Abstract
The present work investigated new sustainable opportunities for wood protection against xylophagous organisms (cellulolytic fungi and termites) based on the use of natural bioactive compounds present in Milicia excelsa wood and Nerium oleander bark. To achieve this, solid–liquid extractions by ethanol were carried out, obtaining extraction yields of 5.47 ± 0.78% for the extract of M. excelsa and 21.88 ± 0.53% for N. oleander. Gas chromatography coupled with mass spectrometry analyses were carried out to evaluate the chemical composition of both extracts, showing interesting compounds with biological activity such as pyrogallol, 4-acetylresorcinol, karanjin and scopoletin. Likewise, an evaluation of the cellulolytic capacity of different wood-isolated fungi (Aspergillus flavus, Penicillium chrysogenum, Trichoderma longibrachiatum, Mucor circinelloides and Mucor fragilis) was carried out through two screenings, based on their growth rate in carboxymethyl cellulose agar media, and their cellulose-degrading ability via filter paper rupture, being T. longibrachiatum the fungus with the highest growth rate in both substrates. Finally, a protective treatment for pine wood (Pinus sp.) was designed by using the ethanolic extracts separately and combined, respectively, against T. longibrachiatum and Reticulitermes grassei, comparing in both cases the biotic damage with a control. The results demonstrated that the impregnation significantly reduced T. longibrachiatum biomass consumption by over 70% for all treatments. Additionally, the M. excelsa impregnation notably decreased termite activity, with a 81% reduction in the long-term assays.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood has been used since ancient times due to its abundance and versatility. As is well-known, it is composed of three fundamental sections (i.e., bark, sapwood, and heartwood), each conferring a variety of functions based on their distinct chemical composition (Xu et al. 2016; Muilu-Mäkelä et al. 2021; Lourençon et al. 2016). Additionally, its recalcitrant nature results from the complex interaction between cellulose, hemicelluloses, and lignin (Bomble et al. 2017). Nonetheless, this material is susceptible to degradation by abiotic factors such as solar radiation and water, as well as biotic factors, particularly fungi and insects (Pacheco et al. 2021).
Cellulolytic fungi, such as those from the genera Trichoderma, Aspergillus, and Penicillium, secrete extracellular enzymes to convert cellulose into fermentable sugars, playing a significant role in wood decomposition (Houfani et al. 2020; Panchapakesan and Shankar 2016). Besides, termites are the most efficient living organisms at degrading lignocellulosic biomass, with a much higher cellulose degradation rate than other invertebrates and fungi (Brune and Dietrich 2015). In Spain, the predominant termite species, Reticulitermes grassei, causes significant economic damage to rural and urban infrastructures. This highly efficient digestion is aided by a diverse array of intestinal microorganisms, forming a mutually beneficial symbiotic relationship (Duarte et al. 2018; König 2006).
Traditionally, wood protection has relied on using oils, varnishes, and enamels to increase its durability against these biotic factors. This approach not only reduces the pressure on timberlands but also has a positive economic impact by lowering the costs associated with repairing and replacing wood-based structures (poles, railway ties…) (Khademibami and Bobadilha 2022). However, these products often contain xenobiotic and recalcitrant chemical compounds such as creosote, pentachlorophenol (PCP), and chromated copper arsenate (CCA), which are potentially carcinogenic to humans (Ozdemir et al. 2015; Khademibami and Bobadilha 2022). Additionally, these chemicals have been identified in soil and groundwater, raising environmental concerns (Sheng et al. 2013). Consequently, the European Union has imposed strict regulations on biocidal substances used in wood preservation, prompting the need for research into alternative methods (Lasota et al. 2019).
An eco-friendly and sustainable method for wood protection involves the use of essential oils (EOs) from plant species (Aboud 2015). Indeed, EOs may contain a variety of bioactive molecules (e.g., tannins, flavonoids, and terpenoids) known for their potential antimicrobial and insecticidal properties. Moreover, they are generally considered safe for users, hence their utilization in pharmacological and cosmetic applications, among others (Pánek et al. 2014). Milicia excelsa, notable for its impressive size, has historically been cultivated in most tropical African cities for agroforestry purposes (Atindehou et al. 2022). This practice has systematically generated a significant amount of potentially valorizable waste (Tomen et al. 2023). Indeed, its wood-derived EO, in addition to its recognized antitermite activity, makes this waste an interesting source for obtaining chemical compounds with potential biocidal activity (Nagawa et al. 2015). Besides, Nerium oleander constitutes a group of bushes native to the Mediterranean region that have been widely cultivated in urban areas for their ornamental value and resilience. Interestingly, it is one of the most poisonous plant species due to the nature of its secondary metabolites (Aboud 2015; Stefi et al. 2020). Additionally, its EO has been reported to exhibit antifungal, antibacterial, insecticidal, and cytotoxic activities, making it potentially useful for protecting wooden elements against xylophagous organisms (Derwich et al. 2010; Aboud 2015).
Overall, this work aimed to utilize the residues generated from activities related to M. excelsa and N. oleander to demonstrate the suitability of their EOs as wood protectants against cellulolytic fungi and termites. This contributes to the development of future wood protection solutions that are sustainable, effective, and safe for both humans and the environment.
Materials and methods
Materials
M. excelsa wood was provided by the Department of Zoology of the University of Córdoba (Spain) in the form of construction beams, while N. oleander wood was obtained from shrubs located in the city of Córdoba. Wood samples (of corresponding size between 10-mesh and 30-mesh using laboratory sieves) were obtained from saw-cut shavings of M. excelsa, while N. oleander bark particles resulted from manually clearing branches with a coarse-grained metal file.
Ethanol absolute pure (99.5%, M.W. = 46.07, Panreac, Spain) was employed for conducting ethanolic extractions of each type of wood particle. In the microbiological tests, potato dextrose agar (PDA, EP/USP/BAM, Condalab, Madrid, Spain), carboxymethyl cellulose (CMC, sodium salt, average M.W. = 250,000, DS = 0.9, Acros Organics, France), Whatman filter paper (sheets, Whatman® qualitative filter paper Grade 1, Merck, Germany), calcium chloride (CaCl2, ≥ 98.0%, M.W. = 110.99, fused, granular about 0.5–2.0 mm for elementary analysis, Merck, Germany), ammonium sulfate ((NH4)2SO4, ≥ 99.5%, M.W. = 132.14, EMSURE®, ACS, ISO, Reag. Ph Eur, for analysis, Merck, Germany), di-potassium hydrogen phosphate (K2HPO4, ≥ 99.0%, M.W. = 174.18, EMSURE®, anhydrous for analysis, Merck, Germany), yeast extract (LP0021B, Oxoid, France), and bacteriological agar (Agar No. 1, LP0011B, Oxoid, Spain) were used. All culture media were sterilized prior to use according to the standard method (121 °C, 21 min).
