-vent stations, underwater sonar screening revealed a flat smooth seafloor, without gas bubbles, and an upwelled topography (Fig. 1e). The benthic community was trawled using a 2.5-m French beam trawl, with a stretched mesh width of 13, and 7 mm at the cod end (Tsai et al. 2009). The fauna was trawled for 30 min (speed, 1.5 knots) after the trawl net reached the seabed. A 2.5 m × 925 m area was trawled for each station. All specimens collected were frozen at −20 °C on board the trawler immediately after they were collected. Trawling has limitations for accurate selective sampling (cf., using ROVs); therefore, the fauna sampled from the vent stations contained fauna from both vent and non-vent areas. Certain species collected in trawl samples from the vent stations were vent-associated species (as supported by the literature), which were absent from all of the non-vent stations. Hereafter, we refer to upper bathyal vent stations as upper bathyal vents and surrounding non-vent regions. We confirmed that the fauna collected from non-vent stations did not contain any vent species. Hereafter, we refer to non-vent stations as exclusive non-vent stations.
Qualitative gut content analysis, functional morphology of chelae, and feeding modes of crustacean species collected
We dissected five specimens of each crustacean collected and the tonguefish Symphurus (list of species shown in Table 1) to perform gut content analysis. We did not dissect the vent species (except for X. testudinatus) for gut content analysis because of low catches. We dissected the foreguts and gastric mills of the crustacean species and the stomach of the tonguefish, and rinsed out the contents using distilled water, following the methods described by Sahlmann et al. (2011). We divided the gut content into six categories: sediment, crustacean parts, fish tissue, fine organic matter, unidentified organic matter, and polychaete tissues (Table 1). We recorded the absence and presence of each category for each specimen. We did not score the relative abundance of each category of gut content, because the objective was to deduce the feeding modes of these species from their gut contents, but not the detailed feeding ecology of the species.
We examined various aspects of the chelae and pereopods of the collected crustacean species under stereomicroscopes to determine their functional morphology, including the shape and form of the cutting edges of the chelae (e.g. strong or weak chelae, and chelae cutting edges with sharp denticles, fine setae, or a smooth surface) and the setal types on the tips of the pereopods (Sahlmann et al. 2011). These structural differences reflect the feeding modes of crustaceans. Based on a literature review of the gut contents and morphology of the feeding appendages of crustaceans, we classified the specimens according to their feeding modes as bacteria-symbiont fauna, scavengers, deposit feeders, detritivores, predators, and plankton feeders (Tables 1, 2). Scavengers and predators often have similar gut contents because they both feed on animal tissues. However, the morphologies of their feeding appendages differ; predators often have sharper and stronger chelae or sharper chelae cutting edges compared with scavengers. Omnivores do not have sharp and strong chelae but exhibit diverse gut contents.
Laboratory δ13C and δ15N stable isotope analyses
We examined all samples to determine δ13C and δ15N stable isotope values (Table 2). We extracted soft tissue from the claws of the decapod crustaceans and extracted tissue from the caudal fin muscle of the fish. All tissues were dried at 60 °C. Zooplankton samples were also dried at 60 °C. The carapace surface of the vent crab X. testudinatus collected from upper sublittoral vents was covered with dense white epibiotic biofilm (Fig. 1c). Bacteria-like filaments were observed when investigating these biofilm under scanning electron microscopes (unpublished data, also see Tsuchida et al. 2011). This biofilm was scraped off, dried at 60 °C, and analysed to determine δ13C and δ15N values. All samples were homogenized prior to stable isotope analysis. The samples used for stable isotope analysis were not acidified to remove carbonates as our preliminary studies showed no effect of acidification treatment on δ13C (see also Mateo et al. 2008).
Stable δ13C and δ15N isotope analyses were conducted by the National Isotope Centre, GNS Science, New Zealand. The analyses were performed using a Europa Geo 20-20 continuous flow Isotope ratio mass spectrometer coupled with an elemental analyser (EA). The international standards for carbon and nitrogen were the Vienna Pee Dee Belemnite and atmospheric N2, respectively. Stable isotope ratios are presented in standard notation (Fry 2006):
where X denotes the heavier isotope (either δ13C or δ15N), and R represents the ratio (either 13C/12C or 15N/14N). Standards were Pee Dee Belemnite for δ13C (±0.1 ‰), and N2 gas (atmospheric) for δ15N ± 0.3 ‰).
Data analysis
Kruskal–Wallis test was conducted to compare δ13C and δ15N stable isotope values of fauna in the upper bathyal hydrothermal vent and surrounding non-vent regions and the exclusively non-vent stations. To examine whether there is an ontogenetic shift in δ13C and δ15N values in the vent crab X. testudinatus, Pearson’s correlation analysis was conducted to test for significant correlations between δ13C and δ15N values and carapace length of upper bathyal and upper sublittoral vent crabs. Mann–Whitney rank-sum tests were conducted to examine the evidence for differences in δ13C and δ15N values between vent crabs from upper bathyal and upper sublittoral habitats.
Results
Qualitative analysis of gut contents and the functional morphology of feeding appendages
The vent crab X. testudinatus was the dominant species in both upper sublittoral and upper bathyal vents. The cutting edges of the fingers of its chelae had small conical teeth without any strong crushing cusps, and the fingertips bore fine setae (Fig. 2). The guts of X. testudinatus from upper sublittoral and upper bathyal vents contained both fine unidentified organic substances and crustacean parts (Table 1). The red and black sea anemones from upper sublittoral vents had tentacles and were believed to be plankton feeders.
Functional morphology of upper bathyal vent and non-vent crustacean species used for stable isotope analysis. a Vent-associated species, A. chelys. b Non-vent species, C. longimana. c Non-vent species, H. sibogae. d Non-vent species, I. novemdentatus. e Non-vent species, Metanephrops formosanus. f Vent-associated species, P. ventilatus. g Vent-associated species, X. testudinatus. h Vent-associated species, Nihontrypaea thermophila. i non-vent species, H. equalis. j Non-vent species, Munida japonica. k Non-vent species, P. gladiator, l Non-vent species, P. japonica
Nihonotrypaea thermophila exhibited unequal first chelipeds (Fig. 2). The major cheliped was heavy and massive, whereas the minor cheliped was slender and weak. Although the fingers of both chelae were hooked and terminated in a subacute tip, the cutting edges of the major chela were unarmed in the fixed finger, but bore one or two blunt, molar-like teeth in the movable finger (Fig. 2). The cutting edges of the minor chela exhibited a row of small corneous teeth interspaced with weak tubercles. Both chelae had tufts of long and short setae. Mouthparts (including the mandible, maxillules, and maxillipeds) were densely setose.
The first chelipeds of P. ventilatus were greatly dissimilar, with the right cheliped being much longer in large males than juvenile hermit crabs (Fig. 2). However, the cutting edges of the fingers of both chelipeds bore a row of small calcareous teeth of dissimilar sizes, in addition to 1–2 corneous teeth (Fig. 2). The chelipeds and mouthparts were covered with dense bacteriophore setae (i.e. densely packed long plumose setae; see Segonzac et al. 1993; Lemaitre 2004).
