Abstract
Menopause is associated with bone loss. Prebiotics increase Ca, inorganic phosphorus (Pi), and Mg absorption, improving bone health. These increases would supply an extra amount of minerals, decreasing bone resorption and possibly reversing ovariectomy-induced bone loss. The present experimental study sought to evaluate the effect of adding a prebiotic GOS/FOS® mixture to a normal or a low Ca diet on Ca, Pi, and Mg absorption, in osteopenic rats. Four groups of n = 8 rats each were OVX, and 8 rats were SHAM operated. All rats were fed a commercial diet for 45 days. They were then fed one of the following diet for 45 days: C-0.5%: SHAM fed AIN 93 M containing 0.5%Ca; O-0.5% and O-0.3%: OVX rats fed AIN 93 M, containing 0.5% or 0.3%Ca, respectively; GF-0.5% and GF-0.3%: OVX rats fed AIN 93 M, containing 0.5% or 0.3%Ca+ 2.5% GOS/FOS®, respectively. At the end of the experimental time point, Ca, P, and MgAbs% was significantly higher in GF-0.5% and GF-0.3% as compared to the remaining groups (p < 0.01). Irrespective of diet Ca content, CTX decreased whereas femur Ca and P content, tibia BV/TV and GPC.Th, lumbar spine and proximal tibia BMD, bone strength, bone stiffness, and elastic modulus increased in the GF-0.5% and GF-0.3% groups as compared to O-0.5% and O-0.3%, respectively (p < 0.05). This prebiotic mixture would be a useful tool to prevent the increase in bone loss associated with menopause and aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone loss is the result of an imbalance between bone resorption and bone formation that increases bone loss, decreases bone mineral density (BMD), and increases susceptibility to osteoporotic fractures. The severity of bone loss usually increases with age and is most prevalent in postmenopausal women. Endocrine and dietary factors play an important role not only in the development but also in the prevention of bone loss and osteopenia/osteoporosis. Estrogen stimulates intestinal calcium (Ca) transporters and enhances the renal production of 1,25-dihydroxyvitamin D (1,25diOHD), the active metabolite of vitamin D and cofactor required for Ca absorption (CaAbs) in the gastrointestinal tract [1]. Moreover, estrogen negatively regulates the production of pro-osteoclastogenic cytokines (such as IL-6 and IL-1), inhibiting osteoclast generation and inducing osteoclast apoptosis. Estrogen withdrawal impairs CaAbs and markedly increases osteoclastic activity and bone remodeling; however, bone formation can not be maintained at the same accelerated rate as resorption. This relative deficit in bone formation as compared to bone resorption increases bone loss and decreases BMD [2]. The ovariectomized (OVX) rat model has been approved by the Food and Drug Administration (FDA) as a preclinical model for studying postmenopausal osteoporosis. Removal of the ovaries declines estrogen production and increases bone remodeling and bone loss [3, 4]. In this regard, previous studies conducted by our research group have shown OVX rats to lose approximately 20% of their bone mass, becoming osteopenic 40 days post-ovariectomy [5].
According to the literature, Ca consumption of a great percentage of the population is far below the recommended amounts, regardless of socioeconomic status [6]. Habitual low Ca intake decreases serum levels of Ca, which induces the release of parathyroid hormone (PTH), increasing the rate of bone turnover and bone loss. Ca obtained from diet would seem to be better than Ca obtained from supplements to correct a low Ca intake. Firstly, Ca supplements acutely increase serum Ca concentration, which reduces the levels of PTH and bone resorption markers, without cumulative benefits in bone mass and osteoporosis prevention [7]. Moreover, although the link between the use of Ca supplements and a higher risk of cardiovascular risk remains controversial and is not endorsed by professional societies such as the National Osteoporosis Foundation and the American Society for Preventative Cardiology [8], it has been proposed that Ca supplement may increase blood Ca levels more quickly than dietary Ca [9]. This increase could lead to greater deposition of Ca in coronary arteries and an increased risk of coronary heart disease. In addition, some people experience gastrointestinal side effects from Ca supplement, which contributes to low compliance [10, 11]. Finally, absorbability of Ca supplements is generally lower than that of dietary Ca from milk or dairy products. Several components of milk maintain Ca in solution, favoring Ca absorption. Milk also provides a simultaneous intake of inorganic phosphorus (Pi), which is essential to bone health [10]. The use of some functional ingredients that improve CaAbs, as opposed to Ca supplementation in tablet form, become a key strategy for optimizing CaAbs and for increasing Ca retention in bone.
