Abstract
Pathophysiological conditions can modify the skeletal chemical concentration. This study analyzes the elemental composition in two anatomical regions from dry femoral bone using a portable X-Ray Fluorescence (pXRF) and evaluates its impact in the bone mineral density (BMD). The left femora of 97 female skeletons (21–95 years old individuals) from the Coimbra Identified Skeletal Collection were studied. Diagenetic biases were discarded at the outset and BMD was determined with Dual-energy X-ray absorptiometry. Chemical measurements were performed at the midpoint of the femoral neck and at the midshaft using a pXRF device, and comparisons were made considering the age and the BMD values. Only elements with a Technical Measurement Error ≤ 5% were selected: P, S, Ca, Fe, Zn, As, Sr, Pb and the Ca/P ratio. Statistically significant differences were found between regions, with higher concentrations of P, Ca, Zn and S at the midshaft, and the Ca/P ratio at the femoral neck. The concentration of P is higher in individuals < 50 years, while S and Ca/P ratio increase in individuals ≥ 50 years. The decrease of P with age can be simultaneously related to the decline of its concentration in osteoporosis. Decreased BMD is also associated with higher levels of S and Pb. Osteoporosis enhances the absorption of osteolytic elements in specific locations. This fast and non-destructive technique has proved effective for the comprehension of chemical changes related to bone mass loss. This study highlights the potential of identified skeletal collections to improve the knowledge about bone fragility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis (OP) is a metabolic bone disease, typified by a decline in bone mass and deterioration of bone microarchitecture, increasing bone fragility and the risk of fracture [1]. Bone fragility and associated fractures are major challenges for public health, especially impacting the global morbidity and mortality of postmenopausal women and aged individuals from both sexes [2]. OP is a complex disorder of multifactorial etiology, being attributed to various endocrine, metabolic, mechanical and inflammation factors that result in an imbalance between bone resorption and bone formation. Risk factors include aging, estrogen withdrawal after menopause, genetics, reproductive history, physical activity, and nutritional status. Studies on OP tend to focus on its main clinical repercussions [3]. However, these events are punctuated by fluctuations in the elemental composition of the mineral matrix of bone tissue, whose knowledge is still inadequate in many aspects [4].
The pure hydroxyapatite molecule—Ca10(PO4)6(OH)2—has the capability of exchanging ions during the reabsorption and deposition of new bone and modify its mechanical-physiochemical properties in order to assist the auxological development of individuals [5]. Factors such as extracellular concentration of trace and ultratrace elements, ionic affinity and the mineralization degree [6] are essential to understand the biophysical bases underlying the bone properties. The concentration of chemical elements in the bone tissue is subordinated to biological factors including age and sex [7], environment (e.g., lead pollution [8]) and pathologies, such as osteoarthritis [9] and osteoporosis [10, 11]. It has also been demonstrated that bone composition displays variability between different animal species [12], as well as among type [13], region [14] and layer of the bone under analysis [11, 13, 14].
Bone mineral oscillations are subject to a very narrow threshold among benefit, damage and toxicity [5], especially in osteoporotic patients whose biochemical imbalance can be associated with: (1) a deficient nutrition or an inappropriate intestinal absorption; (2) the influence of certain medication or supplementation; and (3) the uncoupling between osteoblasts/osteoclasts activity triggered by hormonal and physicochemical factors, which leads to an excess and faster resorption over bone formation [10]. In non-osteoporotic individuals, calcium (Ca) varies from 82,000 μg/g [15] to 336,000 μg/g, whereas phosphorous (P) ranges from 50,700 μg/g [16] to 140,000 μg/g [17] according to the bone and region under study. It has been reported that women with a lower consumption of both Ca and P exhibit an increased bone mass loss [18]. In addition to nutritional deficiencies, impaired bone resorption or deposition could be attributed to genetic conditions, hormonal disorders and aging. For example, in the glucocorticoid-induced osteoporosis, calcium homeostasis is adversely affected by parathormone stimulation or through a deficient intestinal absorption of the element [19]. Likewise, estrogen has an effect on renal phosphorus excretion, being its deficiency linked to a lower excretion [20]. The reduction in phosphorus content may be the result of osteomalacia, an adult genetic or non-genetic vitamin D deficiency with inadequate bone mineralization [21].
Furthermore, many studies have shown that the levels of trace and ultra-trace elements also suffer a great variation in their concentrations when osteoporotic and non-osteoporotic individuals are compared: zinc (Zn) and strontium (Sr) tend to decrease, whereas lead (Pb), sulphur (S) and nickel (Ni) increase in osteoporosis [7, 12,13,14]. However, the evidence about chemical elements interaction either with the bone matrix or the effect of these elements by excess or defect is still controversial because it has been mainly obtained from animal experimentation, whose concentrations differ from humans; and in those publications specifically focused on humans, the sample size is considerably small.