The first chelipeds of the vent shrimp A. chelys were sexually dimorphic, being larger in females than in males. The fingers of the first cheliped were curved, with the tip slightly spooned. The cutting edges of the fingers bore a fine row of closely set teeth and closed without a gap. The second chelipeds were shorter and more slender than the first chelipeds, with fingers terminating in small corneous unguises that crossed each other when closed (Fig. 2). The cutting edges of the second cheliped bore a row of pectinate minute corneous teeth. The mouthparts were covered with tufts of short or long setae.
Tonguefish (S. multimaculatus >20 samples) were captured through upper bathyal vent sampling. The gut contents of these specimens contained a considerable amount of polychaete tissue and crustacean parts, suggesting that they were predators (Table 1).
We examined nine crustacean species, namely Munida japonica, C. longimana, Metanephros formosanus, P. japonica, H. sibogae, H. equalis, P. gladiator, I. novemdentatus, and P. angulatus, collected from exclusive non-vent stations for analyses of gut contents and to examine the functional morphology of feeding appendages (Table 1; Fig. 2). We classified these exclusive non-vent species as predators, scavengers, or deposit feeders based on their gut contents and the functional morphology of their mouthparts and feeding appendages.
Macrofauna stable isotope values
Upper sublittoral vents
In upper sublittoral vents, both the green algae C. catenata and the red algae Gelidiopsis sp. exhibited low δ13C values, but had high δ15N values (Table 2; Fig. 3a). The epibiotic biofilm on the carapace of the vent crab X. testudinatus from upper sublittoral vents exhibited low mean δ13C (−21.4 ‰) and δ15N (−1.2 ‰) values. The surface zooplankton collected above the upper sublittoral vents had mean δ13C values of −19.3 and 7.4 ‰ for δ15N. In upper sublittoral vents, both red and black sea anemones displayed δ13C values of ca. −19.0 ‰ and δ15N values between 8.7 and 9.2 ‰ (Table 2; Fig. 3a). Upper sublittoral Xenograpsus exhibited considerable variation in both δ13C and δ15N values (Fig. 3). The carapace length of Xenograpsus from upper sublittoral vents had no significant correlation with δ13C (n = 22; Pearson’s correlation coefficient = 0.2, P > 0.05) or δ15N values (n = 22; Pearson’s correlation coefficient = 0.27, P > 0.05) (Fig. 4).
Mean ± standard deviation of δ13C and δ15N for species from a upper sublittoral vents and b upper bathyal vent and surrounding non-vent region and exclusive non-vent stations near Kueishan Island. Note vertical lines highlight δ13C derived from CBB cycles ranged between −30 and −22 ‰, and δ13C ranged between −22 and −14 ‰ is derived from mixed carbon sources (see Reid et al. 2013)
The δ13C and δ15N values of the vent crab X. testudinatus from upper sublittoral and upper bathyal vents were significantly different (Mann–Whitney rank-sum test: δ13C : U = 75, P < 0.05; δ15N: U = 116.5, P < 0.05). X. testudinatus collected from upper sublittoral vents exhibited higher δ13C and δ15N values (mean δ13C of −15.9 ‰ and mean δ15N of 6.4 ‰) than their upper bathyal counterparts (mean δ13C of −17.7 ‰ and mean δ15N of 2 ‰).
Upper bathyal vents and non-vent regions
Among the species of the upper bathyal vent and surrounding non-vent region, the upper bathyal vent mussel B. taiwanensis was the symbiont-bearing host displaying the lowest δ13C and δ15N values (δ13C of −26.1 ‰ and δ15N of −9.2 ‰; Fig. 3b). The callianassid shrimp N. thermophila displayed a mean δ13C value (−26.0 ‰) similar to that of B. taiwanensis (−26.1 ‰), but exhibited more variation than did the latter (this was also true for the δ15N value, Fig. 3b; Table 2). The vent shrimp A. chelys exhibited higher δ13C and δ15N values (mean δ13C of −22.1 ‰ and δ15N of 1.2 ‰) than did B. taiwanensis and N. thermophila (Fig. 3b). The hermit crab P. ventilatus and the tonguefish S. multimaculatus exhibited much higher δ13C and δ15N values than A. chelys; these values were close to those of exclusive non-vent fauna (Fig. 3b). The upper bathyal vent crab Xenograpsus exhibited large variation in δ13C and δ15N values, with ranges covering the clusters of vent and exclusive non-vent-endemic fauna. The carapace length of upper bathyal X. testudinatus was significantly correlated with δ13C values (n = 22; Pearson’s correlation coefficient 0.479, P < 0.05; Fig. 4), but was not correlated with the δ15 N signature (n = 22; Pearson’s correlation coefficient = 0.3, P > 0.05; Fig. 4). Surface zooplankton collected from the upper bathyal vents had a mean δ13C of −19.4 ‰ and δ15N of 6.6 ‰.
The species collected from the exclusive non-vent stations are generally predators and scavengers (Table 1), and their mean δ13C and δ15N values were higher and had narrower ranges than vent fauna (Fig. 3b), ranging from −16.0 to −17.0 and 9.0 to 12.0 ‰, respectively (Table 2). Four species of predators (Carcinoplax longimana, Portunus gladiator, Ibacus novemdentatus, and Puerulus angulatus) exhibited high δ13C and δ15N values (Fig. 3b). Pasiphaea japonica is a pelagic plankton-feeding shrimp with a mean δ13C value similar to that of Hymenopenaeus equalis, which is also pelagic but exhibited different δ15N values (Table 2). The upper bathyal shrimp Heterocarpus sibogae, which is a scavenger in general upper bathyal habitats, exhibited the lowest δ13C value (−17.6 ‰). Surface zooplankton collected from the exclusive non-vent regions had mean δ13C value of −19.1 ‰ and a mean δ15N value of 7.4 ‰.
Comparing the δ13C and δ15N values of the fauna from the upper bathyal vent and the surrounding non-vent regions and the exclusive non-vent station, fauna at the vent and surrounding non-vent region exhibited lower δ13C (−25.0 to −20.0 ‰) and δ15N (−3.0 to +5.0 ‰) values and higher intraspecific variation (Fig. 3b) than the exclusive non-vent fauna (δ13C from −18.0 to −14.0 ‰, δ15N from +9.0 to +14.0 ‰). The mean δ13C and δ15N values of the fauna from the vent and surrounding non-vent region, as well as those of the exclusive non-vent fauna, formed two distinct clusters (Fig. 3b). The δ13C and δ15N values of fauna from vent and surrounding non-vent regions were significantly different from those of the exclusive non-vent assemblages (Kruskal–Wallis test for both δ13C and δ15N signatures, P < 0.05).
