Abstract
The aim of this study was to optimize cultivation conditions for Mn-oxidizing peroxidases and laccase production and selective degradation of beech wood and wheat straw lignin by Trametes gibbosa. To promote lignin degradation, the effect of different carbon and nitrogen sources, type of cultivation, enzyme dynamics and inducers were studied. Solid-state cultivation was optimal for lignin degradation. Wheat straw was the optimal carbon source for peroxidase activities stimulating the synthesis of numerous isoforms. The dynamics of enzymatic activities showed that 19-day-old fermentation is optimal and that activities were directly associated with enzyme production. Alteration of nitrogen sources and inducers was applied to increase the rate of lignin degradation and decrease cellulose degradation. The presence of nitrogen in the form of (NH4)2SO4 and concentration of 10 mM significantly increased the extent and selectivity of delignification of wheat straw compared with cellulose (44.1 vs. 36.1%). The most effective and selective wheat straw degradation (52.0% of lignin vs. 31.3% of cellulose) was achieved by enriching the optimum medium, as defined above, with 1.0 mM p-anisidine. The optimum conditions for wheat straw processing to achieve delignification and production of highly active ligninolytic enzymes by T. gibbosa were (NH4)2SO4/wheat bran medium supplemented with p-anisidine. This study indicated the significant potential of optimizing external factors as a promising tool for induction and selectivity in the degradation of lignocelluloses by T. gibbosa as a pretreatment for several biotechnological processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Generation of high amounts of various plant residues and other wastes, environment disruption and rapid consumption of fossil fuels and natural fibre sources are results of intensive enhancement of agricultural and industrial production. Dominant plant raw materials differ from region to region. Thus, rice is the most frequent crop in Asia, maize in America and wheat in Europe, which leads to accumulation of their residues in enormous quantities (Gupta and Verma 2015). Annual production of agricultural and forestry wastes ranges between 150 and 170 × 109 tons, and as 97% is used neither for food and feed nor in the processing industries, it can be concluded that it becomes a serious environmental ballast (Pauly and Keegstra 2008). Resistance of plant residues to degradation is directly related to the presence of lignin, the most recalcitrant natural aromatic insoluble heteropolymer, which comprises 10–30% of the plant cell wall, with an annual production of approximately 20 billion tons and total amount in terrestrial ecosystems estimated to be 300 billion tons (Higuchi 1990; Kaplan 1998). However, these lignocellulosic wastes are readily available, renewable and cheap and could be excellent resources for the production of food, feed, paper pulp, biofuels and numerous chemicals but only after treatment to fermentable carbohydrates. Despite the rapid lignin removal and generation of products prepared for saccharification by physical and chemical methods, nowadays more attention is given to alternative pretreatments based on white-rot fungi or their ligninolytic enzymes. These organisms are the most effective lignocellulose degraders, because those processes in some species and strains can be considerably selective, resulting in higher cellulose utilization, being environmentally friendly and economically justified (Wan and Li 2012).
Numerous studies have shown that the synthesis and activity of lignocellulolytic enzymes depend primarily on the fungal species and strain, but also on cultivation type (submerged and solid state, which differ in water availability, temperature and aeration level), physicochemical properties of the substrate (composition and pH), amount and age of inoculum, and the presence of additional nutrient sources and inducers (Pandey 2003). Wan and Li (2012) emphasized that some white-rot basidiomycetes degrade lignin non-selectively and thus simultaneously depolymerize both cellulose and hemicellulose, which become available by delignification, and holocellulose loss could range between 17 and 50%, resulting in decreased yield of saccharification products. In contrast, species that are selective lignin degraders slightly hydrolyse holocellulose, though the ratio of degraded lignin to holocellulose varies among species and even among strains of the same species. This way the achievement of high lignin degradation and low holocellulose loss is a key prerequisite in pretreatment of lignocellulosics with white-rot fungi and its further use in various biotechnological processes.
Trametes gibbosa (Pers.) Fr. is a white-rot basidiomycete broadly distributed on logs of deciduous species, especially beech, in Europe, Asia and North America (Phillips 1981). It could play a significant role in various biotechnological processes due to its high potential to produce active lignin peroxidase, Mn-oxidizing peroxidases and laccase, extracellular enzymes essential for delignification as well as degradation of lignin-like phenolic and non-phenolic compounds (Niladevi 2009).
The goal of this research was to optimize cultivation conditions for producing active Mn-oxidizing peroxidases and laccase by T. gibbosa and to achieve a higher efficiency and selectivity of lignocellulose degradation.
Materials and methods
Reagents
All chemicals were of analytical grade and were purchased either from Sigma-Aldrich (St. Louis, MO, USA) or from Merck Millipore (Darmstadt, Germany), unless otherwise stated.
Organism and cultivation conditions
The basidiocarps were collected on Suva Mt., Serbia, and identified as Trametes gibbosa according to the macroscopic features and the micromorphology of the reproductive structures (Phillips 1981; Courtecuisse 1999). Small fragment of fresh fruiting body was extracted on Malt agar medium (MA) for isolation of pure culture of T. gibbosa BEOFB 310, which is then maintained in the Culture Collection of the Institute of Botany, Faculty of Biology, University of Belgrade (BEOFB).
