Abstract
With the increasing number of elderly individuals worldwide, the prevalence of age-related loss of muscle mass, referred to as sarcopenia, is expected to increase. Sarcopenia is a relatively new recognized syndrome, which is thought to affect 13% individuals worldwide, and the significant efforts made by different groups have advanced our understanding of the diagnosis, treatment, and natural history of this condition. However, the challenge is now to standardize its measurement and diagnosis to facilitate research in this area and a greater understanding of this condition and its management between clinicians and researchers. The Global Leadership Initiative on Sarcopenia (GLIS) is at the forefront of an international effort to produce standardized definition of sarcopenia. Setting a definition for sarcopenia entails several considerations and trade-offs. In this critical review, we have addressed key challenges driving the process of standardizing the definition, while delving into future avenues in sarcopenia research. Establishing a clear consensus on the working definition of sarcopenia is essential not only for advancing research in this field but also for assessing the prognostic implications of diagnosing sarcopenia and determining the most suitable treatment for affected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “Sarcopenia,” derived from the Greek words “sarx” (flesh) and “penia” (poverty), was coined in 1988 by Irwin Rosenberg [1]. However, it was not indexed in PubMed until 2010 and prior to that time, the MeSH vocabulary only included terms such as “muscular atrophy” to describe this condition. In the initial working definition, sarcopenia was described as “the loss of appendicular muscle (lean) mass (ASM, kg) by < 2 standard deviations (SD) per height squared (m2) in elderly individuals, assessed by dual X-ray absorptiometry (DXA) scans” [2]. A study by Janssen and colleagues utilized data from the Third National Health and Nutrition Examination Survey (NHANES III) and employed this operational definition of sarcopenia. They study found a strong association between low lean mass and physical disability, particularly among women [2, 3].
Sarcopenia, a condition prevalent among older adults, is associated with several unfavorable clinical outcomes, including prolonged hospital stays, frequent readmissions, reduced quality of life (QoL), higher susceptibility to malnutrition, falls, and fractures, and elevated mortality rates [4,5,6,7,8].
The current estimated global prevalence of sarcopenia among older adults (> 60 years) ranges between 10 and 16%, as indicated in a recent systematic review and meta-analysis [9]. Among community dwellers aged 60 years and older, the estimated prevalence of sarcopenia is approximately 11% in men and 9% in women [10]. However, among hospital inpatients, the rates are considerably higher, with upwards of one in every four adult men and women having this condition [10]. Nursing home residents have shown even higher rates, with a prevalence estimate of this condition affecting one half of elderly men and one third of elderly women [10].
Current Conceptual Definition of Sarcopenia
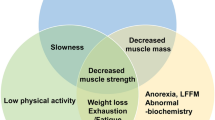
Sarcopenia is currently recognized as a condition with progressive loss of both muscle mass and strength [11] that is associated with impaired quality of life (QoL) and increased disability, frailty, and mortality [12, 13]. A major milestone was achieved in 2016 when sarcopenia was assigned a diagnostic code of ICD-10-MC in several countries [11]. Acknowledgment of sarcopenia as a distinct syndrome was a significant step forward to reach consensus on the diagnostic tools and the prognostic measures of sarcopenia.
Etiology of, and Risk Factors for, Sarcopenia
The pathophysiology of sarcopenia is complex and remains poorly understood. Although sarcopenia occurs with advancing age, it can be accelerated by various factors including physical inactivity, malnutrition, hormonal imbalances, inflammatory processes associated with aging, reduced vascular supply, cellular senescence, and other hallmarks of aging biology [13,14,15]. Several sociodemographic and anthropometric factors may also play a role in the development of this condition. Female sex, low education level, being underweight, and low birth weight have been associated with an increased odd of developing sarcopenia [16]. In addition, investigators in the early 2000s focused on exploring potential biological etiologies associated with this condition including the loss of motor units innervating muscles, inflammatory diseases, oxidative stress, endocrine disorders, nutritional factors, and obesity [17, 18]
International Definitions of Sarcopenia
Over the past decade, multiple definitions, and criteria for diagnosing sarcopenia have been proposed [14]. A number of consensus groups, including the European Working Group on Sarcopenia in Older People (EWGSOP [2010]), the revised EWGSOP2 (2019), the Asian Working Group for Sarcopenia (AWGS), the International Working Group on Sarcopenia (IWGS), the Foundation for the National Institutes of Health (FNIH), Sarcopenia Definition on Outcome Consortium (SDOC), and the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) expert working group, have aligned their individual definitions of sarcopenia around three main components: muscle mass, muscle strength, and physical performance [19,20,21,22,23,24,25]. However, the lack of true consensus among these widely used definitions has hindered research and delayed the integration of sarcopenia into regular clinical practice. It remains crucial to establish a globally accepted and unified definition of sarcopenia to further move the field of inquiry forward and ultimately benefit patients with this condition.