We previously demonstrated that during growth a combination of short-chain galacto-oligosaccharides (GOS), the bioactive component of human milk, and long-chain fructo-oligosaccharides (FOS) exhibited prebiotic properties that increased intestinal Abs of Ca and other minerals, which are essential for bone health [4, 5]. These prebiotic properties were evidenced by a twofold increase in cecal wall weight, an increase in lactobacilli growth, and a considerable decrease in cecal pH. It is important to point out that the prebiotic GOS/FOS mixture contains two types of sugars of different chain lengths that induce sequential fermentation in the proximal colon (the short-chain sugar) and in the distal parts of the colon (the long-chain sugar) [12]. As a result, the GOS/FOS mixture was found to increase the efficacy of mineral absorption in a large part of the large intestine [13]. Based on the above, the hypothesis of the present experimental study in a model of postmenopausal osteopenia was that consumption of this prebiotic mixture could increase Ca, phosphate (P), and magnesium (Mg) absorption. This effect would lead to increased availability of minerals, which in turn would prevent further increases in bone resorption, preserving not only bone mass but also bone architecture, due to the simultaneous increase in Ca and Mg absorption. The aim of the present experimental study was to evaluate the effect of adding a prebiotic GOS/FOS mixture (GOS/FOS®) to a normal and a low Ca diet on Ca, Pi, and Mg absorption and on several aspects of bone health, including resorption, retention and biomechanical properties of bone, using an experimental model of postmenopausal osteopenia.
Materials and Methods
Diets
All experimental diets were isocaloric by formulation and were prepared according to the American Institute of Nutrition Rodent Diets Recommendations for maintenance settled in 1993 (AIN-93M) [14]. CaCO3 (Analytical grade, Anedra, Argentina) was added to obtain the two dietary levels of Ca: 0.5% (NCa) or 0.3% (LCa). Diets containing prebiotics were prepared by adding 5.3 g% of a mixture of GOS/FOS® (9:1) (NV Nutricia) (batch No 110710 and HPPGJ1AGJ, respectively) (Table 1).
Experimental Design
A total of 40 aged virgin female Wistar rats, approximately 2 months of age (195.0 ± 9.0 g), were supplied by the Laboratory Animal Service of the Oral Biochemical Department, School of Dentistry, Buenos Aires University (Argentine). The animals were allowed free access to deionized water and food and were housed in individual stainless steel cages in a temperature (21 ± 1 °C) and humidity (60 ± 10%) controlled room under a 12-h-light/dark cycle throughout the entire experimental period.
Thirty-two rats were OVX by a dorsal approach under light anesthesia (0.1 mg/100 g body weight (BW) of ketamine hydrochloride + 0.1 mg/100 g BW of acepromazine maleate) (Holliday-Scott S A, Buenos Aires, Argentina) and fed a commercial diet for 45 days in order to induce bone loss and osteopenia. Control rats (n = 8) were SHAM operated and fed the same commercial diet for 45 days.
After this period, SHAM rats were fed the AIN 93M diet, whereas OVX rats were randomly assigned to one of the following groups (n = 8/group) for an additional period of 45 days (Table 1).
-
C-0.5%: SHAM rats fed a semisynthetic diet prepared according to the American Institute of Nutrition Diet (AIN 93 M) [13] containing 0.5% Ca.
-
O-0.5%: OVX rats fed AIN 93 M, containing 0.5% Ca.
-
O-0.3%: OVX rats fed AIN 93 M, containing 0.3% Ca.
-
GF-0.5%: OVX rats fed AIN 93 M, containing 0.5% Ca + 2.5% GOS/FOS®.
-
GF-0.3%: OVX rats fed AIN 93 M, containing 0.3% Ca + 2.5% GOS/FOS®.
The diets were offered as powder. Food consumption and body weight (BW) were recorded twice a week.
Tibia weight was measured “ex vivo” at the end of the study. Fresh fecal samples were obtained weekly. Densitometry analysis was performed at T = 50. Fasting blood samples were collected under light anesthesia at the end of the study, and the serum samples were stored at − 20 °C until assay. The animals were then killed by CO2 inhalation and cecum, femurs, and tibiae were resected in order to perform histological, biochemical, and biomechanical studies.