The inoculum was prepared by inoculation of 100.0 mL of synthetic medium (glucose—10.0 g/L; NH4NO3—2.0 g/L; K2HPO4—1.0 g/L; NaH2PO4 × H2O—0.4 g/L; MgSO4 × 7H2O—0.5 g/L; yeast extract (≥10% of nitrogen)—2.0 g/L; pH 6.5) with 25 mycelial agar plugs (Ø 0.5 cm, of 7-day-old culture on MA), incubation at room temperature (22 ± 2 °C) on a rotary shaker (100 rpm) for 7 days, washing of the resultant biomass with sterile distilled water three times, and homogenization with 100.0 mL water in a laboratory blender. This suspension was used for medium inoculation (3.0 mL per flask in solid state and 5.0 mL in submerged fermentation of selected lignocellulosic wastes) (Ćilerdžić et al. 2011).
T. gibbosa was cultivated under solid state and submerged conditions in the medium with beech (Fagus moesiaca (K. Malỳ) Czeczott) sawdust (particle size of 2.5–5.0 mm) or wheat straw (pieces of 2.0–10.0 mm) as carbon sources to determine the optimum cultivation type and carbon source. Solid-state cultivation was carried out in 100-mL flasks with a substrate composed of 2.0 g of selected plant residue and 10.0 mL of the modified synthetic medium (without glucose and with optimum nitrogen source and concentration, 10 mM of nitrogen in the form of (NH4)2SO4) at 25 °C for 7 days (Knežević et al. 2014). Submerged cultivation was performed in 250-mL flasks with 5.0 g of plant waste and 50.0 mL of the modified synthetic medium, on a rotary shaker at room temperature for 7 days.
The dynamics of Mn-oxidizing peroxidases [Mn-dependent peroxidase (EC 1.11.1.13; MnP) and Mn-independent peroxidase (EC 1.11.1.16; MnIP)] and laccase (EC 1.10.3.2) activities were determined during 23 days of T. gibbosa BEOFB 310 cultivation under previously defined optimum conditions.
The optimum nitrogen source and concentration was determined by enriching the modified synthetic medium containing the selected plant residue as carbon source, but without added nitrogen, with NH4NO3, (NH4)2SO4 or peptone, at concentrations of 10, 15, 20, 25, 30 and 40 mM for inorganic nitrogen sources and 0.25, 0.5, 1.0, 2.0, 3.0 and 4.0% for organic nitrogen. The optimum carbon source was supplemented with wheat bran in the ratios of 1:9, 2:8 and 3:7 (w/w) to test whether an additional nitrogen source would stimulate the production and activity of T. gibbosa ligninolytic enzymes, under previously defined optimum cultivation conditions.
The potential of phenylmethylsulfonyl fluoride, vanillic acid, guaiacol and p-anisidine to induce enzyme activities was tested by their addition to the optimum medium at a final concentration of 1.0 mM, and the effect of veratryl alcohol by medium enrichment to the final concentration of 0.5% (w/v), 72 h after the beginning of T. gibbosa cultivation.
The medium without nitrogen source and inducer was used as the control.
Enzyme activity and total protein production assays
Extraction of synthesized ligninolytic enzymes after solid-state fermentation of selected plant residues was performed by sample stirring with 50.0 mL of sterile distilled water in a magnetic stirrer at 4 °C for 10 min. The extracts as well as flask contents after submerged cultivation were centrifuged (4 °C, 3000 rpm, 15 min), and the supernatants were used for determining Mn-oxidizing peroxidases and laccase activities as well as total protein contents spectrophotometrically (CECIL CE2501 (BioQuest)).
Mn-oxidizing peroxidase activities were determined with 3.0 mM phenol red (ε 610 = 22,000 M−1 cm−1) as a substrate, in a succinate buffer pH 4.5 (disodium salt of succinate acid, albumin from bovine serum (BSA) and DL-lactic acid sodium salt) as described by Stajić et al. (2010).
The activity of laccase was determined by monitoring the A436 change related to the rate of oxidation of 50 mM 2,2′-azino-bis-[3-ethylbenzothiazoline-6-sulphonate] (ABTS) (ε 436 = 29,300 M−1 cm−1) in 0.1 M phosphate buffer (pH 6.0) at 35 °C as described by Stajić et al. (2010).
The enzymatic activity of 1 U was defined as the amount of enzyme that transformed 1 μmol of substrate per minute.
Total protein quantities in extracts were determined according to the method of Bradford (1976). Total protein content is presented as mg/mL and was used for determination of specific enzymatic activities which were defined as enzyme activity per mg of total proteins (U/mg).
Electrophoresis
The profiles of Mn-oxidizing peroxidases and laccase of T. gibbosa were screened for the optimum cultivation conditions. Enzyme isoforms and their isoelectric points (pI) were determined by isoelectric focusing (IEF) using a Mini IEF Cell-Model 111 (BIO-RAD) as described by Knežević et al. (2016).
Determination of hemicellulose, cellulose and lignin contents in selected plant wastes
Determination of hemicellulose content
The content of hemicellulose was determined by a modified Van Soest method (Goering and Van Soest 1970; Van Soest et al. 1991). A mixture of dried and ground sample (1.0 g) and 100 mL of a solution of neutral detergent (NDS) (EDTA—18.6 g/L; SDS—30.0 g/L; 2-ethoxyethanol—10.0 mL; NaH2PO4 × H2O—4.56 g/L; Na2B4O7 × 10H2O—6.81 g/L; pH 6.9–7.1), 0.5 g Na2SO3 and a few drops of 1-octanol was heated until boiling and then refluxed for an hour to remove soluble sugars, proteins, pectin, lipids and vitamins from the sample. The sample residues of neutral detergent fibres (NDF) were filtered, washed three times with boiled water and twice with cold acetone, dried at 105 °C for 8 h and weighed. The samples were further treated with acidic detergent solution (ADS) (CTAB—20.0 g dissolved in 1000.0 mL of 0.5 M H2SO4; pH 6.9–7.1), heated to boiling, refluxed for an hour, filtered and washed with boiled water and a few times with acetone. After that, samples were dried at 105 °C overnight and weighed. The weight of acidic detergent fibres (ADF) was determined gravimetrically as the residue remaining after extraction. Hemicellulose content was calculated as NDF-ADF.