In this critical review, we aim to shed light on the variations in how sarcopenia is identified and diagnosed in different populations by: (a) providing an overview of the various accepted international and region-specific definitions of sarcopenia; (b) discussing the measurement tools utilized and the diagnostic cut points associated with these working definitions; and (c) describing the prevalence of sarcopenia across different international regions based on the use of these different diagnostic criteria.
Sarcopenia Across the Globe
Europe
The initial effort to establish a definition for sarcopenia came from the Special Interest Group (SIG) of the European Society Clinical Nutrition and Metabolism (ESPEN) in 2010 [26]. This group emphasized the need for a clear definition to enable the application of effective diagnostic and treatment approaches for sarcopenia [27]. Their definition described sarcopenia as a combination of reduced muscle mass and reduced strength among older adults.
Shortly after the ESPEN SIG released their definition of sarcopenia, the European Working Group on Sarcopenia in Older People (EWGSOP) published their original consensus paper on the definition and diagnosis of sarcopenia. The 2010 EWGSOP report defined sarcopenia as the presence of low muscle mass in addition to either low muscle strength or low physical performance and used specific cutoff values for muscle mass, strength, and performance. Low muscle mass was defined as a skeletal muscle mass index (SMI) that is 2 SD below the mean SMI of young healthy adults of the same sex and ethnicity [21]. Low muscle strength was defined as a handgrip strength of < 20 kg in women and < 30 kg in men, and low physical performance was defined as a gait speed of < 0.8 m/s (Table 1). In the EWGSOP classification of sarcopenia, two major categories are distinguished: primary (age-related) sarcopenia and secondary (disease-related) sarcopenia. Furthermore, to facilitate appropriate case identification and treatment, this group further classified sarcopenia into acute (lasting less than 6 months) and chronic (lasting more than 6 months) [21]. This classification framework emphasized the importance of accurate diagnosis and timely intervention in managing sarcopenia effectively. In Europe, the prevalence of sarcopenia among older adults was reported as 22% using EWGSOP criteria and 11% using the Foundation for the National Institutes of Health (FINH) criteria [16].
In 2018, the EWGSOP2 consensus paper revised the definition of sarcopenia and proposed new diagnostic criteria. In this revision, sarcopenia was defined as a disease of the muscles characterized by the presence of low muscle mass, low muscle strength, and/or low physical performance. In addition, they recommended the use of a continuous measurement of muscle strength instead of the categorical definition proposed in the previous consensus report [21, 22]. The EWGSOP2 group was the first to suggest using the simple self-report SARC-F questionnaire for identifying persons at risk for sarcopenia [27]. The questionnaire covers five aspects related to sarcopenia, including an individual’s muscle strength, ability to walk without assistance, getting up from a chair, climbing stairs, and instances of falling [27]. SARC-F is a convenient and cost-effective tool that can be used in community health care frameworks and clinical contexts [27].
Furthermore, the EWGSOP2 report recommends that muscle mass may be measured using bioelectrical impedance analysis (BIA) or DXA, and the cutoff values for low muscle mass remain the same as in the original consensus. The cutoff points for low muscle mass were based on population-based studies of ASMM in healthy young adults, with a cutoff point usually set at − 2 SD or − 2.5 SD for a more conservative diagnosis. The EWGSOP2 also recommends the use of the handgrip strength test as the preferred method for measuring muscle strength and suggests a cutoff value of < 16 kg for women and < 27 kg for men based on < 2 SD from a reference normative population [28].