In order to better understand the changes in mineral content derived from the process of bone mass loss, this study presents a compositional analysis carried out with a portable X-ray Fluorescence (pXRF) device in the neck and midshaft of the femoral bone of adult female individuals from the Coimbra Identified Skeletal Collection. The aims are to assess potential differences in the elemental composition between these two bone regions, and to evaluate variations conditional to the age at death of the individuals, and the occurrence of osteoporosis as previously diagnosed by Dual-energy X-ray absorptiometry (DXA).
Material and Methods
The Coimbra Identified Skeletal Collection (CISC) comprises 505 skeletons recovered from the Municipal Cemetery of Conchada (Coimbra, Portugal). Biographical data for the skeletal individuals are available, e.g., sex, age at death, cause of death and occupation. Most of the individuals in the collection were Portuguese nationals with low socioeconomic status and employed in non-qualified manual labor [22]. Ninety-seven left femora from adult women of the CISC were randomly selected and used to implement this research. Only individuals with at least one femur showing no macroscopical signs of post-depositional modifications were included. The individuals studied show no signs of osteomalacia or other pathological conditions, as diagnosed by paleopathological criteria.
Ages at death ranged from 21 to 95 years old (mean: 54.04 ± 20.45 years; Table 1). All individuals died during the twentieth century and were buried in wooden coffins in earth-cut shallow graves for at least five years. The absence of diagenetic biases was previously verified through macroscopical and imaging evaluation of the trabecular and endocortical integrity of the bone. Conventional radiography suggested that soil erosion was insignificant or null, and microradiographs of the dry cortex obtained in the anterior midshaft of the femur showed normal mineralization [23, 24]. Once bone preservation was established, BMD at the femoral neck was evaluated with DXA. For this purpose, a Hologic QDR 4500C Elite densitometer was used and femora were placed on a low-density container and positioned in parallel to the X-ray emission source [25]. BMD was then classified according to the World Health Organization standards [26] in three groups: normal (N = 33), osteopenic (N = 35) and osteoporotic (N = 29) individuals.
Elemental measurements were performed at the midpoint of femoral neck and at the midshaft, without any prior knowledge of the biographical data or BMD status, using a pXRF Thermo Scientific Niton™ XL3t Goldd + XRF analyzer (Fig. 1). The pXRF is a surface to near‐surface technique in which the X‐ray penetration depth depends on the energy of the X‐ray and the density of the material. This device gauges the chemical elements on a circular flat surface (8 mm diameter) in a non-invasive and non-destructive way. According to its technical specifications, the Thermo Scientific Niton XL3t 900 with GOLDD + works at voltages from 6 to 50 kV while the built-in silver anode operates at a maximum of 50 kV and 200 µA, using four different energy settings over 30 s each for light, low, main and high Z elements, and allowing plus light element analysis (Mg, Al, Si, P, S) without helium or vacuum purging. The software NITON Data Transfer version NDT_REL_8.2 was used to convert spectral data to composition analysis in parts per million, or μg/g, whereas calibration was performed through the “Test All mode” in order to identify a maximum of 43 elements from magnesium to uranium. The Ca/P ratio is also included in this mode, yet elements with an atomic number lower than magnesium are provided together in a percentage called “Balance”. To obtain the most accurate measurements, the source of emission should be held as perpendicular and close to the cortical bone as possible (no periosteum remains on dry bone) to reduce air pollution. Thus, the femur is stabilized in a V-structure with a flexible and adaptable coating, which allows an adapted and optimized measurement to the natural curvature of the bone (Fig. 1).
In order to test the reliability and reproducibility of the method, the accuracy and intra-observer error were determined using the relative Technical Measurement Error (rTEM) as previously described [25]. The first 20 measurements (ten in each femoral regions aforementioned) were obtained twice in an alternate order. Only those elements with a rTEM ≤ 5% were considered for study. With these compositional values three different comparisons were performed: (1) femoral regions (femoral neck [Fem.N] vs. femoral diaphysis [Fem.D]), (2) age (< 50 vs. ≥ 50 years), and (3) BMD status (normal [NOR] vs. osteoporosis [OP]). To simplify the comparison between different bone mass conditions—and considering that the classification between BMD categories has been defined by artificial cut-off points—the osteopenia category will not be considered in the comparative analyses (although it will be included in the Lineal Discriminant Analysis, giving a global vision of the data distribution).