Determination of cellulose and lignin contents
Acidic detergent fibres (ADFs) were used for determination of cellulose and lignin contents. Lignin content was defined by Klason or 72% H2SO4 method (Kirk and Obst 1988). In total, 1.0 mL of 72% H2SO4 was added for each 100.0 mg of sample. The mixture was incubated at 30 ± 0.5 °C in a water bath for an hour, stirred frequently and after that diluted using 28.0 mL of water for each 1.0 mL of acid. Secondary hydrolysis was performed in an autoclave at 120 °C for an hour. The hot solution was filtered through a tared Gooch crucible, and the Klason lignin residues were washed with hot water. The sample was dried at 105 °C to a constant weight, and lignin content (LC) was expressed as the percentage by weight of that present in the original sample. The cellulose content was calculated as ADF–LC.
Statistical analysis
The assays were carried out in five replicate aliquots of inoculum that were used for each variant of medium composition, and the results are expressed as the mean ± standard error. One-way analysis of variance (ANOVA) followed by Tukey’s HSD post hoc test were performed to test the significance of differences among treatments. P values less than 0.01 were considered statistically significant. All statistical analysis was done using STATISTICA software, version 6.0 (StatSoft, Inc., Tulsa, USA).
Results
Effect of cultivation type and plant residue
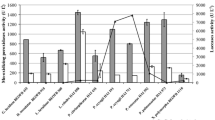
Activities of Mn-oxidizing peroxidases and laccase were detected after 7 days of submerged and solid-state fermentation of beech sawdust and wheat straw with T. gibbosa. The conditions of solid-state cultivation were optimum for the production and activity of the analysed ligninolytic enzymes. However, after submerged fermentation of wheat straw, only insignificant MnP activity (61.9 ± 3.3 U/L) was detected (Fig. 1A). Under the optimum conditions of solid-state cultivation, wheat straw was a better carbon source for MnP and MnIP activity (3303.1 ± 216.1 and 623.1 ± 46.4 U/L, respectively) and beech sawdust for laccase activity (354.1 ± 23.7 U/L).
During solid-state fermentation of both plant wastes, T. gibbosa synthesized two MnP isoforms pI (4.4 and 4.6) and one MnIP with pI of 4.2. This species did not produce Mn-oxidizing peroxidases during submerged cultivation in the medium with beech sawdust as carbon source, while during submerged fermentation of wheat straw only one MnP isoform (pI 4.1) was noted (Fig. 1Ba, Bb). In the case of laccases, three isoforms (pI 4.4, 4.5 and 4.6) were detected by isoelectric focusing of the extract obtained after 7 days of solid-state fermentation of wheat straw, two (pI 4.5 and 4.6) after solid-state cultivation on beech sawdust, while no laccase was found under conditions of submerged fermentation of both plant wastes (Fig. 1Bc).
Changes in hemicellulose, cellulose and lignin contents occurred after 7 days of solid-state and submerged fermentation of both plant residues with T. gibbosa (Table 1). The extents of lignin degradation were in accordance with activities of the ligninolytic enzymes, i.e. degradation rates were significantly higher after solid-state fermentation of the plant wastes. The highest levels of delignification (10.9 ± 0.2%) as well as degradation of hemicellulose and cellulose (10.5 ± 0.3 and 7.9 ± 0.2%, respectively) occurred in wheat straw after solid-state fermentation (Table 1).
Effect of cultivation period
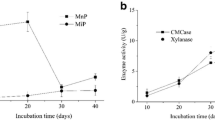
The dynamics of MnP, MnIP and laccase activities were monitored during 23 days of solid-state fermentation of wheat straw with T. gibbosa. Ligninolytic enzyme activities changed through several stages during that time (Fig. 2). In the first phase, from day 4 to day 7 of wheat straw fermentation, MnP activity increased considerably and reached a maximum value of 2332.1 ± 128.0 U/L. The activity declined rapidly till day 9 when it reached 745.0 ± 95.4 U/L. During the same period, a sharp decline of laccase activity occurred, reaching a minimum of 854.5 ± 82.0 U/L on day 8 of fermentation. In this phase, MnIP activity was moderate and stable, in the range from 405.3 to 458.3 U/L. The second or stationary phase, from day 10 to day 15 of wheat straw fermentation, was characterized by similar activities of all studied enzymes. During the third phase that began on day 16 of fermentation, activities of all enzymes increased, reaching maxima for MnIP and laccase on day 19 (934.4 ± 79.2 and 2245.0 ± 100.3 U/L, respectively). The fourth phase was characterized by a significant decrease of MnIP and laccase activities and continual growth of MnP activity that reached a second maximum (1649.0 ± 201.5 U/L) on day 22 of fermentation (Fig. 2).