Extraction of plant ethanolic compounds
The natural wood preservatives used in this study were obtained through solid–liquid extraction by ethanol, based on the methodology described by Erman and Turkoglu (2021) with minor adaptations. Initially, 5 g of each oven-dried (72 h, 105 ± 5 °C) sawdust were taken into cellulose thimbles, introducing them into a Soxhlet-type extractor using absolute ethanol as solvent. Six replicates were performed for each type of fiber. The extracted material was oven-dried (24 h, 105 ± 5 °C) and the extraction yield was determined. The resulting liquid phase was vacuum rotavaporated (Laborota 4000, Heidolph) to recover the solvent at 60 °C, noting the final volume to calculate the concentration (C, g/mL) of each extract by gravimetric difference as follows:
where m0 is the added-sawdust initial weight (g), m1 is the cellulose thimble initial weight (g), m2 is the weight (g) of the cellulose thimble including sawdust after Soxhlet extraction, and V is the final extract volume (mL) obtained after rotavaporation.
Chemical characterization of ethanolic extracts (GC–MS analysis)
Both ethanolic extracts were analyzed on an Agilent Technologies GC–MS Instruments 5977B GC/MSD (Spain) equipped with an HP-5 ms Ultra Inert column (30 m × 250 μm × 0.25 μm). GC–MS spectra were obtained using He as a carrier gas, with a flow rate of 1.8 mL/min, a split of 1:20, 1 μL as injection volume at 300 °C, with an oven temperature of 200 °C and an electronic impact at 70 eV as the ionization mode. Identification of the components was achieved by comparing mass spectral fragmentation patterns with those stored in the data bank NIST Standard Reference Database 1Av17 (Data Version NIST v17, Software Version 2.3). Furthermore, it was possible to determine the relative content (referred to the % of the total area) of the main compounds by integration of peaks, according to the method followed by Koudehi et al. (2020).
Selection of cellulolytic fungi
Five strains were provided by the Department of Agricultural Chemistry, Edaphology and Microbiology of the University of Cordoba from its collection of cellulolytic fungi isolated from wood: Aspergillus flavus, Penicillium chrysogenum, Trichoderma longibrachiatum, Mucor circinelloides, and Mucor fragilis.
The cellulolytic activity of these fungi was tested by two secondary screenings to assess the most promising candidate (active against potential wood degradation) for subsequent protection assays. During the first screening, the aim was to determine which one of the initial strains exhibited the highest growth rate on CMC medium, indicative of the cellulolytic capacity of the microorganism (Carbonero-Pacheco et al. 2023). Concisely, the fungal strains were cultured on PDA media (72 h, 28 ± 2 °C), strategically placing the inocula at the center of 90 mm diameter Petri plates to promote their radial growth. Then, a circular sample (1 cm diameter) from the margin of each culture was placed in the center of plates containing CMC agar medium (CMC, 1.25%; CaCl2, 0.0125%; yeast extract, 0.0625%; (NH4)2SO4, 0.0625%; K2HPO4, 0.125%; agar, 1.5%, in deionized water), incubating them at 28 ± 2 °C until the hyphae of the fastest fungus grew on the entire plate surface. To assess the radial growth of each fungus in CMC, their development was measured daily using a digital caliper micrometer (Mitutoyo Manufacturing, Tokyo, Japan). The obtained data were used to calculate the radial growth rate (mm/day) for each isolate. Three replicates were conducted for each tested fungi strain.
Regarding the second screening, qualitative tests were carried out for all fungi capable of growing in the CMC agar medium, adapting the methodology followed by Guder and Krishna (2019). This screening was carried out because different authors suggest that growth on CMC medium may be due not only to the cellulolytic capacity of the microorganism but also to other enzymatic activities (Johnsen and Krause 2014). For this, test tubes (160 × 13 mm) with 8 mL of a ready-to-use basal mineral salt medium (standard saline solution -see detailed composition in Table S1 of the Supplementary Information (SI)-, 50 mL; trace elements solution -see detailed composition in Table S2 of the SI-, 1 mL; ammonium nitrate, 1 g, in 1000 mL of deionized water) and a filter paper strip (7 cm long × 1 cm wide, 1–2 cm protruding from the level of the liquid) were inoculated and incubated for 35 days at 28 ± 2 °C. Thus, microbial growth would confirm their cellulolytic activity and support the results obtained during the first screening. This test was performed in triplicate.
Response of xylophagous organisms to N. oleander and M. excelsa ethanolic extracts
Pine wood (Pinus sp.) blocks (1 cm3) were impregnated with the previously obtained ethanolic extracts following the indications of Fidan et al. (2016), thus applying the method stated in ASTM-D 1413. Hence, three different impregnation treatments were performed: (1) using the ethanolic extracts from M. excelsa, (2) using those extracted from N. oleander, and (3) with a 50% volume mixture of both extracts. In short, after dehydration (72 h, 60 ± 5 °C), pine wood blocks were submerged in 1% volume of extract in ethanol and placed in a vacuum thermostatic desiccator Vacuo-Temp JP SELECTA at 133,32 mbar and 25 ± 1 °C during 30 min. Once the impregnation time was over, blocks were oven-dried (72 h, 60 ± 5 °C). The retention percentage (R, %) of each EO in pine wood was determined following the methodology specified by Yildiz et al. (2020), using the following equation:
where m1 is the weight (g) of the pine wood cubes before the impregnation treatment, and m2 is the weight (g) of the cubes after the impregnation.