Discussion
We investigated three trophic systems in Kueishan Island waters: namely upper sublittoral vents, upper bathyal vents and the surrounding non-vent region, and the exclusive non-vent upper bathyal environment. The major groups in these trophic systems exhibited distinct stable isotope values, with little isotopic overlap apparent in the assemblages from the three trophic systems (Fig. 4).
Trophic structure according to δ13C- and δ15N-stable isotope analysis
Upper sublittoral vent fauna
Carbon fixed by enzymatic reactions catalysed by the ribulose-1, 5, biphosphate carboxylase/oxygenase form I (RuBisCo form I) of the CBB cycle will result in δ13C ranging between −30.0 and −22.0 ‰ (Robinson et al. 2003; Roeske and O’Leary 1984), while carbon fixed by rTCA cycles will result in δ13C ranging from −14.0 to −2.0 ‰ (House et al. 2003). In upper sublittoral vents, the epibiotic biofilm on the vent crab carapace exhibited low δ13C (−21.4 ‰) and δ15N (−1.0 ‰) values (Fig. 2), indicating that biofilm is one of the primary components at the base of the trophic system in upper sublittoral vents. At the hydrothermal vents at the East Scotia Ridge, Southern Ocean, δ13C and δ15N values of epibionts sampled on the ventral setae of the decapod Kiwa sp. (Reid et al. 2013) varied from −18.9 to −9.9 and 3.3 to 5.2 ‰, respectively. In a hydrothermal vent at the Okinawa Trough, the δ13C and δ15N values of bacterial biofilm on the ventral setae of the galatheid crab Shinkaia crosnieri ranged between −21.1 and −21.4, and 2.1 and 2.3 ‰, respectively (Tsuchida et al. 2011). The δ13C value of the epibiont on Kiwa sp. in East Scotia Ridge suggests that the carbon is fixed from the rTCA cycle. In the present study and in work from the Okinawa Trough (Tsuchida et al. 2011), the biofilm on the vent crab Xenograpsus and the galatheid crab Shinkaia had δ13C values of ~−21.0 ‰, indicating the carbon is fixed from the CBB cycle.
Abundant beds of green and red algae (C. catenata and Gelidiopsis sp.) were observed adjacent to the upper sublittoral vents, and these algae exhibited low δ13C values (−35.0 to −25.0 ‰), but high δ15N values. Seaweeds often show large variation in δ13C values (−11.0 to −39.0 ‰), and such variation remains difficult to explain (Farquhar et al. 1989; Carvalho and Eyre 2011). Some studies (Farquhar et al. 1989; Carvalho et al. 2009; Carvalho and Eyre 2011) have suggested that physiological reactions (e.g. photosynthesis and respiration) and environmental factors (e.g. water velocity) may cause the variations in algal carbon-stable isotope values.
Tarasov et al. (2005) suggested that primary production in upper sublittoral vents is mainly supported by photosynthetic primary producers, whereas chemosynthetic components often play a secondary role. In this study, the red and green algae in the upper sublittoral hydrothermal vents exhibited low carbon and nitrogen values (Table 2), indicating that they are located at the bottom of the trophic system and are the primary producers in upper sublittoral vents. No dominant grazer has been identified in the upper sublittoral vent region. The δ13C values of the algae and organisms collected in upper sublittoral vents did not overlap, suggesting that these organisms do not feed on algae. No gastropod grazers were seen and collected from frequent intensive diving in the upper sublittoral vents. The trophic relationships between algae and higher-level fauna in upper sublittoral vents require further investigation using mixing model analysis, based on adequate potential food sources sampled. Further sampling is needed to identify any potential grazers in these upper sublittoral vents.
Red and black anemones are suspension feeders that use their tentacles to prey on plankton in the surrounding water or plankton killed by the hot water emitted from upper sublittoral vents. In the present study, the mean δ13C (−19.3 ‰) and δ15N (7.4 ‰) values of zooplankton were close to the black and red sea anemones (δ13C −19.0 to −19.1 ‰, δ15N 8.7–9.2 ‰), suggesting a close trophic relationship between zooplankton and the sea anemones. In the present study, we have no data on temporal variation on zooplankton stable isotope values. Tropical zooplankton can exhibit seasonal variation in δ13C (−29.0 to −18.0 ‰) (Bouillon et al. 2000). Further temporal samplings of zooplankton in the sublittoral vent regions can reveal a better trophic relationship between the zooplankton and sea anemones.
The δ13C and δ15N values of X. testudinatus in the upper sublittoral and upper bathyal vents and the surrounding non-vent region displayed the greatest variation (Table 2; Fig. 3). This suggests that X. testudinatus is a generalist omnivore (probably including carcasses of non-vent organisms) that feeds on more than the plankton killed by vent plumes, as previously suggested (Jeng et al. 2004). Another vent crab of the same genus (X. nagatama), from the upper sublittoral vents of the Tonga Arc, exploits a wide range of food resources from photosynthetic and chemosynthetic systems (Comeault et al. 2010). The gut contents of X. testudinatus from both upper sublittoral and upper bathyal vents contained fine organic matter and large crustacean parts, confirming that vent crabs do not feed solely on dead plankton, but may also feed on dead crustacean bodies and detritus as well. The guts of X. testudinatus have high activity of the digestive proteolytic enzymes, which are believed to be an adaptation to digest irregular food supplies (Hu et al. 2012).
Laboratory observations of X. testudinatus kept in an aquarium demonstrated that these crabs can feed on and effectively dissemble dead fish using their chelae, suggesting that vent crabs are able to feed on carrion in the natural environment. Comparing the mean δ13C and δ15N values of the zooplankton with those of X. testudinatus, the mean δ13C and δ15N values of crabs were distinctly separated far from the zooplankton cluster. Therefore, upper sublittoral vent crabs and zooplankton did not show a strong direct trophic relationship.
Fauna from upper bathyal hydrothermal vents, the surrounding non-vent region, and the exclusive non-vent stations
In the present study, we support that at the basal trophic level of the upper bathyal hydrothermal vent off Kueishan Island is a chemo-autotrophic-based system, which energy of the trophic system is produced by chemosynthetic bacteria associated with the mussel B. taiwanensis. The hermit crab P. ventilatus and the vent crab X. testudinatus are generalist omnivores that occupy higher trophic levels compared with vent shrimps and mud shrimps. The tonguefish S. multimaculatus is probably located at the top level of the trophic system in upper bathyal vents (Fig. 3b). Particulate organic matter and phytodetritus can be important energy sources at the basal of the food chain in upper bathyal vents. In the present study, we did not collect phytodetritus and particulate organic matter due to the limitation of sampling using trawls. In the waters of NE Taiwan and the South China Sea, Lin et al. (2014) suggested that phytodetritus and particulate organic matter are important food sources for consumers in the continental slope and deep-sea basin, respectively. The role of phytodetritus and particulate organic matter in the trophic structure of upper bathyal vents in Kueishan Island deserves further research.