For the assessment of physical performance, the EWGSOP2 recommends the use of the Short Physical Performance Battery (SPPB), which includes measures of gait speed, chair stand, and balance. The EWGSOP2 recommends that a score of less than or equal to 8 out 12 on the SPPB indicates low physical performance. This consensus report also proposes a new category of “probable sarcopenia” for individuals who have low muscle strength without meeting the criteria for low muscle mass or low physical performance to capture individuals who may be at risk for developing sarcopenia or who may have early-stage sarcopenia [21, 22]. Estimates of the prevalence of sarcopenia using the EWGSOP2 criteria have yet be reported.
North America
Following the work of Baumgartner and Janssen [2, 3], investigators in North America, particularly the United States, endorsed the conclusions of the IWGS [24]. Based on expert consensus, this group endorsed the use of an appendicular lean mass cutoff point for females (< 5.67 kg/m2) and males (< 7.23 kg/m2) together with a habitual gait speed (< 1 m/s) as criteria for sarcopenia. In 2014, the Foundation for the National Institutes of Health (FNIH) reported on the results of the FNIH Sarcopenia Project, which was initiated in 2010 to conduct a series of analyses using a large database of community-dwelling older adults including 11,427 males, mean age = 75.2 years; 15,198 females, mean age = 78.6 years from several large US epidemiological cohorts [25]. This working group established cut points for clinically relevant low muscle strength and demonstrated associations with slow walking speeds (defined as < 0.8 m/s), and clinically relevant low lean mass (measured by whole-body DXA scans). They examined how low lean mass was related to clinically relevant weakness and applied these criteria to assess concurrent and predictive validity [25].
The FNIH Sarcopenia Project proposed the following evidence-based criteria for defining clinically relevant weakness and low lean mass based on the findings from a large population of community-dwelling older adults: isometric grip strength (< 26 kg for males and < 16 kg for females); grip strength to BMI ratio (weak BMI) (< 1 for males and < 0.56 for females); appendicular lean mass (ALM) (< 19.75 kg for males and < 15.02 kg for females) or ALM to BMI ratio (ALM:BMI) (< 0.79 for males and < 0.51 for females). The predictive validity of these cutoff points demonstrates that weakness, defined in this manner, is associated with an increased risk of mobility limitation, independent of low lean mass. In North America, the estimated prevalence of sarcopenia among older adults was 14% through use of the EWGSOP criteria and 9% through use of the FINH criteria [16], further highlighting how the specific cut points influence the prevalence with a specific population.
Limitations in the findings from the FNIH Sarcopenia Project included the relatively healthy cohort of older adults and the limited ability of the derived cutoff points to predict relevant clinically meaningful outcomes. This led to the conduct of additional analyses by the Sarcopenia Definitions and Outcomes Consortium (SDOC) [30].
The SDOC set out to develop evidence-based cutoff points for lean mass and strength to identify persons at risk for mobility disabilities and other adverse health outcomes, including falls, self-reported mobility limitations, hip fractures, and death. The SDOC assembled a large body of data from a number of observational epidemiologic studies, clinical trials, and special populations and utilized data-driven analytical approaches to generate cutoff points for low lean mass, reduced muscle strength related to their ability to predict slow walking speed. Using classification and regression tree analysis in pooled data from eight separate epidemiological cohorts (n = 18,831, 17% with self-reported mobility limitations), they found that various measures of grip strength (grip strength/BMI, grip strength/total body fat, and absolute grip strength) were the primary discriminating variables for slow walking speeds in men and women [30]. This working group further showed that low grip strength, using these cutoff points, and slow gait speed were associated with an increased risk of mobility limitations, falls, fractures, and all-cause mortality [31]. Weakness combined with slow gait speed tended to increase the relative risk of falls, fracture, mobility limitations, and mortality while lean body mass measured by DXA was unrelated to these outcomes. Inasmuch, the SDOC consortium endorsed low grip strength and slowness in gait speed as key components of sarcopenia. Prevalence of sarcopenia using the SDOC criteria for low grip strength ranged from 30 to 66% in women and 23–61% in men with higher prevalence occurring with increasing age [32].