To evaluate the biocide effect of the wood treatment, both impregnated and unimpregnated pine wood blocks were dehydrated (oven-drying, 72 h, 60 ± 5 °C), weighed, and finally tested against xylophagous organisms. The fungal response to each impregnation treatment was evaluated according to the method proposed by Brischke et al. (2022), slightly modified. Briefly, 2% agar was used as the culture medium for conducting six different assays, each replicated three times. The assays included: (1) inoculating the fungus in Petri dishes; (2) culturing unimpregnated pine wood blocks; (3) culturing inoculated, unimpregnated pine wood blocks; (4) culturing inoculated pine wood blocks impregnated with M. excelsa ethanolic extracts; (5) culturing inoculated pine wood blocks impregnated with N. oleander ethanolic extracts; and (6) culturing inoculated pine wood blocks impregnated with the oil mix. First, a constant mass of each pine wood block was achieved through dehydration. Subsequently, the blocks were cultivated in the agar medium depending on the assay. Finally, the required blocks were inoculated with the fungus (previously grown on PDA, 48 h, 28 ± 2 ºC) that exhibited the highest growth rate on CMC agar medium and demonstrated the ability to grow on the filter paper strip. In all cases, the Petri dishes were sealed with parafilm and incubated for 60 days at 28 ± 2 °C. At the end of the incubation time, an isolation of the microorganisms present on each of the surfaces of the blocks was carried out on PDA medium (48 h, 28 ± 2 °C). Subsequently, the pine wood blocks were carefully removed from the agar, cleaned of adhering mycelium, oven-dried for 72 h at 60 ± 5 °C, and weighed. The evaluation of the eventual external damage suffered in each block was carried out with a binocular stereomicroscope (Olympus SD 30). Then, the antimicrobial effect was determined based on the percentage of sample mass loss (ML, %) as
where m0 and m1 represent the weight (g) of the samples before and after decay, respectively.
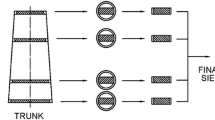
Simultaneously, the degradation capacity of R. grassei specimens was tested to evaluate the potential antitermitic effect of the impregnation process. To achieve this, the methodology proposed by Cárdenas et al. (2018) was followed with some modifications. Shortly, an ecosystem colonized by R. grassei was sampled across a forest area of the Rabanales University Campus (Córdoba, Andalusia region, southern Spain; standard UTM coordinates “30N 348506X 4197911Y”) in autumn 2020. This forest area predominantly comprised tree species from the genera Pinus, Eucalyptus, and Populus. A baiting station containing corrugated cardboard was placed next to the base of a pine tree showing signs of termite activity to capture them, ensuring they belonged to the same colony (Carbonero-Pacheco et al. 2023). The termites were maintained in rearing containers at a temperature of 28 ± 1 ºC and RH of 80 ± 5%, in complete darkness. They were fed on pine wood ad libitum, using sterile vermiculite moistened with distilled water in a ratio of 1:3 (w/v) as substrate. The termites were maintained under these conditions for a maximum of one week before the start of the assays (Cárdenas et al. 2020). Then, a choice test was conducted by placing two cubes at the opposite ends of a plastic container (17.0 cm length × 9.5 cm width × 3.0 cm height), as shown in Fig. 1. In each case, one of the cubes was impregnated with one of the treatments, while the other cube remained untreated. Each cube was placed in a 50 mm diameter Petri dish with holes to allow the insects to enter and exit. Both cubes were separated by 3 g of moistened sterile vermiculite (at the same ratio mentioned previously), allowing the termites to select their preferred food source. The lid of each plastic container was punctured to facilitate air entry. Finally, a group of 150 termites (5:45 soldier-to-worker ratio) was introduced into each plastic container, keeping the assays under the same temperature and humidity conditions as mentioned above (Chouvenc et al. 2011). Thus, trials were carried out at 15, 30, and 45 days, performing three replications for each period and for the three impregnation treatments proposed. Following each trial period, the number of surviving termites was counted using fine-tipped entomological forceps. Blocks were carefully removed and cleaned before being dehydrated in an oven for 72 h at 60 ± 5 °C. Once dehydrated, they were weighed to determine the mass consumption by the termites. If the consumption of impregnated cubes negatively impacted termite survival, such treatment was categorized as termiticidal. Conversely, if there was a reduced preference for the impregnated cubes without affecting termite survival, it was considered as a repellent treatment.
Statistical analysis
Data were shown as means ± SD. Statistical analyses were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software Inc., San Diego, USA). The Shapiro–Wilk test was used to assess the normality of the data, and the Bartlett test was applied to check the equality of variances. For the termite (consumption and survival) and fungal (consumption) datasets, the Student’s t-test and one-way ANOVA followed by Tukey’s post-hoc test were utilized, respectively. In all cases, a p-value of ≤ 0.05 was considered statistically significant.
Results and discussion
Yield and composition of extracts
The ethanolic extracts from M. excelsa were obtained with an extraction yield of 5.5 ± 0.8% and a final concentration of 0.13 g/mL. Regarding those obtained from N. oleander bark, they presented a higher extraction yield (21.9 ± 0.5%), but with the same final concentration as in the case of M. excelsa. Areola et al. (2015) obtained a slightly higher extraction yield (8.2%) in their investigation while macerating the bark of M. excelsa in 70% (v/v) ethanol for 72 h with regular shaking. Otherwise, Kgosana (2019) obtained an extraction yield of 22.6% by applying a decoction process on N. oleander leaves, involving heating water to 80–100 °C. This shows how the plant material, the chosen solvent, and the extraction method can affect the number of extractable compounds that can be obtained.
Concerning the impregnation process, the retention values (R) obtained for each EO in the pine wood cubes were 12.48%, 18.37%, and 16.59% for M. excelsa, N. oleander, and the mixture, respectively. These variations in R values are consistent with the literature and can be attributed to the complex binding process of each EO to the specific type of wood, making the retention value unique to each combination (Sen et al. 2009). Yildiz et al. (2020) demonstrated that the impregnation of pine wood with aqueous and methanolic extracts of other plant species following the same standard methodology yielded significantly higher R values. This is due to the importance of the concentration of the initial impregnation solution, as it is well known that more concentrated impregnation solutions result in higher R values (Yildiz et al. 2020).