Bathymodiolus taiwanensis
The genus Bathymodiolus (Mytilidae, Bathymodiolinae) comprises large mussels that are endemic to deep-sea hydrothermal vents and seep environments ranging from 200 to 3,000 m in depth (Desbruyères et al. 2006; Cosel 2008). The Bathymodiolinae subfamily is widely known as a host of chemoautotrophic bacteria. Two types of chemoautotrophic bacteria are symbiotic with Bathymodiolinae mussels, namely thiotrophic bacteria (Cavanaugh et al. 1992; Nelson et al. 1995; Mckiness et al. 2005) and methanotrophic bacteria (Childress et al. 1983; Streams et al. 1997; Barry et al. 2002). Dual symbiosis involving both thiotrophic and methanotrophic bacteria has also been observed (Fisher et al. 1993; Pond et al. 1998; Fiala-Médioni et al. 2002; Duperron et al. 2006). The δ13C values of Bathymodiolinae mussels range between −37.3 and −20.0 ‰ in hydrothermal vent assemblages, whereas those of cold seep (i.e. methanotropic) assemblages range from −68.1 to −37.5 ‰ (McKiness et al. 2005; Duperron 2010). Bathymodiolus taiwanesis is currently found only near the Kueishan Island upper bathyal vents and is the smallest and shallowest-dwelling Bathymodiolinae (Cosel 2008). The lowest δ13C value of B. taiwanensis was −26.1 ‰, confirming that these mussels are endosymbiotic hosts (involving the CBB cycle for carbon fixation) that occupy the basal region of the trophic system in the upper bathyal hydrothermal vents off Kueishan Island. The mussels may also be able to carry out filter feeding (see Page et al. 1991). The exact species of symbiotic bacteria associated with B. taiwanensis remains unknown, and this topic requires further investigation.
Nihonotrypaea thermophila
Eight species of burrowing mud shrimp (thalassinideans, recently classified as Axiidea and Gebiidea) have been reported in reducing environments such as hydrothermal vents, cold seeps, and whale falls (Komai and Fujiwara 2012). However, their roles in these reducing environments remain unclear. N. thermophila was the first vent-endemic mud shrimp reported (Lin et al. 2007). The unequal first chelae of this species bear either molar-like or weak teeth, indicating that it is not an efficient predator (Fig. 2). Mud shrimps are obligate burrowers in soft substratum and depend on assorted detritus as their main food source (Shimoda et al. 2007). Their food sources are species specific and depend on the specificity of each habitat (Griffis and Suchanek 1991; Abed-Navandi and Dworschak 2005; Shimoda et al. 2007). Hydrothermal vents exhibit a high diversity of free-living microorganisms including bacteria (attached on the surface and in the subsurface). These microorganisms are an essential food resource for many organisms and bacterivores, and usually display a wide range of δ13C and δ15N values (Tunnicliffe 1991; Van Dover and Fry 1994; Van Dover 2000; Bergquist et al. 2007). The large variation in δ13C and δ15N values of N. thermophila suggest that they are generalist omnivores, consuming a wide range of food resources and likely feed on various bacteria with different metabolic and carbon-fixing pathways.
Alvinocaris chelys
Vent shrimp often aggregates on the vent chimney wall (Desbruyères et al. 2006). These shrimps display a great diversity of feeding modes and include bacterial grazers, episymbiotic bacteria bearers, and scavengers. δ13C and δ15N values can be highly variable among different vent shrimp species (Van Dover et al. 1988; Polz et al. 1998; Gebruk et al. 2000; Colaço et al. 2002). For example, Mirocaris fortunate is a detritivore that feeds on detritus (δ13C: −23.9 to −9.4 ‰; δ15N: 0–8.4 ‰; Gebruk et al. 2000; Colaço et al. 2002). Alvinocaris markensis (δ13C: 0.21.2 to −14.2 ‰; δ15N: 3.7–9.2 ‰) and Chorocaris chacei (δ13C: −18.0 to −11.7 ‰; δ15N: 4.1–8.0 ‰) are scavengers or predators (Gebruk et al. 2000; Colaço et al. 2002). Rimicaris exoculata is a bacterial grazer or episymbiotic bacteria bearer (δ13C: −18.1 to −10.0 ‰; δ15N: 3.9–8.2 ‰; Van Dover et al. 1988; Polz et al. 1998; Gebruk et al. 2000; Colaço et al. 2002).
The vent shrimp A. chelys of Kueishan Island had the third lowest δ13C (mean: −22.1 ‰) and δ15N (mean: 1.2 ‰) values of the upper bathyal hydrothermal vent assemblage. The mean δ13C value of A. chelys, which was less than −20 ‰, was similar to that of A. markensis and M. fortunate. The first and second pereopods of A. chelys bear small chelae with cutting edges that provide a fine row of closely set teeth or minute corneous teeth. These weak feeding appendages suggest that this shrimp is not a predator, but more likely a scavenger or deposit feeder. The low δ13C value of A. chelys suggests that they feed on free-living microorganisms or meiofauna on the substratum. The difference in δ13C value between A. chelys and B. taiwanensis was greater than 4.0 ‰, implying that these two species have no direct relationship and that the symbiotic bacteria in B. taiwanensis is not a food source of A. chelys.
Paragiopagurus ventilatus
Paragiopagurus ventilatus is currently the only hermit crab reported to be endemic to hydrothermal vents. This vent-endemic hermit crab was previously found only in the Kueishan Island hydrothermal vents at a depth of 128–281 m, but was recently observed in the Nikko seamount of the Mariana Trough at a depth of 410 m (Komai et al. 2011). The P. ventilatus of the Nikko seamount inhabit the empty tubes of the vent-endemic siboglinid tubeworm L. satsuma. Laboratory observations have proven that P. ventilatus can ingest various food sources, including bacterial mat, tuna meat, and euphausiids (Komai et al. 2011). P. ventilatus has dense bacteriophore setae in its mouthparts and chelipeds (Lemaitre 2004). The bacteriophore setae are long, plumose, feather-like setae that can gather epibiotic bacteria on the surface (Segonzac et al. 1993). However, the δ13C (−18.6 ‰) and δ15N (7.9 ‰) values of P. ventilatus were the highest among the vent-endemic assemblage, being close to those of non-vent fauna, suggesting that this hermit crab is a generalist omnivore with a mixed diet including microbial organisms and macroscopic food sources from both vent and surrounding non-vent environments.