In addition to these studies conducted in the United States, a cross-sectional analysis of data from The Frailty Dynapenia and Sarcopenia in Mexican Adults was conducted to validate the FRAIL scale [33], which includes 5 components: fatigue, resistance, ambulation, illness, and loss of weight. To our knowledge, there are still no diagnostic guidelines that are specific to Mexico. The study found that, according to the FRAIL scale, the prevalence of sarcopenia was 8.9% in the total sample and 9.6% among older adults. However, the study sample consisted of primarily urban residents and may not have represented the national Mexican population, which includes a large proportion of rural and semirural individuals [33].
South America
Currently, South America does not have any regionally specific guidelines for diagnosing sarcopenia; therefore, some countries have adopted European guidelines for estimating the prevalence of sarcopenia and have found varying estimates depending on the criteria utilized. In South America, the prevalence of sarcopenia was 21% using EWGSOP and 29% using FINH criteria [16].
A recent systematic review and meta-analysis of 53,134 older adults from seven countries (Argentina, Brazil, Chile, Colombia, Ecuador, Peru, and Venezuela) found that almost 1 in 2 persons are prefrail and 1 in 5 are frail, with hospitalized older adults and nursing home residents being the most affected [34]. There is an overlap between sarcopenia and the classic frailty phenotype definition, which also includes both low gait speed and low grip strength. Inasmuch, although sarcopenia was not assessed in all studies, one can infer the potential magnitude of the high prefrailty, and frailty estimated rates and the need to establish a region-specific diagnostic framework [34].
Asia
In considering the diagnosis of Sarcopenia in Asian populations, it is essential to consider specific factors tied to their unique anthropometric features and the cultural or lifestyle distinctions that differentiate them from Western cohorts (body size, diet, physical activity). In 2018, Japan published clinical practice guidelines for the diagnosis of sarcopenia [35]. Which should be considered when an individual has low muscle mass plus either low muscle strength or low physical performance [19, 20].
In 2019, the Asian Working Group for Sarcopenia (AWGS) proposed revised cutoff points for the diagnosis of sarcopenia in older adults based on muscle mass, muscle strength, and physical performance. The organization defined low muscle mass as a muscle mass index (appendicular skeletal muscle mass divided by height squared) of < 7.0 kg/m2 for men and < 5.7 kg/m2 for women. They recommended using handgrip strength as a measure of muscle strength and defined weak grip strength as < 28 kg for men and < 18 kg for women. For the assessment of physical functioning, they recommended using the Short Physical Performance Battery (SPPB) and defined low physical performance as an SPPB score of < 9 [19, 20]. The prevalence of Sarcopenia among Asian individuals was found to be 21% based on the EWGSOP criteria and 10% when following the FINH criteria [16].
Oceania
In Oceania, which consists of comprising Australasia, Melanesia, Micronesia, and Polynesia, the prevalence of Sarcopenia exhibited marked differences depending on the diagnostic criteria used. Recognizing the importance of accurate diagnosis, the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) Expert Working Group advocates for a comprehensive assessment approach, including muscle mass, muscle strength, and physical performance [23]. They endorsed the definition of sarcopenia proposed by EWGSOP2, which includes measurements of muscle mass, muscle strength, and physical performance. The ANZSSFR has undertaken two Delphi processes to establish a consensus on the operational definition of sarcopenia for individuals living in Australia and New Zealand. The original Delphi process identified the original European Working Group on Sarcopenia in Older People definition as the preferred definition for diagnosing sarcopenia in Australia and New Zealand.
Current diagnostic criteria rely on the use of DXA or BIA to measure muscle mass, with grip strength and gait speed used as measures of muscle strength and physical performance, respectively. Other tests, such as measures of muscle strength, physical performance, and muscle quality, may also be incorporated into the diagnostic process if deemed necessary [23]. The ANZSSFR recommends that individuals aged 65 years and older, or those who are younger but have risk factors for sarcopenia, such as a history of falls, hospitalization, or prolonged bed rest, should undergo screening for sarcopenia using similar diagnostic measures [23]. When the EWGSOP criteria were employed, it was estimated that 40% of the population had sarcopenia, while utilization of the FINH criteria yielded an estimate of only 5% [16].