Through GC–MS analysis, the main active compounds present in both samples were identified, as well as their relative content (% referred to the total area) was determined as shown in Tables 1 and 2, respectively. For M. excelsa extract (Table 1), 7 main bioactive compounds were identified, being the main constituents pyrogallol (15.06%), 3,4-dihydroxybenzaldehyde (14.24%), and methyl vanillate (5.92%). In addition, it was possible to identify other phenolic and aromatic compounds related to antimicrobial activities, such as dihydrojasmone, coniferyl alcohol, 2-metoxyresorcinol, and syringaldehyde (Pinto et al. 2021; Lubbers et al. 2019; Golpayegani et al. 2014). Furthermore, antitermitic activity has been reported for 2-methoxy-resorcinol, coinciding with the potential termiticidal properties of M. excelsa extractives reported by other authors (Nagawa et al. 2015; Golpayegani et al. 2014).
Regarding the extract of N. oleander (Table 2), the chromatography allowed us to identify a total of 16 chemical compounds. Among them, several hemicellulosic derivatives (30% of the total) were identified, such as ethyl α-D-glucopyranoside, 3-O-methyl-D-glucose, and trehalose. Also, it was possible to identify different fatty acids and derivatives related to antimicrobial activities such as ethyl laurate, methyl linoleate, ethyl linoleate, and ethyl oleate in addition to others that have also been related to insecticidal activities such as ethyl palmitate (Kumari et al. 2022). Likewise, it was possible to identify other chemical compounds related to antimicrobial activities such as 5-hydroxymethylfurfural, ethyl 9,9-diethoxy-nonanoate, and 4-acetylresorcinol (Lubbers et al. 2019; Zhou et al. 2009; Morris 1992). Also, antitermitic activities have been cited in the literature for the compounds karanjin and scopoletin (Ahmed et al. 2022; Qazi et al. 2018).
Secondary screening of cellulolytic fungi
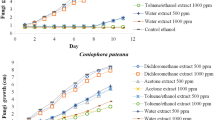
All the fungi tested were able to grow on CMC agar, thus demonstrating their carboxymethyl cellulolytic activity. However, the growth rate results obtained after 8 days of incubation showed different abilities between them to hydrolyze the polymer, being T. longibrachiatum the fungus presenting the highest growth rate on CMC agar (10.9 ± 0.1 mm/day), followed by M. fragilis (7.4 ± 0.2 mm/day), M. circinelloides (7.3 ± 0.9 mm/day), A. flavus (4.5 ± 0.0 mm/day) and, finally, P. chrysogenum (1.8 ± 0.0 mm/day).
Considering the filter paper assay, the growth of cellulolytic fungi manifested as pigmented spots appearing on the strips confluence between culture medium and the air. In this regard, three fungi were able to colonize the cellulose strip, specifically T. longibrachiatum, A. flavus, and P. chrysogenum (Fig. 2). However, only T. longibrachiatum managed to break the strip at the level of the meniscus, agreeing with the high growth rate previously observed in the CMC agar assays. These results totally coincide with the controversy shown in the bibliography since, despite its wide use, some authors state that growth in CMC agar may be due to agarase rather than cellulase capacity (Johnsen and Krause 2014), an effect contrasted with the absence of growth of M. circinelloides and M. fragilis in the cellulose strips. Moreover, these results align with the screening conducted by Pachauri et al. (2017), who showed that T. longibrachiatum was the strain isolated from wood with the highest cellulolytic activity. In fact, its cellulase activity has been used for its potential to degrade other lignocellulosic residues (Li et al. 2020). Overall, T. longibrachiatum was chosen as the model fungus to establish the protective power of the impregnation treatments.
Fungal response to protection treatments by impregnation
The degradation rate of the wood samples by T. longibrachiatum is depicted in Fig. 3. All the impregnation treatments determined significant protection (p ≤ 0.05) against T. longibrachiatum. Indeed, the unimpregnated wood exhibited a consumption rate of 7.29 ± 1.11% wt., while the impregnation with M. excelsa, N. oleander, and the mixture EOs determined a significant reduction (p ≤ 0.05) in wood consumption, with rates of 1.93 ± 0.47% wt., 2.01 ± 0.25% wt., and 2.09 ± 0.62% wt., respectively. These substantial decreases observed in the treated groups, exceeding 70% in all cases, underscore the potential of these essential oils as environmentally friendly and sustainable wood protectants against cellulolytic fungi (Khademibami and Bobadilha 2022). The results obtained for agar media lacking pine wood cubes showed no fungal growth, indicating the absence of any cellulase activity-promoting nutrient. Furthermore, after each test, axenic cultures of T. longibrachiatum were obtained in all cases, demonstrating that the degradation observed in each cube was exclusively attributed to this fungus.
These degradation results can be explained by the presence in the extracts of different active molecules identified by GC–MS (Tables 1 and 2). Thus, the presence of dihydrojasmone (4.19%), pyrogallol (15.06%), methyl vanillate (5.92%), and 3,4-dihydrobenzaldehyde (14.24%) in the ethanolic extract of M. excelsa, reported as potent antimicrobials, was crucial for its contrasted antifungal activity in the consumption of the blocks (Pinto et al. 2021; Kocaçalışkan et al. 2006; Theodori et al. 2006; Syafni et al. 2012). Similarly, the behavior of T. longibrachiatum in response to impregnation with N. oleander EO could be attributed to certain chemical compounds identified in its ethanolic fraction, such as 3-O-methyl-D-glucose, which exhibited the highest relative content in the sample (Altameme et al. 2015). Likewise, 4-acetylresorcinol (9.70%) and ethyl linoleate (5.52%) have also been related to fungicidal activities (Morris 1992; Ko and Cho 2017). Lastly, the mixture of both ethanolic extracts did not have a greater antifungal effect than the extracts separately (p > 0.05), although it managed to reduce the activity of T. longibrachiatum by 71.29% (Table 3). The presence of chemical compounds with demonstrated bactericidal activity in both ethanolic extracts, such as pyrogallol, 3,4-dihydroxybenzaldehyde, coniferyl alcohol, 4-acetylresorcinol, ethyl linoleate, and ethyl 9,9-diethoxynonanoate, makes this mixed treatment potentially effective against cellulolytic bacteria (Kocaçalışkan et al. 2006; Syafni et al. 2012; Lubbers et al. 2019; Morris 1992; Ko and Cho 2017; Zhou et al. 2009). These cellulolytic microorganisms are characterized by a rapid growth rate, easy adaptation to extreme environments, and secretion of large amounts of extracellular cellulases, also playing a significant role in wood decay (Ghio et al. 2012). Finally, the examination of wood cube surfaces showed that there were little discernible differences in the visual damage or deterioration between the unimpregnated and impregnated blocks. This phenomenon could be attributed to the specific colonization pattern of T. longibrachiatum. As stated in the bibliography, fungal colonization can initiate at the cut ends of wood, propagating from one cell to another within the internal regions. Consequently, cellulose degradation can proceed without causing apparent changes to the surface of the material (Daniel 2016).