Xenograpsus testudinatus
Xenograpsus testudinatus has a wide vertical distribution (15–300 m deep). The δ13C and δ15N values of X. testudinatus from upper sublittoral and upper bathyal vent habitats were significantly different (also see Fig. 3), indicating that the upper sublittoral and upper bathyal assemblages of X. testudinatus have different food sources. Upper bathyal X. testudinatus exhibited large variation in their δ13C and δ15N values, implying that their food sources varied. This pattern is similar to the vent decapod Kiwa sp. in the East Scotia Ridge of Antarctica waters (Reid et al. 2013). Kiwa sp. exhibited great variation in δ13C between sites, probably reflecting variation in the carbon fixation pathways at the base of the food web (Reid et al. 2013). The carapace length of Xenograpsus had a significant positive correlation with δ13C values, suggesting there was an ontogenetic shift in their diet where smaller Xenograpsus crabs consumed and assimilated food that was 13C-depleted relative to their larger conspecifics. However, upper sublittoral Xenograpsus did not exhibit an ontogenetic shift in δ15N values. The varying δ15N values of individual X. testudinatus specimens were scattered in almost all trophic levels (Fig. 4), suggesting that their food sources likely included bacteria, phytodetritus, bivalves, and other decapod crustacean species.
Xenograpsus testudinatus displayed relatively high mobility compared with other vent-endemic species near Kueishan Island. The type specimen of X. testudinatus was collected from a trammel net at a depth of 15 m at a non-vent site in northeastern Taiwan. Other researchers have observed X. testudinatus in great abundance near the upper sublittoral vents near Kueishan Island (Ng et al. 2000). Although the occurrence of X. testudinatus in non-vent areas is considered to be abnormal (Ng et al. 2000), this demonstrated that X. testudinatus is highly mobile and can live in non-vent environments. The δ13C and δ15N values observed in upper bathyal Xenograpsus samples varied considerably, which may partly reflect wide area from which samples were collected including both vents and the surrounding non-vent region, through trawling. A recent proteomic study demonstrated that the protein expression profiles of X. testudinatus exhibit very high spatial variations among different sublittoral vents. The protein profile of crabs changed from the profile in their original habitat to new profile, after 12 h of culture in laboratory conditions (Lin 2011). This rapid protein expression response suggests that X. testudinatus is highly mobile and can quickly acclimate to different environments (Lin 2011). The high abundance of X. testudinatus near vents suggests that this crab can tolerate toxic vent environments and, thus, escape from predation (Hu et al. 2012). Because X. testudinatus is able to move across upper bathyal vents and non-vent environments, they may transfer energy and nutrients between upper bathyal hydrothermal vents and non-vent environments. Vent crabs may be an important food source for non-vent predators; however, this needs further studies to confirm the hypothesis. Such a trophic role has not been reported for any other vent crab. In the East Pacific Rise, the vent-endemic bythograeid crab has a movement range of up to 600 m from the central vent regions (Lichtman et al. 1984), but whether this crab can transfer chemosynthetic carbon to nearby non-vent regions remains unclear (Van Dover 2000).
Symphurus multimaculatus
The tonguefish S. multimaculatus was previously considered a rare species in Taiwan (Lee et al. 2009). However, we collected more than 20 specimens of this tonguefish from the upper bathyal vent sites near Kueishan Island. Munroe et al. (2011) reported that the vent tonguefish S. maculopinnis often lives within 30 m of vents, but their food source remains unclear. This study showed that the gut content of S. multimaculatus contained many polychaetes and crustacean parts. Thus, the tonguefish S. multimaculatus is likely one of the top predators in active vent regions.
Comparisons of trophic structures among different upper bathyal vent systems
Trophic structures of upper bathyal vents have been studied at the Menez Gwen vents (800 m deep) in the Mid-Atlantic Ridges (Colaço et al. 2002) and at the Mohns Ridge vents (556–720 m deep) in the Arctic Ocean (Sweetman et al. 2013). The trophic structure at the Menez Gwen vents is dichotomic, with the chemosynthetic bacteria-associated mussel Bathymodiolus azoricus and vent shrimps (bacterial feeders) being the two major components of the basal part of the food chain. The upper trophic levels include crabs and whelks, which are scavengers or carnivores from non-vent regions, and can enter the vents for foraging (Colaço et al. 2002). Such a trophic structure is similar to that of the upper bathyal vent and surrounding non-vent regions in Kueishan Island waters. In the upper bathyal vents near Kueishan Island, the basal trophic structure consists of symbiont-bearing Bathymodiolus mussels. The mud shrimp N. thermophila is a key consumer that feeds on chemoautotrophic bacteria and other microorganisms. The vent shrimp A. chelys is a deposit feeder that probably consumes microorganisms from surrounding substrates.
In contrast to the Kueishan Island waters, the vents at the Mohns Ridge do not include chemosynthetic mussels. The high-temperature vent sites at the Mohns Ridge exhibit a lower diversity compared with the upper bathyal vents in the Kueishan Island waters. The vents at the Mohns Ridge are dominated by the bacterial-feeding gastropod P. griegi (Sweetman et al. 2013).
The sampling methods used in this study were based on trawling due to an absence of research submersibles in Taiwan. Most sampling methods used in studies of the trophic ecology of other hydrothermal vents involved deep-sea submersibles, and it was reported that a trophic complexity of vent fauna existed in small spatial scales (Gaudron et al. 2012; Sweetman et al. 2013; Reid et al. 2013). Thus, in the present study, samples from small spatial scales could not be collected through trawling, causing a large variation in the stable isotope values for some of the vent fauna. In addition, all of the consumers of symbiotic bacteria cannot be collected using trawling, resulting in the inconsistent trophic levels observed in this study (Van Dover and Fry 1989; Van Dover 2000; Bergquist et al. 2007). Future studies should use ROVs for deep-water sampling in Taiwan to collect adequate potential samples of consumers and their putative prey, thus allowing isotope mixing models to be used to further examine the trophic systems in the vent ecosystems.