Africa
To date, there is a lack of established guidelines for diagnosing sarcopenia in Africa. As a result, researchers have employed various approaches, including the Short Physical Performance Battery (SPPB), which is scored from 0 to 12. In Gambia, the prevalence of sarcopenia in men and women varied significantly depending on the diagnostic criteria applied. According to the criteria set by the FNIH, the frequency of sarcopenia was 20% in men and 45% in women, while the EWGSOP criteria yielded rates of 19% in men and 10% in women. These discrepancies highlight the inconsistency in diagnosing sarcopenia and emphasize the need for a globally accepted definition with ethnic-specific cutoff points [36, 37].
Discussion
Considerations in Disseminating Operational Guidelines for Diagnosing Sarcopenia
The original definitions of sarcopenia primarily focused on assessing muscle mass, whereas the current emphasis has shifted toward evaluating muscle function, which includes both strength and power [38]. When developing and disseminating guidelines for sarcopenia, guidelines should provide clear and thorough criteria for diagnosis, including specific measurement tools, thresholds, and relevant clinical considerations. For example, the IWGS uses a cut-point in gait speed of < 1.0 m/s to define slowness [24]. In contrast, EWGSOP2 uses a cut-point in gait speed of ≤ 0.8 m/s to define poor physical performance [22]. Identifying common language for sarcopenia and harmonizing diagnostic thresholds is critical to minimize ambiguity, enhance reproducibility, and promote consistency in clinical decision-making. A key finding of this review was the heterogeneity of specific cut points that have been proposed to assess sarcopenia worldwide. One clear result of the use of the differing cut points has been the strong impact on the resultant prevalence in a given population. Recently, Westburry et al. highlighted the impact of the cut points proposed by the EWGSOP2 and SDOC using pooled data from the Health Aging and Body Composition (Health ABC) Study, Osteoporotic Fractures in Men (MrOS) Study cohorts (Sweden, USA), the Hertfordshire Cohort Study (HCS) and the Sarcopenia and Physical impairment with advancing Age (SarcoPhAge) Study and found prevalence varied widely (1.1% to 5.3%) depending on the cut point applied [32].
A key aspect to consider is the diversity of ethnicities and patients with various comorbidities when developing guidelines for the diagnosis and management of sarcopenia. The guidelines should provide clear guidance on how to adapt assessments and interventions to account for these variations. By doing so, health care professionals can tailor their approaches to suit the specific needs of individuals from different ethnic backgrounds and with various health conditions. For instance, EWGSOP2 recommended the use of knee flexion/extension for special cases when the grip strength measurement is unattainable [22].
To address this challenge, the Global Leadership Initiative on Sarcopenia (GLIS) was established in 2021 as an international effort to develop an inclusive definition of sarcopenia that can be universally accepted and endorsed by all existing consensus groups that have previously proposed their own definitions. As an initial step, the GLIS has published a summary of recommendations for defining common terms used in sarcopenia research and clinical practice. By bringing together these diverse perspectives, GLIS aims to guide sarcopenia diagnosis, research, and clinical practice worldwide [38, 39].
The future holds promise for significant progress in addressing sarcopenia. This includes prescribing effective exercise regimens, translating scientific findings into clinical studies, and focusing on the unique challenges of sarcopenia in low- and middle-income countries. These advances have the potential to improve patient outcomes and enhance the overall well-being of individuals across diverse populations. By fostering collaboration and innovation, the efforts of GLIS and other initiatives can contribute to significant advances in the field of sarcopenia [39]. The GLIS steering committee recently published a paper on standardized terminology to improve understanding and enhance communication between clinicians and researchers [39].
Inclusivity
Creating a standardized working definition for sarcopenia entails several considerations and trade-offs, which involve balancing inclusiveness and specificity. A comprehensive and inclusive definition may encompass a larger population but might also include individuals who do not exhibit clinically significant sarcopenia. On the other hand, a more specific definition may exclude certain individuals, who may benefit from select interventions.