Termite response to protection treatments by impregnation
As shown in Fig. 4 (A), the impregnation treatment with the ethanolic extracts of M. excelsa minimized the consumption of the pine wood blocks by R. grassei, being null in the 15-day tests and only 5% wt. in the 45-day tests, determining that in the three periods of time the consumption of the unimpregnated wood was significantly higher (p ≤ 0.05). Furthermore, based on the survival rates of the specimens (Fig. 4 B), it can be concluded that the impregnation treatment had a significant impact on termite mortality (p ≤ 0.05), leading to a reduction of 18.67%, 32.67%, and 43.34% in the initial population after 15, 30, and 45 days of testing, respectively. These results agree with the reported antitermitic activity of 2-methoxyresorcinol, whose relative content in our sample was only 1.04%, thus demonstrating its high ability to inhibit the foraging of termites at low concentrations (Golpayegani et al. 2014). In addition to this, the presence of proven antimicrobial compounds in the sample could have affected the complex intestinal microbiota of termites, preventing them from degrading cellulose and ultimately resulting in starvation (Rosengaus et al. 2011).
Regarding the impregnation with the ethanolic extracts of N. oleander, a different consumption pattern was observed. During the 15 day tests, a significant difference was observed in the consumption between the unimpregnated (12.54 ± 2.12% wt.) and the impregnated cubes, where consumption was completely absent. After an in-depth bibliographic review of the main chemical compounds identified by the GC–MS analysis, antitermite and insecticide activity was found for piceol, karanjin, scopoletin, and ethyl palmitate (Mageroy et al. 2017; Ahmed et al. 2022; Qazi et al. 2018; Kumari et al. 2022). Consequently, the combined action of these compounds resulted in the strong repellent effect observed in short-term assays. However, after 30 days of testing, the consumption between both types of wood was similar (approximately 15% wt.). This behavior was observed even after 45 days of testing, with no significant differences in consumption between the two types of wood (p > 0.05). This effect can be attributed to a high volatility of the EO combined with a low retention in pine wood, factors that greatly affect the efficacy of natural wood preservatives (Bahmani and Schmidt 2018). Additionally, the treatment did not significantly affect the survival of the insects (p > 0.05), as a population of 130 ± 12 individuals was maintained even after 45 days of testing.
Finally, the impregnation tests using the mixture EOs revealed a significant difference in consumption between the two types of wood throughout all the different time periods, with a significant preference for the unimpregnated blocks (p ≤ 0.05). Interestingly, this difference in consumption reached its peak in the 15-day assays, where the impregnated cubes showed a consumption rate of 1.34 ± 0.62% wt. This aligns with the previously discussed results for the treatment with the N. oleander EO. Considering the survival results, the treatment with both ethanolic extracts significantly (p ≤ 0.05) impacted termite survival after 30 and 45 days, with a reduction in the initial number of individuals by 18% and 29%, respectively. Overall, the mixed treatment provided both repellent and termiticidal effects, with an efficacy higher than the N. oleander impregnation but lower than the M. excelsa impregnation.
The observed behavior of R. grassei could be explained by the ecology of the plant species used in this work. These termites share biotope with N. oleander, but not with M. excelsa as it is an exotic tree. Thus, the insects could show resistance to a fraction of the chemical compounds of N. oleander as they are adapted to them, while they do not have resistance mechanisms against the chemical compounds of M. excelsa (Kasseney et al. 2011).
Limitations and future research perspectives
The findings elucidated in this work provide a strong starting point for future research, which could address existing limitations. For example, while the Soxhlet method is widely used for extracting such EOs, its optimization (e.g., extraction time, type of solvent) and the evaluation of its impact on wood protective properties would be a promising area for further investigation (Daud et al. 2022). Furthermore, the identification of the specific bioactive compounds responsible for the observed activities would be highly valuable. This could enhance effectiveness and reduce the required quantity of each EO during the preparation of the protective solutions (Diniz do Nascimento et al. 2020). Additionally, while impregnation treatments were specifically applied to pine wood, other wood types commonly attacked by xylophagous organisms (such as aspen, oak…) should also be considered (Khademibami and Bobadilha 2022). Moreover, determining the protective capacity of these extracts against other cellulolytic wood-degrading fungi (e.g., following the European standard EN 113 for basidiomycetes) would be crucial to broaden their antifungal spectrum (Marais et al. 2020). This same consideration applies to their potential antitermite activity, given the diverse species causing significant economic damage to infrastructure in different countries (Subekti et al. 2018). Lastly, advanced imaging techniques (scanning electron microscopy, atomic force microscopy, 3D imaging analysis…) could offer enhanced resolution and detailed insights into structural changes occurring at the microscopic level during the antifungal assays (Daniel 2016; Charpentier-Alfaro et al. 2023). Exploring these topics would facilitate advancements in their application within the wood protection sector.
Conclusion
This work reports for the first time the fungicidal and termiticidal power of the ethanolic extracts from M. excelsa and N. oleander individually and combined. These extracts were evaluated through wood impregnation tests against T. longibrachiatum and R. grassei, thus assessing the resistance to decay in pine wood, a material widely used in construction. The presence of active compounds with reported antifungal activity both in the ethanolic extract of M. excelsa and in N. oleander determined that T. longibrachiatum consumed significantly less pine wood from the impregnated blocks (2.01 ± 0.08% wt.) than from the unimpregnated ones (7.29 ± 1.11% wt.) after 60 days of in vitro culture, highlighting the potential of the treatments as wood protectants against wood-degrading fungi. Besides, impregnating pine wood with M. excelsa EO resulted in repellent and termiticidal activities against R. grassei, with only 5% of the wood consumed by the insects, and nearly half of the population dying after 45 days of exposure. Conversely, pine samples impregnated with N. oleander EO demonstrated strong repellent activity against termites during the 15-day exposure tests, yet did not significantly impact the survival rate throughout the experiments (p > 0.05). This highlights the importance of the ecology of the tested plants and insects. Finally, combining both EOs yielded interesting antimicrobial and anti-termite efficacy results, protecting wood against both types of cellulolytic organisms. This combined treatment potentially offers an industrial advantage by requiring less wood from M. excelsa, which grows slower. In summary, this work has demonstrated three sustainable, safe, and effective alternatives for the wood protection sector, capable of ensuring low xylophagy by cellulolytic fungi and termites.