References
Abed-Navandi D, Dworschak PC (2005) Food sources of tropical thalassinidean shrimps: a stable-isotope study. Mar Ecol Prog Ser 291:159–168. doi:10.3354/meps291159
Barry JP, Buck KR, Kochevar RK, Nelson DC, Fujiwara Y, Goffredi SK, Hashimoto J (2002) Methane-based symbiosis in a mussel, Bathymodiolus platifrons, from cold seeps in Sagami Bay, Japan. Invertebr Biol 121:47–54. doi:10.1111/j.1744-7410.2002.tb00128.x
Bergquist DC, Eckner JT, Urcuyo IA, Cordes EE, Hourdez S, Macko SA, Fisher CR (2007) Using stable isotopes and quantitative community characteristics to determine a local hydrothermal vent food web. Mar Ecol Prog Ser 330:49–65. doi:10.3354/meps330049
Bouchet P, Cosel VR (2004) The world’s largest Lucinid is an undescribed species from Taiwan (Mollusca: Bivalvia). Zool Stud 43:704–711
Bouillon S, Mohan PC, Sreenivas N, Dehairs F (2000) Sources of suspended organic matter and selective feeding by zooplankton in an estuarine mangrove ecosystem as traced by stable isotopes. Mar Ecol Prog Ser 208:79–92
Campbell BJ, Cary SC (2004) Abundance of reverse tricarboxylic acid cycle genes in free-living microorganisms at deep-sea hydrothermal vents. Appl Environ Microbiol 70:6282–6289
Carvalho MC, Eyre BD (2011) Carbon stable isotope discrimination during respiration in three seaweed species. Mar Ecol Prog Ser 437:41–49. doi:10.3354/meps09300
Carvalho MC, Hayashizaki K-I, Ogawa H (2009) Short-term measurement of carbon stable isotope discrimination in photosynthesis and respiration by aquatic macrophytes, with marine macroalgal examples. J Phycol 45:761–770. doi:10.1111/j.1529-8817.2009.00685.x
Cavanaugh CM, Wirsen CO, Jannasch HW (1992) Evidence of methylotrophic symbionts in a hydrothermal vent mussel (Bivalvia: Mytilidae) from the Mid-Atlantic Ridge. Appl Environ Microbiol 58:3799–3803
Chan BKK, Lin IC, Shih TW, Chan TY (2008) Bioluminescent emissions of the deep-water pandalid shrimp, Heterocarpus sibogae De Man, 1917 (Decapoda, Caridea, Pandalidae) under laboratory conditions. Crustaceana 81:341–350. doi:10.1163/156854008783564064
Childress JJ, Fisher CR (1992) The biology of hydrothermal vent animals: physiology, biochemistry and autotrophic symbioses. Oceanogr Mar Biol Annu Rev 30:337–441
Childress JJ, Fisher CR, Brooks JM et al (1983) A methanotrophic marine molluscan (Bivalvia, Mytilidae) symbiosis: mussels fueled by gas. Science 233:1306–1308. doi:10.1126/science.233.4770.1306
Colaço A, Dehairs F, Desbruyères D (2002) Nutritional relations of deep-sea hydrothermal fields at the Mid-Atlantic Ridge: a stable isotope approach. Deep Sea Res I 49:395–412. doi:10.1016/S0967-0637(01)00060-7
Comeault A, Stevens CJ, Juniper SK (2010) Mixed photosynthetic-chemosynthetic diets in vent obligate macroinvertebrates at shallow hydrothermal vents on Volcano 1, South Tonga Arc-evidence from stable isotope and fatty acid analyses. Cah Biol Mar 51:351–359
Cosel VR (2008) A new Bathymodioline mussel (Bivalvia: Mytiloidea: Mytilidae: Bathymodiolinae) from vent sites near Kueishan Island, north east Taiwan. Raffles Bull Zool 19:105–114
Cosel VR, Bouchet P (2008) Tropical deep-water lucinids (Mollusca: Bivalvia) from the Indo-Pacific: essentially unknown, but diverse and occasionally gigantic. Trop Deep Sea Benthos 25:115–213
Dando PR, Hughes JA, Thiermann F (1995) Preliminary observations on biological communities at shallow hydrothermal vents in the Aegean Sea. In: Parson LM, Walker CL, Dixon DR (eds) Hydrothermal vents and processes. Special Publication 87. Geological Society, London, pp 303–317
de Buron I, Morand S (2002) Deep-sea hydrothermal vent parasites: where do we stand? Cah Biol Mar 43:245–246
Desbruyères D, Segonzac M, Bright M (2006) Handbook of deep-sea hydrothermal vent fauna. Denisia 18:1–544
Duperron S (2010) The diversity of deep-sea mussels and their bacterial symbioses. In: Kiel S (ed) The vent and seep biota. Topics in geobiology 33. Springer Science Business Media B.V., Dordrecht, pp 137–167
Duperron S, Bergin C, Zielinski F, Blazejak A, Pernthaler A, McKiness ZP, DeChaine E, Cavanaugh CM, Dubilier N (2006) Dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environ Microbiol 8:1441–1447. doi:10.1111/j.1462-2920.2006.01038.x
Farquhar GD, Ehleringer R, Hubic KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. doi:10.1146/annurev.pp.40.060189.002443
Fiala-Médioni A, McKiness ZP, Dando P, Boulegue J, Mariotti A, Alayse-Danet AM, Robinson JJ, Cavanaugh CM (2002) Ultrastructural, biochemical, and immunological characterization of two assemblages of the mytilid mussel Bathymodiolus azoricus from the Mid-Atlantic Ridge: evidence for a dual symbiosis. Mar Biol 141:1035–1043. doi:10.1007/s00227-002-0903-9
Fisher CR, Brooks JM, Vodenichar JS, Zande JM, Childress JJ, Burke RA Jr (1993) The co-occurrence of methanotrophic and chemoautotrophic sulfur oxidizing bacterial symbionts in a deep-sea mussel. Mar Ecol 14:277–289. doi:10.1111/j.1439-0485.1993.tb00001.x
Fisher CR, Childress JJ, Macko SA, Brooks JM (1994) Nutritional interactions in Galapagos Rift hydrothermal vent communities: inferences from stable carbon and nitrogen isotope analyses. Mar Ecol Prog Ser 103:45–55
Fry B (2006) Stable isotope ecology. Springer Science+Business Media, LLC, New York
Gage JD, Tyler PA (1991) Deep-sea biology: a natural history of organisms at the deep sea floor. Cambridge University Press, Cambridge
Gaudron SM, Lefebvre S, Jorge AN, Gaill F, Pradillon F (2012) Spatial and temporal variations in food web structure from newly opened habitat at hydrothermal vents. Mar Environ Res 77:129–140
Gebruk AV, Southward EC, Kennedy H, Southward AJ (2000) Food sources, behaviour, and distribution of hydrothermal vent shrimps at the Mid-Atlantic Ridge. J Mar Biol Assoc UK 80:485–499
Govenar B (2012) Energy transfer through food webs at hydrothermal vents: linking the lithosphere to the biosphere. Oceanography 25:246–255. doi:10.5670/oceanog.2012.23
Griffis RB, Suchanek TH (1991) A model of burrow architecture and trophic modes in thalassinidean shrimp (Decapoda: Thalassinidea). Mar Ecol Prog Ser 79:171–183
Hashimoto J, Miura T, Fujikura K, Ossaka J (1993) Discovery of vestimentiferan tube-worms in the euphotic zone. Zool Sci 10:1063–1067