The dynamics of protein production confirmed that enzyme activities were directly associated with enzyme production during wheat straw fermentation with T. gibbosa.
Effect of nitrogen source and concentration
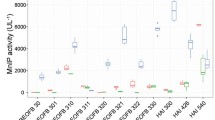
The effects of two inorganic (NH4NO3, (NH4)2SO4) and one organic (peptone) nitrogen source as well as their concentrations on the activities of Mn-oxidizing peroxidases and laccase were determined after 7 and 19 days of wheat straw solid-state fermentation by T. gibbosa, as those were the optimum conditions for enzymatic activities (Fig. 2). In the presence of all nitrogen sources and concentrations tested after both 7 and 19 days, laccase activities were significantly lower than Mn-oxidizing peroxidase activities, and MnIP activities were always lower than MnP activities (Fig. 3).
A slight increase of Mn-oxidizing peroxidase activities with increase of nitrogen concentration was noted after 7-day cultivation in the NH4NO3-enriched medium (Fig. 3Aa). A similar effect of this nitrogen source was observed after 19 days of wheat straw fermentation, i.e. MnP and MnIP activities increased slightly until a nitrogen concentration of 25 mM after which they decreased. NH4NO3 had no significant effect on laccase activity, except on day 19 of cultivation in the medium enriched with 10 mM of nitrogen when the activity was 204.8 ± 10.5 U/L (Fig. 3Ba).
However, after 7 days of T. gibbosa cultivation, (NH4)2SO4 caused a significant increase of MnP and MnIP activities with maxima at a nitrogen concentration of 15 mM (4479.3 ± 149.2 and 1430.9 ± 95.6 U/L, respectively). With further concentration enhancement, those activities began to decline (Fig. 3Ab). The effect of (NH4)2SO4 was the same on day 19 of cultivation, i.e. activities of MnP and MnIP increased to a nitrogen concentration of 10 mM when maxima reached 4933.5 ± 159.1 and 1638.7 ± 129.7 U/L, respectively. Compared with the two other nitrogen sources, (NH4)2SO4 was the most effective as MnP and MnIP activities were above 4000.0 and 1000.0 U/L, respectively, at all tested concentrations (Fig. 3Ab, Bb). This nitrogen source had no stimulatory effect on laccase activity.
After 7 days of cultivation in the medium enriched with peptone, MnP, MnIP and laccase activities increased as peptone concentration increased until a concentration of 1.0%, giving maxima of 3964.5 ± 123.9, 1421.3 ± 112.2 and 605.2 ± 28.4 U/L, respectively. Thereafter, sharp decreases of the activities of MnP and MnIP occurred, giving minima of 59.9 ± 4.7 and 19.7 ± 5.7 U/L, respectively, at 4.0% peptone, while laccase activity was not detected (Fig. 3Ac). Similarly, after 19 days of cultivation, 0.25% peptone caused an increase in MnP activity which declined steeply with further increases of peptone concentrations reaching a minimum at 4.0% of 28.8 ± 3.5 U/L (Fig. 3Bc). MnIP activity also was not affected significantly by the organic nitrogen source, except at a concentration of 4.0%, which caused a significant decrease of activity to 24.2 ± 2.8 U/L. Laccase activity increased until a peptone concentration of 3.0% (922.3 ± 34.3 U/L) (Fig. 3Bc).
In the presence of inorganic nitrogen sources, MnP, MnIP and laccase activities depended on the portion of active molecules in the total enzyme molecules present, while in the presence of the organic nitrogen source those activities depended on the rates of enzyme production.
Profiles of Mn-oxidizing peroxidases and laccases of T. gibbosa were determined by isoelectric focusing of extracts obtained after 19 days of solid-state fermentation of wheat straw in the presence of NH4NO3, (NH4)2SO4 and peptone at their nitrogen concentrations optimal for enzymes activity (25 mM, 10 mM and 0.25%, respectively) (Fig. 4). Three MnP isoforms (pI 4.2, 4.3 and 4.4) were synthesized in the medium enriched with (NH4)2SO4 and two (pI 4.2 and 4.3) in the presence of the other two nitrogen sources (Fig. 4a), while one MnIP isoenzyme with pI 4.2 was expressed with all nitrogen sources (Fig. 4b). However, profiles of laccases significantly varied depending on the nitrogen source (Fig. 4c). Namely, two isoforms were detected in the presence of NH4NO3 (pI 4.6 and 4.7) and peptone (4.7 and 4.8), and three (pI 4.5, 4.6 and 4.7) in (NH4)2SO4-enriched medium.
Based on these isoenzyme profiles, two conclusions could be drawn. First, the highest level of MnP activity occurring in the presence of 10 mM nitrogen in the form of (NH4)2SO4 was caused by both the maximum level of enzyme production and the number of synthesized isoforms. Second, the larger number of laccase isoforms was not associated with the level of enzyme activity, namely in the presence of (NH4)2SO4 more isoforms but lower enzyme activity were found than in the presence of peptone.
In the presence of inorganic nitrogen source at concentrations optimal for the highest enzymes activity, levels of lignocellulose degradation were significant after 19 days of wheat straw solid-state fermentation with T. gibbosa and corresponding with activities of the enzymes (Table 2). The highest lignin degradation (44.1 ± 1.9%) was obtained in the presence of (NH4)2SO4 and the lowest in the presence of NH4NO3 (32.6 ± 0.9%).