Data Availability
No datasets were generated or analysed during the current study.
References
Aboud AS (2015) Antimicrobial activities of aqueous and ethanolic extracts from Nerium oleander Used in the treatment of burns infections isolates. J Pharm Chem Biol Sci 2:248–258
Ahmed S, Tabassum MH, Hassan B (2022) Evaluation of Antitermite Properties of Wood Extracts from Pongamia pinnata (L.) Pierre (Leguminosae) against Subterranean Termites. An Acad Bras Cienc. https://doi.org/10.1590/0001-3765202220190591
Altameme HJ, Hameed IH, Abu-Serag NA (2015) Analysis of bioactive phytochemical compounds of two medicinal plants, Equisetum arvense and Alchemila valgaris Seed using gas chromatography-mass spectrometry and fourier-transform infrared spectroscopy. Malays Appl Biol 44:47–58
Areola J, Babalola O, Ilesanmi O, Oyedapo O (2015) Toxicity studies of the ethanolic stem-bark extract of Milicia excelsa (Welw.) CC Berg in Wistar rats. Am J Biochem 5:131–137. https://doi.org/10.5923/j.ajb.20150506.01
Atindehou MML, Azihou AF, Dassou HG, Toyi SM, Dangnigbe P, Adomou AC, Ouedraogo A, Assogbadjo AE, Sinsin B (2022) Management and protection of large old tree species in farmlands: Case of Milicia excelsa in southern Benin (West Africa). Trees for People 10:100336. https://doi.org/10.1016/j.tfp.2022.100336
Bahmani M, Schmidt O (2018) Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas-Cienc Tecnol 20:325–332. https://doi.org/10.4067/s0718-221x2018005003301
Bomble YJ, Lin CY, Amore A, Wei H, Holwerda EK, Ciesielski PN, Donohoe BS, Decker SR, Lynd LR, Himmel ME (2017) Lignocellulose deconstruction in the biosphere. Curr Opin Chem Biol 41:61–70. https://doi.org/10.1016/j.cbpa.2017.10.013
Brischke C, von Boch-Galhau N, Bollmus S (2022) Impact of different sterilization techniques and mass loss measurements on the durability of wood against wood-destroying fungi. Eur J Wood Prod 80:35–44. https://doi.org/10.1007/s00107-021-01745-8
Brune A, Dietrich C (2015) The gut microbiota of termites: digesting the diversity in the light of ecology and evolution. Annu Rev Microbiol 69:145–166. https://doi.org/10.1146/annurev-micro-092412-155715
Carbonero-Pacheco J, Aguilar J, Raya MC, Trapero A, Gaju-Ricart M, Agustí-Brisach C (2023) diversity of cellulolytic microorganisms associated with the subterranean termite Reticulitermes grassei. J Fungi 9:294. https://doi.org/10.3390/jof9030294
Cárdenas AM, Gallardo P, Toledo D (2018) Suitability of multiple Mediterranean oak species as a food resource for Reticulitermes grassei Clément (Isoptera: Rhinotermitidae). Bull Entomol Res 108:532–539. https://doi.org/10.1017/s0007485317001043
Cárdenas AM, Gallardo P, Carbonero-Pacheco JR, Trillo M (2020) Response of the subterranean termite Reticulitermes grassei Clément (Isoptera: Rhinotermitidae) to pH of substrate. Pedobiologia 78:150608. https://doi.org/10.1016/j.pedobi.2019.150608
Cardoso VM, Solano AGR, Prado MAF, de Nunan E, A, (2006) Investigation of fatty acid esters to replace isopropyl myristate in the sterility test for ophthalmic ointments. J Pharm Biomed Anal 42:630–634. https://doi.org/10.1016/j.jpba.2006.05.018
Charpentier-Alfaro C, Benavides-Hernández J, Poggerini M, Crisci A, Mele G, Rocca GD, Emiliani G, Frascella A, Torrigiani T, Palanti S (2023) Wood-decaying fungi: from timber degradation to sustainable insulating biomaterials production. Materials 16:3547. https://doi.org/10.3390/ma16093547
Chouvenc T, Bardunias P, Li HF, Elliott ML, Su NY (2011) Planar arenas for use in laboratory bioassay studies of subterranean termites (Rhinotermitidae). Fla Entomol 94:817–826. https://doi.org/10.1653/024.094.0413
Daniel G (2016) Fungal Degradation of Wood Cell Walls. In: Kim YS, Funada R, Singh AP (eds) Secondary Xylem Biology: Origins, Functions, and Applications. Elsevier Inc, https://doi.org/10.1016/b978-0-12-802185-9.00008-5
Daud NM, Putra NR, Jamaludin R, Norodin NSM, Sarkawi NS, Hamzah MHS, Nasir HM, Zaidel DNA, Yunus MAC, Salleh LM (2022) Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci Technol 119:201–214. https://doi.org/10.1016/j.tifs.2021.12.010
Derwich E, Benziane Z, Boukir A (2010) Antibacterial activity and chemical composition of the essential oil from flowers of Nerium oleander. Elec J Env Agricult Food Chem 9:1074–1084
Diniz do Nascimento de Moraes da Costa Galúcio Taube Costa Cruz Andrade Faria LAABKSJMPPSCMLJNEHDALJGD (2020) Bioactive natural compounds and antioxidant activity of essential oils from spice plants: new findings and potential applications. Biomolecules 10:988. https://doi.org/10.3390/biom10070988
Duarte S, Nobre T, Borges PAV, Nunes L (2018) Symbiotic flagellate protists as cryptic drivers of adaptation and invasiveness of the subterranean termite Reticulitermes grassei Clément. Ecol Evol 8:5242–5253. https://doi.org/10.1002/ece3.3819
Erman F, Turkoglu S (2021) Chemical Composition of Essential Oils, Biological and Antioxidant Activities of Ferula orientalis Growing in Erzurum. Fresenius Environ Bull 30:1424–1433
Fidan MS, Yaşar ŞŞ, Yaşar M, Atar M, Alkan E (2016) Characterization of the Combustion Parameters of Impregnated and Varnished Cedar Wood (Cedrus libani). For Prod J 66:290–299. https://doi.org/10.13073/fpj-d-15-00063