Clinical Utility
Achieving a balance between clinical applicability of the definition of sarcopenia and scientific rigor is key. On the one hand, an excessively intricate working definition may pose challenges for routine clinical practice. For example, assessment of lean muscle mass using DXA scans as opposed to BIA is problematic. On the other hand, a simple definition that is easy to use may fail to capture the complexity and underlying mechanisms of this condition. Clinical research necessitates precise and standardized definitions to facilitate comparability and generate robust evidence while the usefulness of a disease definition in routine clinical practice requires a practical definition that can be easily implemented in different health care settings [38, 39].
Consensus and Global Applicability
Sarcopenia affects populations worldwide, yet its prevalence, risk factors, and manifestations may vary across different regions and cultures. Developing a globally applicable definition that also accounts for cultural and contextual variations is challenging [38, 39]. Studies of sarcopenia in low- and middle-income countries need to be encouraged, not only to address local requirements but also to foster a global understanding of sarcopenia [39].
Conclusion
Sarcopenia has emerged as a significant syndrome of aging, and recent findings challenge our current understanding of the appropriate measures to determine to classify sarcopenia. Once a global definition for sarcopenia has been agreed upon, new assessment methodologies, establishment of appropriate thresholds, creation of algorithms, and pertinent outcome measures will be necessary. The GLIS initiative is well-positioned to catalyze changes and implementation in the field with greater understanding of the natural history of disease, predisposing factors, and application of existing and novel intervention strategies to enhance patient-centered outcomes associated with this condition of advanced age.
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127(5):990S-991S
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR et al (1998) Epidemiology of Sarcopenia among the Elderly in New Mexico. Am J Epidemiol 147(8):755–763. https://doi.org/10.1093/oxfordjournals.aje.a009520
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (Sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50(5):889–896. https://doi.org/10.1046/j.1532-5415.2002.50216.x
Ligthart-Melis GC, Luiking YC, Kakourou A, Cederholm T, Maier AB, de Schueren MAE (2020) Frailty, sarcopenia, and malnutrition frequently (Co-)occur in hospitalized older adults: a systematic review and meta-analysis. J Am Med Dir Assoc 21(9):1216–1228
O’Shea E, Trawley S, Manning E, Barrett A, Browne V, Timmons S (2017) Malnutrition in hospitalised older adults: a multicentre observational study of prevalence, associations and outcomes. J Nutr Health Aging 21(7):830–836. https://doi.org/10.1007/s12603-016-0831-x
Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF (2016) Sarcopenia and length of hospital stay. Eur J Clin Nutr 70(5):595–601
Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B (2017) Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle 8(2):251–258. https://doi.org/10.1002/jcsm.12163
Beaudart C, Reginster JY, Amuthavalli Thiyagarajan J, Bautmans I, Bauer J, Burlet N et al (2023) Measuring health-related quality of life in sarcopenia: summary of the SarQoL psychometric properties. Aging Clin Exp Res 35(8):1581–1593. https://doi.org/10.1007/s40520-023-02438-3
Yuan S, Larsson SC (2023) Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism 144:155533
Papadopoulou SK, Tsintavis P, Potsaki G, Papandreou D (2020) Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging 24(1):83–90. https://doi.org/10.1007/s12603-019-1267-x
Vellas B, Fielding RA, Bens C, Bernabei R, Cawthon PM, Cederholm T et al (2018) Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the international conference on frailty and sarcopenia research task force. J Frailty Aging 7(1):2–9
Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y et al (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing 43(6):748–759. https://doi.org/10.1093/ageing/afu115
Senior HE, Henwood TR, Beller EM, Mitchell GK, Keogh JWL (2015) Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 82(4):418–423
Coletta G, Phillips SM (2023) An elusive consensus definition of sarcopenia impedes research and clinical treatment: a narrative review. Ageing Res Rev 86:101883
Englund DA, Zhang X, Aversa Z, LeBrasseur NK (2021) Skeletal muscle aging, cellular senescence, and senotherapeutics: current knowledge and future directions. Mech Ageing Dev 200:111595