Statistical and multivariate analyses were carried out using SPSS v.20.0 and Excel XLSTAT-Base v.2018 software, respectively. The sample was characterized using descriptive statistics, and normality was verified using the Kolmogorov–Smirnov test. To study the differences among groups, the parametric Student's t-test for normally distributed elements, and the Mann–Whitney U nonparametric test for those which did not follow a normal distribution, were used. The correlation between the different elements and the BMD was estimated with Pearson's and Spearman's correlation coefficients. Finally, a Lineal Discriminant Analysis (LDA) was performed in order to identify potential relationships between the group of elements and the BMD.
Results
Of the 43 potentially detected chemical elements, 26 (60%) were quantified in the femora. Of these, seven (35%) had a rTEM ≤ 5% (Table 2): Phosphorus (P), Sulfur (S), Calcium (Ca), Iron (Fe), Zinc (Zn), Strontium (Sr), and Lead (Pb). According to the Kolmogorov–Smirnov's test, P, S, Ca and Sr were normally distributed.
Bone Region, Age, BMD Status and Bone Composition
Tables 3, 4 and 5 summarize the statistical results (mean, standard deviation (s.d.) and 95% confidence interval) for each element according to bone region (femoral neck and diaphysis), age at death and BMD status, respectively. In any case, no significant differences were found for Fe or Sr.
Comparing the concentrations at the midpoint of femoral neck and the midshaft, statistically significant differences were observed in the concentration of P, S, Ca and Zn (Table 3), being these elements in greater abundance in the femoral diaphysis. In contrast, the Ca/P ratio was significantly higher in the femoral neck.
Regarding age-related changes (Table 4), P levels were significantly higher in women under 50 years of age, both in the neck (124,797.90 ± 14,094.58 vs. 116,628.50 ± 15,061.37 μg/g) and in the femoral diaphysis (137,725.15 ± 11,448.10 vs. 132,268.60 ± 9701.31 μg/g), while S appears in greater concentration in women over 50 years (neck: 5216.74 ± 2041.63 vs. 6318.19 ± 2148.41 μg/g; midshaft: 6110.92 ± 2222.30 vs. 7093.37 ± 1990.95 μg/g). The Ca/P ratio was significantly higher in women over 50 years of age (neck: 2.05 ± 0.18 vs. 2.19 ± 0.23; midshaft: 1.89 ± 0.14 vs. 1.96 ± 0.13).
When comparing subjects with normal BMD and with osteoporosis (Table 5), the concentration of P was lower in the midshaft of the individuals with osteoporosis (130,508.50 ± 8774.47 μg/g) than in those with normal BMD (137,976.53 ± 12,392.90 μg/g), while S increases significantly in osteoporotic women in both regions of the femur (neck: 5225.66 ± 2246.07 vs. 6644.87 ± 2113.87 μg/g; midshaft: 6050.28 ± 2351.46 vs. 7496.51 ± 1969.98 μg/g). This results in a higher Ca/P ratio in osteoporotic individuals in both bone regions. Besides, Pb shows significant differences according to BMD to the extent that in the osteoporotic midshaft its concentration almost doubles that found in individuals with normal BMD (90.17 ± 71.90 μg/g vs. 49.05 ± 35.43 μg/g).
Association Between Chemical Concentration and BMD
The linear correlations between the concentration of different chemical elements and the BMD values for each femoral region are presented in Table 6. In both regions, S and Pb are negative and significantly correlated with BMD, increasing their concentration as BMD declines. Phosphorus and Fe are positive and significantly correlated in the midshaft, therefore, as the BMD increases, the concentration of these elements is greater. Finally, the Ca/P ratio displays a negative correlation in both regions.
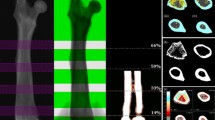
Figure 2a displays the LDA with the values obtained at the midshaft, as this is the region where differences between women with osteoporosis, osteopenia and normal BMD are best appreciated. There is a clear separation between the centroids corresponding to each of the three groups in the different quadrants. Factor 1 (F1) account for 88.36% of the variance, separating mainly the normal BMD group from the osteoporotic one, and positioning the osteopenic individuals in an intermediate position. Factor 2 (F2) accounts for 11.64% of the variance. Considering the highest correlation (Fig. 2b,c) values between the variables and the linear discriminants, it can be observed that for the X axis (F1), P presents the highest positive correlation values. In the negative side, Pb appears more highly correlated, followed closely by S. In the Y axis (F2), the elements register lower correlation values than F1. Nevertheless, Fe is the most correlated element in the positive side, while P correlates in the negative side. These results can be appreciated better in the correlation circle.