Fecal and Cecum Determinations
Fresh feces were directly obtained by rectal stimulation, transferred immediately to sterile tubes, and stored at 4 °C until analysis. Fecal samples were homogenized and diluted in 0.1M phosphate buffer (containing 0.5% cysteine). An aliquot was poured onto selective Lactobacilli MRS-agar (Britania, Argentina) and incubated at 37 °C under an anaerobic atmosphere (5–10% CO2) for 48 h. Lactobacillus (LS) colonies were counted, and the number of colony forming units (CFU) was expressed as log CFU per gram of feces.
A second aliquot was used to assess activity of fecal enzymes, as described elsewhere [4]. Briefly, β-glucosidase activity was evaluated using p-nitrophenyl β-D-glucopyranoside as substrate (Sigma, USA), and β-glucuronidase activity was assessed using p-nitrophenyl-β-D-glucuronide as substrate (Sigma, USA). Tryptophanase activity was assayed using tryptophan as substrate, and urease activity was determined using urea as substrate. The reactions were measured by spectrophotometry at 405 nm, 550 nm and 610 nm, respectively (Metrolab 2100, Argentina).
At the end of the study, the caeca were excised, weighed and split open; pH was directly recorded by inserting a glass electrode into the cecum content (Adwa AD110, Hungary).
Ca, Pi, and Mg Absorption
At the beginning of the experimental period and during the last 3 days of the study, the animals were housed individually in plastic metabolic cages. Food consumption (I) was determined, and feces (F) were collected in order to calculate apparent mineral absorption (Abs) (mg/d). Apparent absorption, expressed as a percentage of intake (Abs %), was calculated according to the following equation: Abs % = (I−F/I) 100. Feces were dried under infrared light and pounded. Diets and feces were digested with nitric acid, and Ca, P, and Mg content was evaluated using Parr bombs [15].
Femur Analysis
The left femurs were cleaned of soft tissue, dried in an oven at 100 °C for 72 h, and defatted by immersion in a chloroform–methanol (3:1) solution for 2 weeks. Defatted samples were dried at 100 °C and weighed (Denver instrument, USA). Length was measured using a Vernier caliper (VIS, Poland). The defatted femurs were digested in a glass tube containing a mixture of HCl–HNO3 (1:1) to evaluate Ca, Mg, and Pi.
Biochemical Determinations
Ca concentration in serum, feces, diet, and femur was determined by atomic absorption spectrophotometry. Lanthanum chloride (6500 mg/L in the final solution) was added as interference suppressor. Pi and Mg concentration in serum, feces, diet, and femur was evaluated by conventional methods, using an automated analyzer (Abbott Laboratories, Abbott Park, IL, USA). Serum bone alkaline phosphatase (BAP) (IU/L) was measured using a colorimetric method after bone isoenzyme precipitation with wheat-germ lectin [16]. Serum CTX (ng/mL) was assessed employing immunoassay (ELISA) (Rat Laps. Osteometer. BioTech, Herlev, Denmark).
Densitometry
At the beginning and at the end of the study, total skeleton bone mineral content (tsBMC) and bone mineral density (tsBMD) were determined “in vivo” by dual energy X-ray absorptiometry (DXA) under light anesthesia (0.1 mg ketamine hydrochloride/100 g BW and 0.1 mg acetopromazine maleate/100 g BW). A whole body scanner and software designed specifically for small animals (DPX Alpha, Small Animal Software, Lunar Radiation Corp. Madison WI) were used as described in a previous report [17]. Briefly, all rats were scanned using an identical scanning procedure. Precision was assessed by measuring one rat five times, repositioning between scans, on the same day and on different days [18]. The coefficients of variation (CV) for BMC and BMD were 3.0 and 0.9%, respectively.
The different subareas were analyzed on the image of the animal on the screen using a region of interest (ROI) for each segment. CVs were 1.8% for lumbar spine, 0.8% for femur and 3.5% for proximal tibia.
Histology
Immediately after euthanasia, the right tibiae were resected, cleaned of soft tissue, weighed, and measured. The tibiae were fixed in 10% buffered formaldehyde solution for 48 h, decalcified in ethylene-diamine tetra-acetic acid (EDTA, Sigma), pH 7.4, for 30 days, and embedded in paraffin. One 8- to 10-µm-thick longitudinally oriented section of subchondral bone, including primary and secondary spongiosa, was obtained at the level of the middle third and stained with hematoxylin-eosin. The section was microphotographed (AXIOSKOP, Carl ZEISS) to perform histomorphometric measurements on the central area of the metaphyseal bone displayed on the digitalized image (Image pro plus 4.5). Bone volume fraction (BV/TV) (%): the percentage of cancellous bone within the total measured area and total width of epiphyseal cartilage (GPC.Th) were measured according to Parfitt et al [19].