The effect of the additional nitrogen source on activities of Mn-oxidizing peroxidases and laccase in T. gibbosa was determined by adding 10, 20 and 30% of wheat bran to the medium with 10 mM of nitrogen in the form of (NH4)2SO4. Wheat bran used in this study contained nitrogen (435.4 ppm), proteins (12%), hemicellulose (30%), cellulose (9%), lignin (8%), copper (11.44 ppm), iron (81.5 ppm), manganese (111.52 ppm), zinc (72.92 ppm) and calcium (537.6 ppm). Medium enrichment with wheat bran significantly stimulated MnP activity in comparison with the control (961.2 ± 95.7 U/L), especially when supplemented at 10% (2945.2 ± 245.1 U/L) (Fig. 5A). In the case of laccase, a high activity (2457.3 ± 199.7 U/L) was found after 19-day wheat bran fermentation with T. gibbosa, small during cultivation with a mixture of straw and bran at a ratio of 7:3 (w/w), while no activity was detected during fermentation of straw alone as well as its mixture with 10 and 20% bran (Fig. 5A).
Effect of wheat bran as an additional nitrogen source on (A) activities and (B) IEF profiles of a Mn-dependent peroxidase, b Mn-independent peroxidase and c laccase in Trametes gibbosa BEOFB 310 after 19 days of solid-state fermentation. (cont—wheat straw + (NH4)2SO4; WB—wheat bran; M—mixture of wheat straw and wheat bran in a 9:1 ratio)
Two MnP isoenzymes of pI 4.2 and 4.3 and one MnIP isoform of pI 4.2 were detected by isoelectric focusing of extracts obtained after 19 days of solid-state fermentation of wheat straw, wheat bran and a mixture of straw and bran in the ratio 9:1 (defined as the optimum for enzyme activity) (Fig. 5Ba, Bb). In the case of laccase, differences in isoform profiles of the extracts were more significant. Namely, three laccase isoenzymes of pI 4.4, 4.5 and 4.6 were synthesized during the fermentation of straw and the mixture of straw and bran, while five isoforms (pI in the range from 4.4 to 4.8) were produced during bran fermentation (Fig. 5Bc).
Thus, Mn-oxidizing peroxidase activities directly depend on the rate of enzyme production and not on the number of synthesized isoforms. Although no laccase activity was detected spectrophotometrically, the ability of T. gibbosa to synthetize five laccase isoforms during wheat bran fermentation could be explained by the production of the isoenzymes in trace amounts.
The addition of 10% of wheat bran to wheat straw led to increase in the selectivity of lignocellulose depolymerization (Table 2). Namely, lignin and hemicellulose degradation increased significantly (48.3 ± 0.8 and 54.9 ± 1.1%, respectively), while the extent of cellulose hydrolysis decreased (27.1 ± 1.3%).
Effect of inducers
The addition of selected inducers to wheat straw/(NH4)2SO4/wheat bran medium had diverse effects on the activities of Mn-oxidizing peroxidases and laccase (Fig. 6A). Only p-anisidine had a significant stimulatory effect on MnP activity whose maximum activity in its presence was 20% higher than in the control (4083.5 ± 234.8 and 3395.3 ± 165.5 U/L, respectively). The other inducers either slightly increased or even decreased the activity of the enzyme. Veratryl alcohol was the only inducer which had a stimulatory effect on MnIP and its addition to the medium increased MnIP activity by 82% in comparison with the control. Veratryl alcohol was also a good inducer of laccase activity, which was 93-fold higher in its presence than in the control (2400.5 ± 160.9 and 25.6 ± 4.9 U/L, respectively). Significant increases of laccase activity of 4.8- and 4.2-fold in comparison with the control were found in samples with vanillic and gallic acids, respectively (Fig. 6A).
Effect of the selected inducers on (A) activities and (B) IEF profiles of a Mn-dependent peroxidase, b Mn-independent peroxidase and c laccase in Trametes gibbosa BEOFB 310 after 19 days of solid-state cultivation in a wheat straw/(NH4)2SO4/wheat bran medium. (cont control, p-a p-anisidine, va veratryl alcohol)
p-Anisidine did not affect the profiles of ligninolytic enzymes of T. gibbosa, which were the same as in the control, wheat straw/(NH4)2SO4/wheat bran extract (Fig. 6B). However, the effect of veratryl alcohol was significant. Namely, in the presence of this inducer, T. gibbosa synthesized one MnP (pI 4.6), three MnIP (pI 3.8, 4.0 and 4.3) and three laccase isoforms (one of pI 4.0–4.5, one of pI 5.6 and one of pI 6.4) (Fig. 6B).
Discussion
These results showed that cultivation conditions are essential factors responsible for the activity of ligninolytic enzymes and effectiveness in delignification. Solid-state cultivation was found to be better for the activities of Mn-oxidizing peroxidases and laccase of T. gibbosa BEOFB 310 and many other fungi (Viniegra-González et al. 2003; Barrios-González 2012). This phenomenon was explained by high similarities of solid-state conditions to those in natural habitats, which are considered to have better availability of oxygen required for fungal metabolic processes, biomass production and a lower level of proteolysis. The findings confirmed results of Acuña-Aegüelles et al. (1995) that during submerged cultivation some enzymes were not produced and those which were synthesized differed significantly in pI values from forms obtained in solid-state cultivation.