House CH, Schopf JW, Stetter KO (2003) Carbon isotopic fractionation by Archaeans and other thermophilic prokaryotes. Org Geochem 34:345–356
Hu MYA, Hagen W, Jeng MS, Saborowski E (2012) Metabolic energy demand and food utilization of the hydrothermal vent crab Xenograpsus testudinatus (Crustacea: Brachyura). Aquat Biol 15:11–25. doi:10.3354/ab00396
Hudson IR, Wigham BD (2003) In situ observations of predatory feeding behaviour of the galatheid squat lobster Munida sarsi using a remotely operated vehicle. J Mar Biol Ass UK 83:463–464. doi:10.1017/S0025315403007343h
Hugler M, Sievert SM (2011) Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Ann Rev Mar Sci Palo Alto Annu Rev 3:261–289
Hwang J-S, Lee C-S (2003) The mystery of underwater world for tourism of Turtle Island, Taiwan. Northeastern Coast National Scenic Area Administration, Tourism Bureau, The Ministry of Transportation, and Communication, Taiwan (in Chinese)
Jeng MS, Ng NK, Ng PKL (2004) Feeding behaviour: hydrothermal vent crabs feast on sea ‘snow’. Nature 432:969. doi:10.1038/432969a
Karl DM (1995) Ecology of free-living, hydrothermal vent microbial communities. In: Karl DM (ed) The microbiology of deep-sea hydrothermal vents. CRC Press, New York, pp p35–p124
Komai T, Chan TY (2010) A new genus and two new species of alvinocaridid shrimps (Crustacea: Decapoda: Caridea) from a hydrothermal vent field off northeastern Taiwan. Zootaxa 2372:15–32
Komai T, Fujiwara Y (2012) New records of callianassid ghost shrimp (Crustacea: Decapoda: Axiidea) from reducing environments in Kyushu, southwestern Japan. Zootaxa 3271:55–67
Komai T, Kitajima M, Nemoto S, Miyake H (2011) New record of Paragiopagurus ventilatus (Crustacea: Decapoda: Anomura: Parapaguridae) from hydrothermal vents on the Nikko Seamount, Mariana Trough, the first hermit crab using siboglinid tubes for housing. Mar Biodivers Rec 3:e122. doi:10.1017/S1755267210001090
Kuo FW (2001) Preliminary investigation of the hydrothermal activities of Kueishantao Island. Dissertation, National Sun Yat-sen University
Lavalli KL, Spanier E (2007) The biology and fisheries of the slipper lobster. Crustacean, Issues 17. CRC Press, New York
Lee MY, Munroe TA, Chen HM (2009) A new species of tonguefish (Pleuronectiformes: Cynoglossidae) from Taiwanese waters. Zootaxa 2203:49–58
Lemaitre R (2004) Discovery of the first hermit crab (Crustacea: Decapoda: Parapaguridae) associated with hydrothermal vents. Cah Biol Mar 45:325–334
Levesque C, Limen H, Juniper SK (2005) Origin, composition and nutritional quality of particulate matter at deep-sea hydrothermal vents on Axial Volcano, NE Pacific. Mar Ecol Prog Ser 289:43–52
Lichtman GS, Normark WR, Spiess FN (1984) Photogeologic study of a segment of the East Pacific Rise axis near 21°N latitude. Geol Soc Am Bull 95:743–752. doi:10.1130/0016-7606(1984)95<743:PSOASO>2.0.CO;2
Lin SY (2011) The study of feeding habits and protein expression pattern of Xenograpsus testudinatus in upper sublittoral hydrothermal vents of Kueishan Island. Dissertation, National Sun Yat-sen University
Lin FJ, Komai T, Chan TY (2007) A new species of a callianassid shrimp (Crustacea: Decapoda: Thalassinidea) from deep-water hydrothermal vents off Taiwan. Proc Biol Soc Wash 120(2):143–158. doi:10.2988/0006-324X(2007)120[143:ANSOCS]2.0.CO;2
Lin HY, Lin PY, Chang NN, Shiao JC, Kao SJ (2014) Trophic structure of megabenthic food webs along depth gradients in the South China Sea and off northeastern Taiwan. Mar Ecol Prog Ser 501:53–66
Mateo MA, Serrano O, Serrano L, Michener RH (2008) Effects of sample preparation on stable isotope ratios of carbon and nitrogen in marine invertebrates: implications for food web studies using stable isotopes. Oecologia 157:105–115. doi:10.1007/s00442-008-1052-8
McKiness ZP, McMullin ER, Fisher CR, Cavanaugh CM (2005) A new bathymodioline mussel symbiosis at the Juan de Fuca hydrothermal vents. Mar Biol 148:109–116. doi:10.1007/s00227-005-0065-7
Micheli F, Peterson CH, Mullineaux LS, Fisher CR, Mills SW, Sancho G, Johnson GA, Lenihan HS (2002) Predation structures communities at deep-sea hydrothermal vents. Ecol Monogr 72:365–382. doi:10.1890/0012-9615(2002)072[0365:PSCADS]2.0.CO;2
Munroe TA, Tyler J, Tunnicliffe V (2011) Description and biological observations on a new species of deepwater symphurine tonguefish (Pleuronectiformes: Cynoglossidae: Symphurus) collected at Volcano-19, Tonga Arc, West Pacific Ocean. Zootaxa 3061:53–66
Nanjo N (2007) Feeding habits of the glass shrimp Pasiphaea japonica in Toyama Bay of the Sea of Japan. Crustacean Res 36:45–51
Nelson DC, Hagan KD, Edwards DB (1995) The gill symbiont of the hydrothermal vent mussel Bathymodiolus thermophilus is a psychrophilic, chemoautotrophic, sulfur bacterium. Mar Biol 121:487–495. doi:10.1007/BF00349457
Ng NK, Huang JF, Ho PH (2000) Description of a new species of hydrothermal crab, Xenograpsus testudinatus (Crustacea: Decapoda: Brachyura: Grapsidae) from Taiwan. Nat Taiwan Mus Spec Publ Ser 10:191–199
Page HM, Fiala-Medioni A, Fisher CR, Childress JJ (1991) Experimental evidence for filter-feeding by the hydrothermal vent mussel, Bathymodiolus thermophiles. Deep Sea Res I 38:1455–1461
Pedersen RB, Rapp HT, Thorseth IH, Lilley MD, Barriga FJAS, Baumberger T, Flesland K, Fonseca R, Fruh-Freen GL, Jorgensen SL (2010) Discovery of a black smoker field and a novel vent fauna at the ultraslow spreading Arctic Mid-Ocean Ridges. Nat Comm 1:126
Polz MF, Robinson JJ, Cavanaugh CM, Van Dover CL (1998) Trophic ecology of massive shrimp aggregations at a Mid-Atlantic Ridge hydrothermal vent site. Limnol Oceanogr 43:1631–1638
Pond DW, Bell MV, Dixon DR, Fallick AE, Segonzac M, Sargent JR (1998) Stable-carbon-isotope composition of fatty acids in hydrothermal vent mussels containing methanotrophic and thiotrophic bacterial endosymbionts. Appl Environ Microbiol 64:370–375
Rau GH, Hedges JI (1979) Carbon-13 depletion in a hydrothermal vent mussel: suggestion of a chemosynthetic food source. Nature 203:648–649. doi:10.1126/science.203.4381.648