Biomechanical Analysis
The right femurs were excised, cleaned of soft tissues, weighed, and frozen (− 20 °C) until analysis. Bone breaking strength, elastic modulus, and stiffness were measured using a three point bending test (Instron, 4411). The load was applied perpendicularly to the long axis, at the mid-length region of the femur (displacement rate of 0.01 mm/s, sampling rate of 100 Hz). The distance between the supporting points was 10 mm.
Statistical Methods
Results were expressed as mean ± standard deviation (SD). Normality of variables was evaluated using the Shapiro Wilk test, and homogeneity of variances was assessed by Levene’s test. Data with a normal distribution were analyzed using one-way analysis of variance (ANOVA). Nonparametric data (count of LS) were analyzed using the Kruskal–Wallis test.
Results
Zoometric Parameters
All animals remained in good health and showed no signs of diet-related side effects, such as diarrhea, throughout the study. Regarding internal organs, no significant differences were found among groups, except for an increase in the size of the colon (data not shown) and in cecum weight in the prebiotic groups, irrespective of diet Ca content (p < 0.05) (Table 2).
No significant differences in food consumption were observed among the five studied groups throughout the study. No significant differences in BW (g) were observed among groups at the beginning of the study, or among the 4 OVX groups at the end of the study, irrespective of prebiotic and Ca content. At the end of the study, BW was significantly lower in the SHAM group than in the OVX groups (p = 0.059) (Table 2).
Prebiotic Effect
At T = 0, no differences in LS counts in fresh feces were observed among the five studied groups. This parameter was significantly increased in GF-0.5% and GF-0.3% as compared to the other studied groups (p < 0.05) from the first week of prebiotic consumption to the end of the study. No differences were observed between GF-0.5% and GF-0.3%, or among C-0.5%, O-0.5%, and O-0.3% (Table 2).
Cecum pH was significantly lower in GF-0.5% and GF-0.3% as compared to the other studied groups (p < 0.05), and no differences were observed between the GF-0.5% and GF-0.3% groups; no differences were observed among SHAM, O-0.5%, and O-0.3% groups (Table 2).
At T = 0, no differences in the activity of the studied enzymes were observed among the five studied groups. At the end of the study, β-glucosidase activity was significantly higher, whereas β-glucuronidase, urease, and tryptophanase activity was significantly lower in GF-0.5% and GF-0.3% than in the remaining groups (p < 0.05). No differences were observed between the GF-0.5% and GF-0.3% groups or among SHAM, O-0.5%, and O-0.3% groups (Table 2).
Biochemical Determinations
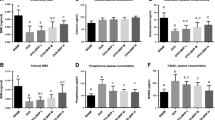
At the end of the study, no differences in serum Ca, P, Mg, total protein and albumin levels were observed among the five studied groups. Consumption of the prebiotic mixture had no significant effects on BAP levels. The highest significant CTX levels were observed in the O-0.3% group (p < 0.05). Irrespective of diet Ca content, prebiotic consumption decreased CTX; the observed decrease only reached statistical significance when comparing GF-0.3% and O-0.3% (p < 0.05). Only GF-0.5% presented CTX levels similar to those of the SHAM group, the remaining GF-0.3%, O-05% and O-0.3% groups had significantly higher CTX levels than the SHAM group (p < 0.05) (Table 3).
Mineral Absorption
No differences in daily food consumption were observed among the five studied groups during the two balance periods (Table 4). CaI was directly related to diet Ca content, whereas Pi and Mg intake was similar in all studied groups.
At T = 0, CaAbs expressed as mg/d was higher in the O-0.5% and GF-0.5% groups as compared to the O-0.3% and GF-0.3% groups, respectively (p < 0.05). No differences in MgAbs or PiAbs were observed between any of the studied groups (Table 4). At the end of the study, fecal Ca, Mg, and Pi excretion was significantly lower, and their corresponding Abs (mg/d) were significantly higher in GF-0.5% and GF-0.3% as compared to the O-0.5% and O-0.3% groups, respectively (p < 0.05) (Table 4).
At T = 0, no significant differences in the percentage of Ca, Mg, and Pi Abs (Abs %) were observed between any of the O groups (Table 4). At the end of the study, Ca, Mg, and Pi Abs % was significantly higher in GF-0.5% and GF-0.3% as compared to the remaining groups (p < 0.05).