Previous studies have shown that wheat straw and beech sawdust are good substrates for the growth of Trametes species and the production of ligninolytic enzymes (Schlosser et al. 1997; Knežević et al. 2013a). These authors also noted higher laccase activities in comparison with Mn-oxidizing peroxidases after solid-state cultivation on beech sawdust. Variations in the profiles of enzymatic activities in T. gibbosa could be caused by differences between the contents of lignin, nitrogen and copper in wheat straw (9.7%, 0.53% and 5.9 ppm, respectively) and beech sawdust (23.0%, 0.71% and 11.74 ppm, respectively). Higher concentrations of copper, water-soluble phenols and compounds of small molecular weight in beech than in wheat are a possible reason for the more active laccase in this substrate (Schlosser et al. 1997). However, the lower level of degradation of beech lignin indicates that the contribution of laccase to delignification is negligible compared with Mn-oxidizing peroxidases. Significant contribution of laccase to delignification was noted only during simultaneous production with peroxidases synthesis (Knežević et al. 2013a).
The level of degraded lignin after 7 days of plant residue fermentation was expectedly low and in accordance with results of Robertson et al. (2008) who noted a more significant rate of delignification during later phases of lignocellulose fermentation despite the high levels of ligninolytic enzyme activities during the earlier phases of cultivation. Namely, synthesis of enzymes began during the phase of substrate colonization when reactive oxygen species are initially attacking cell walls, while the intensive and enzyme-catalysed delignification occurred much later (Robertson et al. 2008).
Significant variations of the activities and contribution of individual enzymes in the enzymatic pool of T. gibbosa BEOFB 310 were detected during solid-state fermentation of wheat straw. Schlosser et al. (1997) noted intensive laccase synthesis during the initial phase of beech wood colonization with T. versicolor when production of peroxidases was either absent or negligible. Two-phase oscillations of laccase activity and two maxima, one in the earlier and the other in the later phase of cultivation of T. versicolor, were also reported by Collins and Dobson (1997). The first maximum could refer to intensive degradation of small molecular weight compounds, which could also be significant carbon sources, inducers of laccase genes transcription, but also hazardous for white-rot fungal species (Piscitelli et al. 2011; Couto et al. 2002). Thus, not only do laccases have a significant role in the supply of fungi with carbon and energy but also in detoxification. The second maximum occurred at an advanced stage of delignification when degradation products induced laccase synthesis according to the principles of feedback (Smith et al. 1998).
Enhanced syntheses of peroxidases are known to occur after the consumption of low molecular weight compounds, when poorly accessible forms of carbon, such as lignin, are available to basidiomycetes (Bonnarme et al. 1991). This can be an explanation for a high level of MnP activity in the initial phase of plant waste fermentation with T. gibbosa BEOFB 310. Decreases of T. gibbosa BEOFB 310 MnP and MnIP activities on days 19 and 22 of cultivation are likely to have been the result of the stationary phase of growth and advanced delignification. Namely, Knežević et al. (2013a, b) showed that species of the genus Trametes have high capacities to degrade wheat straw lignin which indicated a fast consumption of the carbon source, accompanied by a significant decrease of peroxidase activities.
The nitrogen source, its concentration and availability are also important factors that affect the activity of ligninolytic enzymes in species of the genus Trametes and other white-rot basidiomycetes and consequently the level of lignocellulose degradation (Mikiashvili et al. 2005; Stajić et al. 2010). Janusz et al. (2013) reported that nitrogen primarily acts on the level of gene expression, especially mnp genes. However, whether nitrogen stimulates or inhibits the synthesis and activity of these enzymes depends on the fungal species (Levin et al. 2010). Previous studies showed that the intensity of the effect of nitrogen source on Mn-oxidizing peroxidases activities depended on cultivation type, fungal species and its capacity to produce active enzymes. Namely, Viniegra-González et al. (2003) reported that significant activities of ligninolytic enzymes should not be expected during submerged cultivation, in contrast to solid-state cultivation. On the other hand, good producers of Mn-oxidizing peroxidases quickly achieve their maximum production, which leads to saturation of the enzyme, which means that the active sites of enzyme molecules are occupied and increases of substrate concentration do not lead to enhanced rates of enzymatic reactions (Segal 1992). This was demonstrated in T. gibbosa BEOFB 310 where increase of MnP activity in the presence of 10 mM nitrogen in the form of (NH4)2SO4 was only 44% in comparison with the control. This contrasts with two T. versicolor strains where Mikiashvili et al. (2005) noted multiple increases of the activity during submerged fermentation of synthetic medium enriched with NH4NO3, (NH4)2SO4 or peptone.
In the case of laccase, the results for T. gibbosa were in accordance with those of Mikiashvili et al. (2005), who showed that inorganic nitrogen sources do not induce laccase synthesis while peptone as an organic nitrogen source significantly increased the activity of this enzyme.
Johansson and Nyman (1996) showed that Mn-oxidizing peroxidases and laccases in species of the genus Trametes are encoded by structurally different gene families, which may serve to explain the ability of T. gibbosa BEOFB 310 to synthesize a few isoforms of enzymes (pI ranging between 4.2 and 4.4) in comparison with those produced by T. versicolor whose pIs were in the range from 2.93 to 3.17.