a Graphic representation of Linear Discriminant Analysis carried out with elemental concentration registered at midshaft of femoral diaphysis. Centroids for each BMD group are represented with triangles. b Factor loadings of Factor 1 (F1) and Factor 2 (F2) for each element. In bold, the highest values of factor loading. c Correlation circle. Elements sorted by atomic number
Discussion
The last decade has witnessed a remarkable increase in knowledge about osteoporosis, although this condition is still a growing public health problem. For such a reason, understanding the role of elemental composition in bone tissue may be fundamental both to prevent its morbimortality and to improve further treatments. In this work, using the valuable source of an identified skeletal collection, we have reported changes in certain chemical elements associated not only with age, but also with the loss of bone mass.
Accuracy and Reliability of pXRF on Dry Bone
This study is the first to use pXRF in order to quantify the concentration of chemical elements in dry bone from a collection of identified human skeletons, preserving the integrity of the sample, being easily transported, simple to use and ideal to analyze large samples in a short period of time. This technique—and in particular this device—has been successfully used in previous studies [27]. However, there are some limitations and technical issues that must be clarified. pXRF is ineffective in the detection of elements with low atomic mass (“Balance”) and those with rTEM > 5% in bone context when compared to other methodologies such as Neutron Activation Analysis with High-Resolution Spectrometry of Short (or long)-Lived Radionuclides, Particle-Induced Gamma-Ray Emission, Inductively Coupled Plasma Atomic Emission Spectrometry, and Inductively Coupled Plasma Mass Spectrometry [16]. On the contrary, these techniques are destructive and, therefore, avoidable for identified skeletal collections, making pXRF a promising tool for the study of OP in bioarchaeological contexts. Zaichick’s research [16], focused on the ribs, hampers the comparison with other bones such as the femur since the skeletal turnover depends on several factors: the particular shape, size, surface to volume ratio, trabecular/ cortical proportion of the bones and on palaeobiological, biomechanical, genetic and pathological parameters of each individual under analysis. In this sense, it has been considered that the constant load to which the rib cage is subjected during respiration could enhance the costal turnover, although Fahy and collaborators [28] found no significant differences when the rib and the femoral cortex were compared histomorphometrically.
Byrnes and Bush [29] mention that the detection of lighter elements such as P represents a technical limitation in pXRF analysis. However, the Geometrically Optimized Large Area Drift Detector (GOLDD+) technology attached to the Thermo Scientific Niton XL3t 900 device allows the detection of P from 600 μg/g using mining mode for a SiO2 matrix. Besides, in all the skeletons analyzed in this study, values higher than this threshold were found, indicating that no technical biases were present in the P measurements in this study.
Although usually ignored in this type of study, the high values of standard deviations verified in some elements needs to be considered. Because measures showed similar standard deviations after double testing on each surface (technical replicas), the interindividual variability becomes a plausible explanation. In fact, Pemmer et al. [30] also suggested this after evaluating the concentration of Zn and Pb in bone at an ultrastructural level. In this sense, the dietary choices, the predisposition to exogenous and endogenous stressors or the area of residence, just to name a few, could be responsible for the standard deviation dispersion. Furthermore, this phenomenon depends on the order of magnitude obtained for each particular elemental concentration, as well as on the proximity of each elemental concentration to the detection limit of the analyzing device. For elements with different orders of magnitude (e.g., 200,000 μg/g versus 2 μg/g), the fluctuation between two measurements will always be lower in the former than in the latter. That is why lowest standard deviations were found in the main bone components (Ca and P) whose concentrations are expressed in hundreds of thousands, while the minerals with lower concentrations (trace elements) display the highest.
Chemical Differences Between Femoral Regions
The distribution of chemical elements along the bone varies [11, 13, 14], detecting higher concentrations of Ca, P, Zn and S in the midpoint of the femoral diaphysis in the current sample. However, it is important to consider that the differences in concentration among both femoral regions, the neck and the midshaft, may be influenced by the characteristics of the surfaces analyzed [29].
To ensure its biomechanical suitability, the femoral neck is structurally made up of abundant trabecular tissue adapted to respond to body load, covered by a thin cortical layer, in reverse of what occurs in the midshaft [31]. Trabeculae have a higher mineral content, an increased metabolic activity, and a faster turnover rate compared to the cortical bone [6, 14], although the latter is less susceptible to diagenesis. For these reasons, the femoral neck has a greater predisposition to fragility fractures than the midshaft [25]. Since the measurements were made only on the external cortical of both regions, the differential concentration of Ca, P, Zn and S may be attributed to the disparity in cortical bone thickness [29], a parameter that is lower in osteoporosis. However, P levels are significantly lower in osteoporosis whereas S levels are higher. Therefore, despite the differences caused by cortical thickness, it is feasible to establish a relationship between BMD and these elements.