Reid WDK, Sweeting CJ, Wigham BD, Zwirglmaier K, Hawkes JA et al (2013) Spatial differences in east scotia ridge hydrothermal vent food webs: influences of chemistry, microbiology and predation on trophodynamics. PLoS One 8(6):e65553. doi:10.1371/journal.pone.0065553
Robinson JJ, Scott KM, Swanson ST, O’Leary MH, Horken K, Tabita FR, Cavanaugh CM (2003) Kinetic isotope effect and characterization of form II RuBisCO from the chemoautotrophic endosymbionts of the hydrothermal vent tubeworm Riftia pachyptila. Limnol Oceanogr 48:48–54
Roeske CA, O’Leary MH (1984) Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23:6275–6284
Sahlmann C, Chan TY, Chan BKK (2011) Feeding modes of deep-sea lobsters (Crustacea: Decapoda: Nephropidae and Palinuridae) in Northwest Pacific waters: Functional morphology of mouthparts, feeding behaviour and gut content analysis. Zool Anz 250:55–66. doi:10.1016/j.jcz.2010.11.003
Segonzac M, de Saint Laurent M, Casanova M (1993) L’énigme du comportement trophique des crevettes Alvinocarididae des sites hydrothermaux de la dorsale médio-atlantique. Cah Biol Mar 34:535–571
Shimoda K, Aramaki Y, Nasuda J, Yokoyama H, Ishihi Y, Tamaki A (2007) Food sources for three species of Nihonotrypaea (Decapoda: Thalassinidea: Callianassidae) from western Kyushu, Japan, as determined by carbon and nitrogen stable isotope analysis. J Exp Mar Biol Ecol 342:292–312. doi:10.1016/j.jembe.2006.11.003
Stickney RR (1976) Food habits of Georgia estuary fishes II. Symphurus plagiusa (Pleuronectiformes: Cynoglossidae). Trans Am Fish Soc 105:202–207. doi:10.1577/1548-8659(1976)105<202:FHOGEF>2.0.CO;2
Streams ME, Fisher CR, Fiala-Médioni A (1997) Methanotrophic symbiont location and fate of carbon incorporated from methane in a hydrocarbon seep mussel. Mar Biol 129:465–476. doi:10.1007/s002270050187
Sweetman AK, Levin LA, Rapp HT, Schander C (2013) Faunal trophic structure at hydrothermal vents on the southern Mohn’s Ridge, Arctic Ocean. Mar Ecol Prog Ser 473:115–131. doi:10.3354/meps10050
Tarasov VG, Gebruk AV, Mironov AN, Moskalev LI (2005) Deep-sea and upper sublittoral hydrothermal vent communities: two different phenomena? Chem Geol 224:5–39. doi:10.1016/j.chemgeo.2005.07.021
Tsai PC, Yeh HM, Chan BKK, Chan TY (2009) Comparison between the catch composition of the French and ORE type beam trawls on deep-sea decapod crustaceans: implications for quantitative sampling of the deep-sea decapod biodiversity. Crustaceana 82:565–591. doi:10.1163/156854008X390326
Tsuchida S, Suzuki Y, Fujiwara Y, Kawato M, Uematsu K, Wamanaka T, Mizota C, Yamamoto H (2011) Epibiotic association between filamentous bacteria and the vent-associated galatheid crab, Shinkaia crosnieri (Decapoda: Anomura). J Biol Assoc UK 91:23–32
Tunnicliffe V (1991) The biology of hydrothermal vents: ecology and evolution. Oceanogr Mar Biol Annu Rev 29:319–407
Tunnicliffe V, Jensen RG (1987) Distribution and behaviour of the spider crab Macroregonia macrochira Sakai (Brachyura) around the hydrothermal vents of the northeast Pacific. Can J Zool 65:2443–2449. doi:10.1139/z87-369
Van Dover CL (2000) The ecology of deep-sea hydrothermal vents. Princeton University Press, Princeton
Van Dover CL (2002) Trophic relationships among invertebrates at the Kairei hydrothermal vent field (Central Indian Ridge). Mar Biol 141:761–772. doi:10.1007/s00227-002-0865-y
Van Dover CL, Fry B (1989) Stable isotopic compositions of hydrothermal vent organisms. Mar Biol 102:257–263. doi:10.1007/BF00428287
Van Dover CL, Fry B (1994) Microorganisms as food resources at deep-sea hydrothermal vents. Limnol Oceanogr 39:51–57
Van Dover CL, Fry B, Grassle JF, Humphris S, Rona PA (1988) Feeding biology of the shrimp Rimicaris exoculata at hydrothermal vents on the Mid-Atlantic Ridge. Mar Biol 98:209–216. doi:10.1007/BF00391196
Vidal VM, Vidal PM, Isaacs JD (1978) Coastal submarine hydrothermal activity off northern Baja California. J Geophys Res B 83:757–1774. doi:10.1029/JB083iB04p01757
Voight JR (2000) A deep-sea octopus (Graneledone cf. boreopacifica) as a shell crushing hydrothermal vent predator. J Zool Lond 252:335–341. doi:10.1111/j.1469-7998.2000.tb00628.x
Wang TW, Chan TY, Chan BKK (2013) Diversity and community structure of decapod crustaceans at hydrothermal vents and nearby deep-water fish grounds off Kueishan Island, northeastern Taiwan: a high deep-sea biodiversity area in the N.W. Pacific. Bull Mar Sci 89(2):505–528. doi:10.5343/bms.2012.1036
Williams MJ (1982) Natural food and feeding in the commercial sand crab Portunus pelagicus Linnaeus, 1766 (Crustacea : Decapoda : Portunidae) in Moreton bay, Queensland. J Exp Mar Biol Ecol 59:165–176. doi:10.1016/0022-0981(82)90113-7
Yang TF, Lan TF, Lee H-F, Fu C–C, Chuang P-C, Lo C-H, Chen C-H, Chen C-TA, Lee C-S (2005) Gas composition and helium isotopic ratios of fluid samples around Kueishantao, NE offshore Taiwan and its tectonic implications. Geochem J 39:469–480
Acknowledgments
This study was supported by a grant from the Ministry of Science and Technology, Taiwan, a Career Development Award from Academia Sinica, and the Fisheries Agency, Council of Agriculture, Executive Yuan, Taiwan, R.O.C. The authors would like to thank the GNS laboratory, New Zealand, for analysing the stable isotope values of the samples. The authors would like to thank Chen I-Han and Chen Hsi-Nien (Academia Sinica) for supporting the field work in upper sublittoral vents, as well as the commercial trawler Jin Tong Long No. 26 for supporting the trawling surveys. Thanks are extended to Coralia V. Garcia, Wallace Academic Editing, Taiwan, for editing the English of the present paper. We would like to thank the four anonymous reviewers and the Associate Editor, Prof. Chris Harrod for their constructive comments, which greatly improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Harrod.
Rights and permissions
About this article
Cite this article
Wang, TW., Chan, TY. & Chan, B.K.K. Trophic relationships of hydrothermal vent and non-vent communities in the upper sublittoral and upper bathyal zones off Kueishan Island, Taiwan: a combined morphological, gut content analysis and stable isotope approach. Mar Biol 161, 2447–2463 (2014). https://doi.org/10.1007/s00227-014-2479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2479-6