The stimulating effects of wheat bran, as an additional nitrogen source, on fungal growth and activity of ligninolytic enzymes have previously been demonstrated (Locci et al. 2008; Souza et al. 2010; Okamoto et al. 2011; Bakkiyaraj et al. 2013). Namely, besides its significant content of carbon in the forms of hemicellulose (30–52%), cellulose (10–15%), lignin (4–8%), starch (10–20%) and other compounds, wheat bran is also rich in nitrogen, primarily in the form of proteins (15–22%) (Johansson and Nyman 1996; Merali et al. 2015). Therefore, Okamoto et al. (2011) assumed that wheat bran, a good source of amino acids and easily accessible carbon (starch), causes the increase of fungal biomass production but not induction of gene expression which is characteristic of inorganic nitrogen sources. However, as T. gibbosa BEOFB 310 synthesized more laccase isoforms during wheat bran fermentation than during cultivation on wheat straw/(NH4)2SO4 medium, it is likely that wheat bran also affected gene expression.
The results for lignin degradation in the presence of wheat bran indicate that delignification was proportional to the level of enzyme activities and thus imply a significant effect of wheat bran on this process. The presence of wheat bran also stimulated selective delignification of lignocellulosic polymers by T. gibbosa, i.e. a high extent of lignin depolymerization and low cellulose hydrolysis, which is in accordance with results obtained for Pleurotus ostreatus by Locci et al. (2008).
Numerous small molecular weight compounds can also induce the synthesis of active ligninolytic enzymes in white-rot fungi (Collins and Dobson 1997; Couto et al. 2002; Dhakar and Pandey 2013). For compounds such as 2,5-xylidine, guaiacol, catechol, veratryl alcohol, p-anisidine, syringaldazine, vanillic-, tanic- and gallic acids have also been shown to be strong inducers of laccase activity (Collins and Dobson 1997; Couto et al. 2002). However, considering that numerous aromatic compounds in concentrations <2 mM can completely inhibit the growth of T. versicolor, detoxification could be one more important function of laccase as this enzyme catalyses the degradation of these compounds (Collins and Dobson 1997).
According to published results, veratryl alcohol affects the activity of white-rot fungi ligninolytic enzymes in three ways: (i) inducing their production (Hakala et al. 2006); (ii) preventing their inactivation and proteolysis (Cancel et al. 1993); and (iii) serving as a mediator in the process of biodegradation (Esser and Kück 2004). The appearance of a broad laccase band in T. gibbosa BEOFB 310 (pI ranging from 4.0 to 4.5) after treatment with veratryl alcohol was probably the result of point changes in amino acid sequence or small post-translational changes of laccase molecules, which would be expected as this enzyme is encoded by a family of closely located genes originating from gene duplications (Janusz et al. 2013). Previous studies also confirmed that veratryl alcohol has the role of a mediator in the process of lignin degradation by white-rot basidiomycetes, including T. versicolor. This species produces veratryl alcohol during delignification as a secondary metabolite, which has a role of redox mediator in the form of a cation radical (Esser and Kück 2004).
Conclusion
This study indicated the significant potential of optimization of external factors as a promising tool for the induction and selectivity in lignocellulose degradation by Trametes gibbosa. The optimal conditions for wheat straw fermentation are defined as solid-state fermentation with 10 mM of (NH4)2SO4 and 10% of wheat bran as additional nitrogen source. p-Anisidine is recognized as a potent inducer of MnP activity. T. gibbosa BEOFB 310 can be pointed out as a promising producer of ligninolytic enzymes with perspectives in pretreatment of wheat straw for utilization in different biotechnological processes. These results can also be the basis for further in situ research of soil bioremediation.
References
Acuña-Argüelles ME, Gutiérrez-Rojas M, Viniegra-González G, Favela-Torres E (1995) Production and properties of three pectinolytic activities produced by Aspergillus niger in submerged and solid-state fermentation. Appl Microbiol Biotechnol 43:808–814
Bakkiyaraj S, Aravindan R, Arrivukkarasan S, Viruthagiri T (2013) Enhanced laccase production by Trametes hirusta using wheat bran under submerged fermentation. Int J Chem Tech Res 5:1224–1238
Barrios-González J (2012) Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem 47:175–185
Bonnarme P, Perez J, Jeffries TW (1991) Regulation of ligninase production in white-rot fungi. In: Leathanl GF, Himmel ME (eds) Enzymes in biomass conversion: Proceedings of a symposium 1990. Boston, pp 200–206
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cancel MA, Orth AB, Tien M (1993) Lignin and veratryl alcohol are not inducers of the ligninolytic system of Phanerochaete chrysosporium. Appl Environ Microbiol 59:2909–2913
Ćilerdžić J, Stajić M, Vukojević J, Duletić-Laušević S, Knežević A (2011) Potential of Trametes hirsuta to produce ligninolytic enzymes during degradation of agricultural residues. BioResources 6:2885–2895
Collins PJ, Dobson A (1997) Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol 63:3444–3450
Courtecuisse R (1999) Mushrooms of Britain & Europe. HarperCollins Publishers, London
Couto SR, Gundín M, Lorenzo M, Sanromán MA (2002) Screening of supports and inducers for laccase production by Trametes versicolor in semi-solid-state conditions. Process Biochem 38:249–255