Major Elements in Bone and the Ca/P Ratio as a Possible Biomarker of Bone Disease
The values obtained in this study for the major elements, Ca and P, are within the range reported from other studies performed in human bone samples [11, 15]. Calcium represents the core element of the musculoskeletal system, from which it is released when calcium homeostasis is disturbed [32]. In a short-term, bone compensates these deficiencies without any corporal visible effects [33], but can be harmful for the bone quality if persists over time [34]. Meanwhile, phosphorus has a structural, energetic, homeostatic and a cellular signalization role in the body [35]. Even though it is an essential and ubiquitous element, its bioavailability depends on its physical and chemical properties. In an inorganic state—food additives, carbonated drinks or ultraprocessed foods—it is more available than in an organic state—meat, fish, nuts, vegetables, etc.—[36]. The maintenance of mean serum phosphorus homeostasis entails an interaction between intestinal absorption, renal reabsorption and excretion, and redistribution between the extracellular phosphate, intracellular spaces, and the bone phosphate storage pool. Diet, aging, and chronic disease impact the control of serum P [37]. Animal studies have shown that an excess of P in the diet reduces BMD [38], suggesting that P may play a particularly important role in bone health, maintaining it at in appropriate concentrations to prevent bone alterations.
Taking together, Ca/P ratio in humans fluctuates between 1.74 and 4.78, with an average value in the femur of 2.14 [39], similar to both the stoichiometric ratio of hydroxyapatite (2.16) and that obtained in the present work (neck: 2.12 ± 0.21, midshaft: 1.94 ± 0.14). According to the results, calcium and phosphorus are associated in different proportions in femoral bone, so the latter is more concentrated in the midshaft than in the neck (see Table 3). Also, in Tables 4 and 5 it is possible to observe how the concentration of P element in bone decreases with age, as stated by [9], and with OP, as according to Santos et al. [12]. This reflects in the Ca/P ratio, whose values are higher in women over 50 years of age and in osteoporosis, not due to fluctuations in calcium levels, which remain stable, but in phosphorus. This is in contrast to the results obtained in osteoporotic rabbits where this ratio usually decrease [39], as well as regarding the lack of consensus about its age-related changes [40]. The metabolism of Ca and P is coregulated through an integrated hormonal system that regulates transport in the gut, kidney, and bone, and operate in cohort with osteoblasts, osteocytes, and extracellular matrix proteins in order to mineralize osteoid as it is being deposited in the skeleton. Therefore, since the increased bone resorption that characterizes the pathogenesis of osteoporosis should similarly affect the values of calcium and phosphorus, factors other than disease and cellular processes must be considered.
The decrease in the concentration of phosphorus is inversely related to the increase in the concentration of the element S, both of which are anions with the possibility of inter-exchange in the hydroxyapatite molecule. This hypothesis supports previous studies that referred to the Ca/P ratio as a sensitive indicator of bone mineral health [41] and a useful tool when studying major bone elements and bone quality, prompting its inclusion in future clinical research. Nevertheless, this study warns that perhaps calcium is not the main target in the loss of bone mass experienced in osteoporosis. On the contrary, phosphorus takes on a much more variable role in anatomical, age and disease terms, being susceptible to changes experienced by less abundant mineral elements such as sulfur.
Biological Role of Minor Elements in Bone Quality
Calcium and P dynamics do not explain all the discrepancies between the different groups, implying that these elements are not the only ones involved in bone health maintenance. Focusing on the LDA analysis (Fig. 2a), it is possible to appreciate how different elements are clustered in the F1 dimension according to their BMD. Although there is some overlap among the data, this may be explained by the artificial division of the BMD s.d. into cut-off points: ≤ 1 s.d., > 1 s.d. and ≥ 2,5 s.d. for normal, osteopenia and osteoporosis categories from the World Health Organization standards [26].
On one hand, Zn also emerges as an element which correlates positively with BMD (Fig. 2c). This element appears in greater concentration in the midshaft than in the neck, probably due to its relationship with the Haversian canals [30]. Its progressive reduction with age and the loss of BMD in both regions (Tables 3 and 4), together with its position in the correlation circle (Fig. 2c) corroborate its relevance to bone health [42]. Zinc deficiencies lead to bone abnormalities, as well as osteopenia, as it has an osteoblast-stimulating and osteoclast-inhibiting function [32].