Hwang J, Park S (2023) Gender-specific prevalence and risk factors of sarcopenic obesity in the Korean elderly population: a nationwide cross-sectional study. Int J Environ Res Public Health 20(2):1140
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP et al (2022) Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 13(1):86–99. https://doi.org/10.1002/jcsm.12783
Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E et al (2019) Sarcopenia: a time for action an SCWD position paper. J Cachexia Sarcopenia Muscle 10(5):956–961. https://doi.org/10.1002/jcsm.12483
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K et al (2020) Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21(3):300-307.e2
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS et al (2014) Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 15(2):95–101
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Daly RM, Iuliano S, Fyfe JJ, Scott D, Kirk B, Thompson MQ et al (2022) Screening, diagnosis and management of sarcopenia and frailty in hospitalized older adults: recommendations from the Australian and New Zealand society for sarcopenia and frailty research (ANZSSFR) expert working group. J Nutr Health Aging 26(6):637–651. https://doi.org/10.1007/s12603-022-1801-0
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12(4):249–256
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69(5):547–558
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 29(2):154–159
Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE (2016) SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 7(1):28–36
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C et al (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40(4):423–429. https://doi.org/10.1093/ageing/afr051
Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA et al (2020) Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc 68(7):1410–1418. https://doi.org/10.1111/jgs.16372
Manini TM, Patel SM, Newman AB, Travison TG, Kiel DP, Shardell MD et al (2020) Identification of sarcopenia components that discriminate slow walking speed: a pooled data analysis. J Am Geriatr Soc 68(7):1419–1428
Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP et al (2020) Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc 68(7):1429–1437. https://doi.org/10.1111/jgs.16517
Westbury LD, Beaudart C, Bruyère O, Cauley JA, Cawthon P, Cruz-Jentoft AJ et al (2023) Recent sarcopenia definitions-prevalence, agreement and mortality associations among men: findings from population-based cohorts. J Cachexia Sarcopenia Muscle 14(1):565–575
Rosas-Carrasco O, Cruz-Arenas E, Parra-Rodríguez L, García-González AI, Contreras-González LH, Szlejf C (2016) Cross-cultural adaptation and validation of the frail scale to assess frailty in mexican adults. J Am Med Dir Assoc 17(12):1094–1098
Coelho-Junior HJ, Marzetti E, Picca A, Calvani R, Cesari M, Uchida MC (2020) Prevalence of prefrailty and frailty in South America: a systematic review of observational studies. J Frailty Aging 9(4):197–213. https://doi.org/10.14283/jfa.2020.22
Akishita M, Kozaki K, Iijima K, Tanaka T, Shibasaki K, Ogawa S et al (2018) Chapter 1 definitions and diagnosis of sarcopenia. Geriatr Gerontol Int 18(S1):7–12. https://doi.org/10.1111/ggi.13311
Metanmo S, Kuate-Tegueu C, Gbessemehlan A, Dartigues JF, Ntsama MJ, Nguegang Yonta L et al (2022) Self-reported visual impairment and sarcopenia among older people in Cameroon. Sci Rep 12(1):17694
Zengin A, Jarjou LM, Prentice A, Cooper C, Ebeling PR, Ward KA (2018) The prevalence of sarcopenia and relationships between muscle and bone in ageing West-African Gambian men and women. J Cachexia Sarcopenia Muscle 9(5):920–928
Sayer AA, Cruz-Jentoft A (2022) Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing 51(10):220. https://doi.org/10.1093/ageing/afac220
Cawthon PM, Visser M, Arai H, Ávila-Funes JA, Barazzoni R, Bhasin S et al (2022) Defining terms commonly used in sarcopenia research: a glossary proposed by the Global Leadership in Sarcopenia (GLIS) Steering Committee. Eur Geriatr Med 13(6):1239–1244
Funding
R.A.F. is partially supported by the US Department of Agriculture (USDA), under agreement No. 58-8050-9-004, by NIH Boston Claude D. Pepper Center (OAIC; 1P30AG031679), and by NIH University of Florida Claude D. Pepper Center (P30AG028740). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alhmly, H.F., Fielding, R.A. A Critical Review of Current Worldwide Definitions of Sarcopenia. Calcif Tissue Int 114, 74–81 (2024). https://doi.org/10.1007/s00223-023-01163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01163-3