Dhakar K, Pandey A (2013) Laccase production from a temperature and pH tolerant fungal strain of Trametes hirsuta (MTCC 11397). Enzyme Res. doi:10.1155/2013/869062
Esser K, Kück U (2004) The Mycota: a comprehensive treatise on fungi as experimental systems for basic and applied research. Springer, Berlin
Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus, reagents, procedures, and some applications), Agriculture Handbook No. 379, Washington
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev 41:550–567
Hakala TK, Hilden K, Maijala P, Olsson C, Hatakka A (2006) Differential regulation of manganese peroxidases and characterization of two variable MnP encoding genes in the white-rot fungus Physisporinus rivulosus. Appl Microbiol Biotechnol 73:839–849
Higuchi I (1990) Lignin biochemistry: biosynthesis and biodegradation. Wood Sci Technol 24:23–63
Janusz G, Kucharzyk KH, Pawlik A, Staszczak M, Paszczynski AJ (2013) Fungal laccase, manganese peroxidase and lignin peroxidase: gene expression and regulation. Enzyme Microb Tech 52:1–12
Johansson T, Nyman PO (1996) A cluster of genes encoding major isozymes of lignin peroxidase and manganese peroxidase from the white-rot fungus Trametes versicolor. Gene 170:31–38
Kaplan DL (1998) Biopolymers from renewable resources. Springer, Berlin
Kirk TK, Obst JR (1988) Lignin determination. In: Colowick SP, Kaplan NO (eds) Methods in Enzymology 161. Academic Press Inc., San Diego, pp 87–101
Knežević A, Milovanović I, Stajić M, Vukojević J (2013a) Potential of Trametes species to degrade lignin. Int Biodeter Biodegr 85:52–56
Knežević A, Milovanović I, Stajić M, Lončar N, Brčeski I, Vukojević J, Ćilerdžić J (2013b) Lignin degradation by selected fungal species. Bioresour Technol 138:117–123
Knežević A, Stajić M, Vukojević J, Milovanović I (2014) The effect of trace elements on wheat straw degradation by Trametes gibbosa. Int Biodeter Biodegrad 96:152–156
Knežević A, Stajić M, Jovanović VM, Kovačević V, Ćilerdžić J, Milovanović I, Vukojević J (2016) Induction of wheat straw delignification by Trametes species. Sci Rep 6:26529. doi:10.1038/srep26529
Levin L, Melignani E, Ramos AM (2010) Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour Technol 101:4554–4563
Locci E, Laconi S, Pompei R, Scano P, Lai A, Marincola FC (2008) Wheat bran biodegradation by Pleurotus ostreatus: a solid-state Carbon-13 NMR study. Bioresour Technol 99:4279–4284
Merali Z, Collins SRA, Elliston A, Wilson DR, Käsper A, Waldron KW (2015) Characterization of cell wall components of wheat bran following hydrothermal pretreatment and fractionation. Biotechnol Biofuels. doi:10.1186/s13068-015-0207-1
Mikiashvili N, Elisashvili V, Wasser SP, Nevo E (2005) Carbon and nitrogen sources influence the ligninolytic enzyme activity of Trametes versicolor. Biotechnol Lett 27:955–959
Niladevi KN (2009) Ligninolytic enzymes. In: Nigam PS, Pandey A (eds) Biotechnology for agro-industrial residues utilization. Springer Science & Business Media, The Netherlands, pp 398–414
Okamoto K, Nitta Y, Maekawa N, Yanase H (2011) Direct ethanol production from starch, wheat bran and rice straw by the white rot fungus Trametes hirsuta. Enzyme Microb Technol 48:273–277
Pandey A (2003) Solid state fermentation. Biochem Eng J 13:81–84
Pauly M, Keegstra K (2008) Cell wall carbohydrates and their modification as a resource for biofuels. Plant J 54:559–568
Phillips R (1981) Mushrooms and other fungi of Great Britain and Europe. Pan Books Ltd., London
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Curr Genom 12:104–112
Robertson SA, Mason SL, Hack E, Abbott GD (2008) A comparison of lignin oxidation, enzymatic activity and fungal growth during white-rot decay of wheat straw. Org Geochem 39:945–951
Schlosser D, Grey R, Fritsche W (1997) Patterns of ligninolytic enzymes in Trametes versicolor. Distribution of extra- and intracellular enzyme activities during cultivation of glucose, wheat straw and beech wood. Appl Microbiol Biotechnol 47:415–418
Segal IH (1992) Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley-Interscience Publication, New York
Smith N, Shnyreva A, Wood DA, Thurston CS (1998) Tandem organization and highly disparate expression of the two laccase genes lcc1 and lcc2 in the cultivated mushroom Agaricus bisporus. Microbiology 144:1063–1069
Souza JVB, Silva ES, Cavallazzi JRP, Sobrinho AS (2010) Formulation of a liquid medium with wheat bran for the production of laccase by Trametes versicolor in an air-lift bioreactor. J Food Agric Environ 8:394–396
Stajić M, Kukavica B, Vukojević J, Simonić J, Veljović-Jovanović S, Duletić-Laušević S (2010) Wheat straw conversion by enzymatic system of Ganoderma lucidum. BioResources 5:2362–2373
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Viniegra-González G, Favela-Torres E, Aguilar CN, Rómero-Gomez SJ, Díaz-Godínez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30:1447–1457
Acknowledgements
The authors thank Professor Dr. Steve Quarrie, Visiting Professor at the University of Newcastle, UK, for revising and improving English. This study was carried out with financial support of Ministry of Education, Science, and Technological Development of Republic of Serbia, Project No. 173032.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knežević, A., Stajić, M., Milovanović, I. et al. Degradation of beech wood and wheat straw by Trametes gibbosa . Wood Sci Technol 51, 1227–1247 (2017). https://doi.org/10.1007/s00226-017-0921-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-017-0921-x