On the other hand, Pb and S concentrations have been identified with a clear tendency to cause bone damage. The references about Pb are consistent regarding its toxicity, its osteolytic nature [32], its ionic competition with Ca and P [6], and its increase after menopause [43]. Bone concentration of Pb usually range between 0.29 μg/g [10] and 93 μg/g [44], an interval which enclosed the average value (Tables 2, 3 and 4) obtained in this study. Sulphur is a component of proteoglycans in bones and, in a minor proportion, also of collagen. Its concentration has been described from 873 [11] to 2760 μg/g [10] and in this study average is considerably higher (Tables 3, 4 and 5) than described. Slight oscillations by excess have been pointed out as being responsible for weak bone quality and osteoporosis [45].
Final Remarks
The interest in the pathophysiology of bone tissue is transversal to several scientific disciplines and the intricate multifactorial relationship behind mineral oscillations must be approached conjointly. In this work, the usefulness of pXRF has been demonstrated by the easy and fast application for the compositional profiling of cortical dry bone in the process of BMD loss. However, a disadvantage of this method for the clinical practice is the fact that its application resides in ex vivo samples. Nevertheless, the present study has demonstrated that dry bone from an identified skeletal collection can be a valuable source of information for the epidemiological knowledge and also give new insights to the prognosis of the bone mass loss. In parallel, the usefulness of pXRF has been demonstrated by the easy and fast application for the compositional profiling of cortical dry bone in the process of BMD loss.
Based on this investigation, the Ca/P ratio and, specifically, the changes in P concentrations shown in the cortex of the femoral neck and the midshaft appear effective predictors of osteoporosis-related loss of bone mineral density in contrast to Ca fluctuations. Other elements such as Zn play a role in osteoprotective action, while S and Pb have an opposite effect. Lead (Pb) displays concentrations almost double in femora from osteoporotic females.
For future analysis, it will be recommended to expand this application to a cohort of male individuals to better understand between sex changes in femoral cortical bone elemental composition with age and osteoporosis, analyze the elemental bone spectrum in other skeletal regions also affected by osteoporosis-related bone loss and fracture risk, such as lumbar vertebrae, and compare possible divergences that may arise in the involvement of calcium turnover.
Data Availability
Available upon request.
Abbreviations
- pXRF:
-
Portable X-Ray Fluorescence
- BMD:
-
Bone mineral density
- rTEM:
-
Relative technical measurement error
- DXA:
-
Dual-energy X-ray absorptiometry
- OP:
-
Osteoporosis
- CSIC:
-
Coimbra Identified Skeletal Collection
- s.d.:
-
Standard deviation
References
NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. J Am Med Assoc 285:785–795. https://doi.org/10.1001/jama.285.6.785
Svedbom A, Hadji P, Hernlund E et al (2019) Cost-effectiveness of pharmacological fracture prevention for osteoporosis as prescribed in clinical practice in France, Germany, Italy, Spain, and the United Kingdom. Osteoporos Int 30:1745–1754. https://doi.org/10.1007/s00198-019-05064-w
Osterhoff G, Morgan EF, Shefelbine SJ et al (2016) Bone mechanical properties and changes with osteoporosis. Injury 47:S11–S20. https://doi.org/10.1016/S0020-1383(16)47003-8
Greenwood C, Clement J, Dicken A et al (2018) Age-related changes in femoral head trabecular microarchitecture. Aging Dis 9:976–987. https://doi.org/10.14336/AD.2018.0124
Ratnayake JTB, Mucalo M, Dias GJ (2017) Substituted hydroxyapatites for bone regeneration: a review of current trends. J Biomed Mater Res B 105:1285–1299. https://doi.org/10.1002/jbm.b.33651
Bronner F (2002) Metals in bone: aluminum, boron, cadmium, chromium, lead, silicon, and strontium. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principles of bone biology, 2nd edn. Academic Press, Elsevier, pp 359–369
Zaichick S, Zaichick V (2010) The effect of age and gender on 38 chemical element contents in human iliac crest investigated by instrumental neutron activation analysis. J Trace Elem Med Biol 24:1–6. https://doi.org/10.1016/j.jtemb.2009.07.002