Ghio S, Di Lorenzo GS, Lia V, Talia P, Cataldi A, Grasso D, Campos E (2012) Isolation of Paenibacillus sp. and Variovorax sp. strains from decaying woods and characterization of their potential for cellulose deconstruction. Int J Biochem Biophys Mol Biol 3:352–364
Golpayegani AS, Thévenon MF, Gril J, Masson E, Pourtahmasi K (2014) Toxicity potential in the extraneous compounds of white mulberry wood (Morus alba). Maderas-Cienc Tecnol 16:227–238. https://doi.org/10.4067/s0718-221x2014005000018
Guder DG, Krishna MSR (2019) Isolation and Characterization of Potential Cellulose Degrading Bacteria from Sheep Rumen. J Pure Appl Microbiol 13(3):1831–1839. https://doi.org/10.22207/jpam.13.3.60
Houfani AA, Anders N, Spiess AC, Baldrian P, Benallaoua S (2020) Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars-a review. Biomass Bioenergy 134:105481. https://doi.org/10.1016/j.biombioe.2020.105481
Johnsen HR, Krause K (2014) Cellulase Activity Screening Using Pure Carboxymethylcellulose: Application to Soluble Cellulolytic Samples and to Plant Tissue Prints. Int J Mol Sci 15:830–838. https://doi.org/10.3390/ijms15010830
Kasseney BD, Deng T, Mo J (2011) Effect of Wood Hardness and Secondary Compounds on Feeding Preference of Odontotermes formosanus (Isoptera: Termitidae). J Econ Entomol 104:862–867. https://doi.org/10.1603/ec10216
Kgosana KG (2019) The effects of extraction techniques and quantitative determination of oxalates in Nerium oleander and feeds. Onderstepoort J Vet Res 86:1611. https://doi.org/10.4102/ojvr.v86i1.1611
Khademibami L, Bobadilha GS (2022) Recent Developments Studies on Wood Protection Research in Academia: A Review. Front for Glob Change 5:793177. https://doi.org/10.3389/ffgc.2022.793177
Ko GA, Cho SK (2017) Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway. Korean J Physiol Pharmacol 22:53–61. https://doi.org/10.4196/kjpp.2018.22.1.53
Kocaçalışkan I, Talan I, Terzi I (2006) Antimicrobial Activity of Catechol and Pyrogallol as Allelochemicals. Z Naturforsch C 61:639–642. https://doi.org/10.1515/znc-2006-9-1004
König H (2006) Bacillus species in the intestine of termites and other soil invertebrates. J Appl Microbiol 101:620–627. https://doi.org/10.1111/j.1365-2672.2006.02914.x
Koudehi MF, Ardalan AA, Zibaseresht R (2020) Chemical Constituents of an Iranian Grown Capsicum annuum and their Cytotoxic Activities Evaluation. Org Med Chem Int J 9:148–154. https://doi.org/10.19080/omcij.2020.09.555769
Kumari S, Dolma SK, Anmol SU, Reddy SGE (2022) Insecticidal Activity of Extracts, Fractions, and Pure Molecules of Cissampelos pareira Linnaeus against Aphid. Aphis Craccivora Koch Molecules 27:633. https://doi.org/10.3390/molecules27030633
Lasota S, Stephan I, Horn MA, Otto W, Noll M (2019) Copper in Wood Preservatives Delayed Wood Decomposition and Shifted Soil Fungal but Not Bacterial Community Composition. Appl Environ Microbiol 85:e02391-e2418. https://doi.org/10.1128/aem.02391-18
Li W, Zhao L, He X (2022) Degradation potential of different lignocellulosic residues by Trichoderma longibrachiatum and Trichoderma afroharzianum under solid state fermentation. Process Biochem 112:6–17. https://doi.org/10.1016/j.procbio.2021.11.011
Lourençon TV, dos Santos PSB, Labidi J, Gatto DA, Gonçalves MRF (2016) Wood under fresh water: Effect on the chemical properties and on decay resistance. Maderas-Cienc Tecnol 18:733–742. https://doi.org/10.4067/s0718-221x2016005000064
Lubbers RJM, Dilokpimol A, Visser J, Mäkelä MR, Hildén KS, de Vries RP (2019) A comparison between the homocyclic aromatic metabolic pathways from plant-derived compounds by bacteria and fungi. Biotechnol Adv 37:107396. https://doi.org/10.1016/j.biotechadv.2019.05.002
Mageroy MH, Jancsik S, Yuen MMS, Fischer M, Withers SG, Paetz C, Schneider B, Mackay J, Bohlmann J (2017) A Conifer UDP-Sugar Dependent Glycosyltransferase Contributes to Acetophenone Metabolism and Defense against Insects. Plant Physiol 175:641–651. https://doi.org/10.1104/pp.17.00611
Marais BN, Brischke C, Militz H (2022) Wood durability in terrestrial and aquatic environments – A review of biotic and abiotic influence factors. Wood Mater Sci Eng 17:82–105. https://doi.org/10.1080/17480272.2020.1779810
Martínez-Padrón HY, Torres-Castillo JA, Rodríguez-Herrera R, López-Santillán JA, Estrada-Drouaillet B, Osorio-Hernández E (2018) Identification and evaluation of secondary metabolites by gas chromatography-mass spectrometry (GC-MS) in native strains of Trichoderma species. Afr J Biotechnol 17:1162–1171. https://doi.org/10.5897/ajb2018.16546
Morris C (1992) Academic Press Dictionary of Science and Technology. Academic Press, California
Muilu-Mäkelä R, Kilpeläinen P, Kitunen V, Harju A, Venäläinen M, Sarjala T (2021) Indoor storage time affects the quality and quantity of volatile monoterpenes emitted from softwood timber. Holzforschung 75:945–956. https://doi.org/10.1515/hf-2020-0262
Nagawa CB, Böhmdorfer S, Rosenau T (2015) Chemical composition of volatiles extracted from indigenous tree species of Uganda: composition of bark extracts from Psorospermum febrifugum and Milicia excelsa. Holzforschung 69:815–821. https://doi.org/10.1515/hf-2014-0283
Ozdemir T, Temiz A, Aydin I (2015) Effect of Wood Preservatives on Surface Properties of Coated Wood. Adv Mater Sci Eng 2015:631835. https://doi.org/10.1155/2015/631835