Specht AJ, Weisskopf M, Nie LH (2014) Portable XRF technology to quantify Pb in bone in vivo. J Biomarkers 2014:1–9. https://doi.org/10.1155/2014/398032
Nganvongpanit K, Buddhachat K, Brown JL (2016) Comparison of bone tissue elements between normal and osteoarthritic pelvic bones in dogs. Biol Trace Elem Res 171:344–353. https://doi.org/10.1007/s12011-015-0556-4
Noor Z, Sumitro SB, Hidayat M et al (2012) Atomic mineral characteristics of Indonesian osteoporosis by high-resolution inductively coupled plasma mass spectrometry. Sci World J 1:6. https://doi.org/10.1100/2012/372972
Santos C, Fonseca M, Corregidor V et al (2014) Elemental distribution in human femoral head. Nucl Instrum Methods Phys Res B 331:266–270. https://doi.org/10.1016/j.nimb.2014.01.032
Nganvongpanit K, Buddhachat K, Klinhom S et al (2016) Determining comparative elemental profile using handheld X-ray fluorescence in humans, elephants, dogs, and dolphins: preliminary study for species identification. Forensic Sci Int 263:101–106. https://doi.org/10.1016/j.forsciint.2016.03.056
Zaichick V (2006) INAA of Ca, Cl, K, Mg, Mn, Na, P, and Sr contents in the human cortical and trabecular bone. J Radioanal Nucl Chem 269:653–659. https://doi.org/10.1007/s10967-006-0281-8
Brätter P, Gawlik D, Lausch J, Rösick U (1977) On the distribution of trace elements in human skeletons. J Radioanal Chem 37:393–403. https://doi.org/10.1007/BF02520545
Kuo HW, Kuo SM, Chou CH, Lee TC (2000) Determination of 14 elements in Taiwanese bones. Sci Total Environ 255:45–54. https://doi.org/10.1016/S0048-9697(00)00448-4
Zaichick V (2013) Data for the reference man: skeleton content of chemical elements. Radiat Environ Biophys 52:65–85. https://doi.org/10.1007/s00411-012-0448-3
Mahanti HS, Barnes RM (1983) Determination of major, minor and trace elements in bone by inductively-coupled plasma emission spectrometry. Anal Chim Acta 151:409–417. https://doi.org/10.1016/S0003-2670(00)80103-8
Tranquilli AL, Lucino E, Garzetti GG, Romanini C (1994) Calcium, phosphorus and magnesium intakes correlate with bone mineral content in postmenopausal women. Gynecol Endocrinol 8:55–58. https://doi.org/10.3109/09513599409028459
Chotiyarnwong P, McCloskey EV (2020) Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol 16:437–447. https://doi.org/10.1038/s41574-020-0341-0
Dick IM, Prince RL (2001) The effect of estrogen on renal phosphorus handling in the rat. Am J Nephrol 21:323–330. https://doi.org/10.1159/000046269
Shen G, Zhang Y, Hu S et al (2017) Adult-onset hypophosphatemic osteomalacia associated with Sjogren syndrome: clinical case report. Medicine (United States) 96:e6493. https://doi.org/10.1097/MD.0000000000006493
Cunha E, Wasterlain S (2007) The Coimbra identified osteological collections. In: Grupe G, Peters J (eds) Skeletal Series in their Socioeconomic Context. M. Leidorf, Rahden/Westf., pp 23–33
Navega D, Coelho J, d. O, Cunha E, Curate F (2018) DXAGE: a new method for age at death estimation based on femoral bone mineral density and artificial neural networks. J Forensic Sci 63:497–503. https://doi.org/10.1111/1556-4029.13582
Bergot C, Wu Y, Jolivet E et al (2009) The degree and distribution of cortical bone mineralization in the human femoral shaft change with age and sex in a microradiographic study. Bone 45:435–442. https://doi.org/10.1016/j.bone.2009.05.025
Curate JFT, Albuquerque A, Correia J et al (2013) A glimpse from the past: osteoporosis and osteoporotic fractures in a portuguese identified skeletal sample. Acta Reumatol Port 38:20–27
Organization WH (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992]
Catarino L, Gil FPSC, Quinta-Ferreira M, Marques F (2018) Characterization and rehabilitation of the “Porta Férrea” stone materials, University of Coimbra, Portugal. Environ Earth Sci 77:416–429. https://doi.org/10.1007/s12665-018-7587-z
Fahy GE, Deter C, Pitfield R et al (2017) Bone deep: variation in stable isotope ratios and histomorphometric measurements of bone remodelling within adult humans. J Archaeol Sci 87:10–16. https://doi.org/10.1016/j.jas.2017.09.009
Byrnes JF, Bush PJ (2016) Practical considerations in trace element analysis of bone by portable X-ray fluorescence. J Forensic Sci 61:1041–1045. https://doi.org/10.1111/1556-4029.13103
Pemmer B, Roschger A, Wastl A et al (2013) Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone 57:184–193. https://doi.org/10.1016/j.bone.2013.07.038