Pachauri P, Aranganathan V, More S, Sullia SB, Deshmukh S (2020) Purification and characterization of cellulase from a novel isolate of Trichoderma longibrachiatum. Biofuels 11:85–91. https://doi.org/10.1080/17597269.2017.1345357
Pacheco CM, Cecilia BA, Reyes G, Oviedo C, Fernández-Pérez A, Elso M, Rojas OJ (2021) Nanocomposite additive of SiO2/TiO2/nanocellulose on waterborne coating formulations for mechanical and aesthetic properties stability on wood. Mater Today Commun 29:102990. https://doi.org/10.1016/j.mtcomm.2021.102990
Panchapakesan A, Shankar N (2016) Fungal Cellulases: An Overview. In: Gupta VK (ed) New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Cellulase System Properties and Applications. Elsevier B.V., Amsterdam, https://doi.org/10.1016/b978-0-444-63507-5.00002-2
Pánek M, Reinprecht L, Hulla M (2014) Ten essential oils for beech wood protection - efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 9:5588–5603
Pinto ÂV, de Oliveira JC, Costa de Medeiros CA, Silva SL, Pereira FO (2021) Potentiation of antifungal activity of terbinafine by dihydrojasmone and terpinolene against dermatophytes. Lett Appl Microbiol 72:292–298. https://doi.org/10.1111/lam.13371
Qazi SS, Lombardo DA, Abou-Zaid MM (2018) A Metabolomic and HPLC-MS/MS Analysis of the Foliar Phenolics, Flavonoids and Coumarins of the Fraxinus Species Resistant and Susceptible to Emerald Ash Borer. Molecules 23:2734. https://doi.org/10.3390/molecules23112734
Rosengaus RB, Zecher CN, Schultheis KF, Brucker RM, Bordenstein SR (2011) Disruption of the Termite Gut Microbiota and Its Prolonged Consequences for Fitness. Appl Environ Microbiol 77:4303. https://doi.org/10.1128/aem.01886-10
Sen S, Tascioglu C, Tirak K (2009) Fixation, leachability, and decay resistance of wood treated with some commercial extracts and wood preservative salts. Int Biodeterior Biodegradation 63:135–141. https://doi.org/10.1016/j.ibiod.2008.07.007
Sheng ZG, Li Y, Fan RM et al (2013) Lethal synergism between organic and inorganic wood preservatives via formation of an unusual lipophilic ternary complex. Toxicol Appl Pharmacol 266:335–344. https://doi.org/10.1016/j.taap.2012.11.013
Stefi AL, Mitsigiorgi K, Vassilacopoulou D, Christodoulakis NS (2020) Response of young Nerium oleander plants to long-term non-ionizing radiation. Planta 251:1–17. https://doi.org/10.1007/s00425-020-03405-2
Subekti N, Priyono B, Aisyah AN (2018) Biodiversity of Termites and Damage Building in Semarang. Indonesia. Biosaintifika: J Biol Biol Educ 10(1):176–182. https://doi.org/10.15294/biosaintifika.v10i1.12832
Syafni N, Putra DP, Arbain D (2012) 3,4-Dihydroxybenzoic Acid and 3,4-Dihydroxybenzaldehyde from the Fern Trichomanes chinense L.; Isolation, Antimicrobial and Antioxidant Properties. Indones J Chem 12:273–278
Theodori R, Karioti A, Rančiḱ A, Skaltsa H (2006) Linear sesquiterpene lactones from anthemis auriculata and their antimicrobial activity. J Nat Prod 69:662–664. https://doi.org/10.1021/np058084i
Tomen WT, Diboma BS, Bot BV, Tamba JG (2023) Physical and combustion properties investigation of hybrid briquettes from tropical sawdust: case study of iroko (milicia excelsa) and padouk (pterocarpus soyauxii). Energy Rep 9:3177–3191. https://doi.org/10.1016/j.egyr.2023.02.006
Uzuner S, Cekmecelioglu D (2019) Enzymes in the Beverage Industry. In: Kuddus M (ed) Enzymes in Food Biotechnology: Production, Applications, and Future Prospects. Academic Press, California, https://doi.org/10.1016/b978-0-12-813280-7.00003-7
Xu LT, Lu M, Sun JH (2016) Invasive bark beetle-associated microbes degrade a host defensive monoterpene. Insect Sci 23:183–190. https://doi.org/10.1111/1744-7917.12255
Yildiz ÜC, Kilic C, Gürgen A, Yildiz S (2020) Possibility of using lichen and mistletoe extracts as potential natural wood preservative. Maderas-Cienc Tecnol 22:179–188. https://doi.org/10.4067/s0718-221x2020005000204
Zhou L, Zhao J, Xu L, Huang Y, Ma Z, Wang J, Jiang W (2009) Antimicrobial Compounds Produced by Plant Endophytic Fungi. In: De Costa P, Bezerra P (eds) Fungicides: Chemistry, Environmental Impact and Health Effects, 1st edn. Nova Biomedical, Waltham, pp 91–119
Acknowledgements
Authors would like to thank Universidad de Córdoba for the financial support provided through the predoctoral contract "Contrato de Personal Investigador en Formación (PIF), submodalidad 2.2. del Plan Propio de Investigación 2021", and from its “Plan Propio de Investigación Enrique Aguilar Benítez de Lugo 2023, Submodalidad 2.4 UCOLIDERA” awarded to AgroCell project.
Author information
Authors and Affiliations
Contributions
M. P.-O. performed the design, conceptualization, and investigation of the work; L. S. acted during investigation, reviewing and supervision of the manuscript preparation; J. C.-P. participated in the design and conceptualization of termites experiments; A.A.R. supervised chemical characterization and results discussion; A.G. supervised the work from conceptualization and design to writing and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peña-Ortiz, M., Serrano, L., Carbonero-Pacheco, J. et al. Evaluation of ornamental/exotic plant extracts as natural preservative methodology against termites and fungi. Wood Sci Technol (2024). https://doi.org/10.1007/s00226-024-01593-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00226-024-01593-8