Grupe G (1988) Impact of the choice of bone samples on trace element data in excavated human skeletons. J Archaeol Sci 15:123–129. https://doi.org/10.1016/0305-4403(88)90002-7
Dermience M, Lognay G, Mathieu F, Goyens P (2015) Effects of thirty elements on bone metabolism. J Trace Elem Med Biol 32:86–106. https://doi.org/10.1016/j.jtemb.2015.06.005
Soni G, Kaur Kochar G, Gurpreet K (2018) Beneficial effect of calcium supplementation on bone mineral density of calcium deficient adolescents. Int J Food Sci Biotechnol 3:83–88. https://doi.org/10.11648/j.ijfsb.20180303.12
Rockell JEP, Williams SM, Taylor RW et al (2005) Two-year changes in bone and body composition in young children with a history of prolonged milk avoidance. Osteoporos Int 16:1016–1023. https://doi.org/10.1007/s00198-004-1789-9
Mahdi AA, Brown RB, Razzaque MS (2015) Osteoporosis in populations with high calcium intake: does phosphate toxicity explain the paradox? Indian J Clin Biochem 30:365–367. https://doi.org/10.1007/s12291-015-0524-y
Calvo MS (1993) Dietary phosphorus, calcium metabolism and bone. J Nutr 123:1627–1633. https://doi.org/10.1093/jn/123.9.1627
Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG (2008) Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 40:82–91. https://doi.org/10.1080/07853890701689645
Krishnarao GVG, Draper HH (1972) Influence of dietary phosphate on bone resorption in senescent mice. J Nutr 102:1143–1145. https://doi.org/10.1093/jn/102.9.1143
Kourkoumelis N, Balatsoukas I, Tzaphlidou M (2012) Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J Biol Phys 38:279–291. https://doi.org/10.1007/s10867-011-9247-3
Zaichick V, Tzaphlidou M (2003) Calcium and phosphorus concentrations and the calcium/phosphorus ratio in trabecular bone from the femoral neck of healthy humans as determined by neutron activation analysis. Appl Radiat Isot 58:623–627. https://doi.org/10.1016/S0969-8043(03)00092-7
Tzaphlidou M (2008) Bone architecture: collagen structure and calcium/phosphorus maps. J Biol Phys 34:39–49. https://doi.org/10.1007/s10867-008-9115-y
Al-Timimi D, Al-Dabbagh S, Al-Timimi DJ et al (2017) Zinc status as a risk of osteoporosis. Int J Pharma Res Heal Sci 5:1686–1689. https://doi.org/10.21276/ijprhs.2017.02.16
Potula V, Kaye W (2006) The impact of menopause and lifestyle factors on blood and bone lead levels among female former smelter workers: The Bunker Hill Study. Am J Ind Med 49:143–152. https://doi.org/10.1002/ajim.20262
Crawford MD, Crawford T (1969) Lead content of bones in a soft and a hard water area. Lancet 293:699–701. https://doi.org/10.1016/S0140-6736(69)92649-X
Thorpe M, Mojtahedi MC, Chapman-Novakofski K et al (2008) A positive association of lumbar spine bone mineral density with dietary protein is suppressed by a negative association with protein sulfur. J Nutr 138:80–85. https://doi.org/10.1093/jn/138.1.80
Acknowledgements
This study was supported by the ERASMUS+ internship [SZ] performed in the Research Centre for Anthropology and Health (CIAS: PEstOE/SADG/UI0283/2020) at the University of Coimbra and the FCT-Fellowship SFRH/BD/115691/2016 [AMC]. The authors express their gratitude to the Department of Life Sciences and to Sofia Wasterlain for authorizing the study of the identified skeletal collection, to the Department of Earth Sciences, Geosciences Center (CGeo) for the use of the pXRF device, and to Carlos Martínez-Toledano for the achievement of the illustrations. The authors also acknowledge the editorial board members and the two anonymous reviewers who improved the content of this paper through their suggestions and comments.
Funding
FCT-Fellowship SFRH/BD/115691/2016 [AMC], Erasmus + Program [SZ].
Author information
Authors and Affiliations
Contributions
SZ, AMC and ALS designed research and analyzed data. LC and FC performed research and analyzed data. SZ and AMC wrote the manuscript and SZ, AMC, LC, FC and ALS edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Sofía Zdral, Álvaro M. Monge Calleja, Lidia Catarino, Francisco Curate, and Ana Luisa Santos declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zdral, S., Monge Calleja, Á.M., Catarino, L. et al. Elemental Composition in Female Dry Femora Using Portable X-Ray Fluorescence (pXRF): Association with Age and Osteoporosis. Calcif Tissue Int 109, 231–240 (2021). https://doi.org/10.1007/s00223-021-00840-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00840-5