Bone Analysis
Almost all the studied bone parameters were lower in O-0.3% and GF-0.3% as compared to their respective 0.5% Ca counterparts at the end of the study (Table 5).
GF-0.5% and GF-0.3% showed an increase in femur Ca and Pi content as compared to O-0.5% and O-0.3%, respectively (p < 0.05), and only GF-0.5% reached SHAM values. GF-0.3% almost reached O-0.5% values (p = 0.058). Group O-0.3% exhibited the lowest femur Ca and Pi content (p < 0.05). Femur Mg content was not modified by prebiotic consumption, diet Ca content, or OVX (Table 5).
Irrespective of diet Ca content, consumption of the prebiotic mixture increased tsBMC/BW. However, only GF-0.5% reached the tsBMC/BW observed in the SHAM group; O-0.3% showed the lowest value (p < 0.05), and GF-0.3% reached values observed in group O-0.5%. Total skeleton BMD was unaffected by ovariectomy and by prebiotic consumption and was slightly affected by diet Ca content. Lumbar spine and proximal tibia BMDs were increased by prebiotic consumption (p < 0.05). GF-0.3% reached similar lumbar spine and proximal tibia BMD values as O-0.5% (Table 5).
BV/TV and GPC.Th were significantly lower in O-0.3% and GF-0.3% than in their O-0.5% and GF-0.5% counterparts. Regardless of diet Ca content, consumption of the prebiotic mixture increased tibia BV/TV and GPC.Th as compared to their respective O- groups, and both parameters were significantly lower than SHAM values (p < 0.05) (Table 5). The lowest BV/TV value was observed in the O-0.3% group (p < 0.05). Figure 1 shows the higher trabecular number in GF-0.5% and GF-0.3% as compared to O-0.5% and O-0.3%, respectively, regardless of Ca content; nevertheless, GF-0.5% and GF-0.3% did not reach trabecular number observed in SHAM animals.
Irrespective of diet Ca content, bone strength, stiffness, and elastic modulus were increased in GF-0.5% and GF-0.3% as compared to O-0.5% and O-0.3%, respectively (p < 0.05). Only GF-0.5% animals reached SHAM bone strength, stiffness, and elastic modulus values, and all three parameters were similar in GF-0.3% and O-0.5%. All three biomechanical parameters were lowest in the O-0.3% group (Table 5).
Discussion
As observed in previous studies on growth conducted by our research team, the results shown here evidence that consumption of the GOS/FOS® prebiotic mixture enhanced Ca, Pi, and MgAbs, and benefitted bone retention in an animal model of postmenopausal osteopenia associated with estrogen deficiency.
The diets assayed here were isocaloric and supplied the same percentage of protein, lipids, and carbohydrate. Moreover, the presence of GOS/FOS in the diet did not affect diet consumption or BW gain. Indeed, irrespective of the prebiotic and Ca content of the diet, both food consumption and efficiency, defined as weight gained per calorie ingested, were similar in all OVX groups [20].
In our experimental model of postmenopausal osteopenia, an extra amount of Ca was necessary to modulate the increase in PTH levels, preventing further increases in bone turnover and bone loss. Although the highest absorption of bone-related-minerals occurs in the small intestine, about 5–10% of CaAbs could occur in the colon if the insoluble, unabsorbed intestinal mineral were maintained in an ionic form [21].
Prebiotics are non-digestible ingredients that benefit the subject’s health, providing a specific substrate for the growth and metabolism of beneficial gut microflora. The mechanisms likely to explain the favorable effects of fermentable carbohydrates on bacterial activities are linked to changes either in the composition or balance of the bacterial population, i.e., number of Bifidobacterium, or to a modification in the intestinal medium due to the release of SCFA, i.e., intestinal pH [22]. Previous studies in rats reported in the literature demonstrated that FOS used as a carbohydrate source induced the growth of Bifidobacterium and inhibited the growth of the potentially pathogenic bacteria Esherichia coli and Clostridium perfringens. It is of note, however, that GOS differs strongly from FOS since GOS was found not to affect the major bacterial group though it altered the activity of a number of enzymes [22]. Nevertheless, the effect of using any of these fermentable sugars was the same, i.e., an increase in SCFA acidification of luminal pH in the gut. The prebiotic mixture assayed here combines GOS and FOS, mimicking the molecular size distribution of human milk oligosaccharides. The combination of galacto- and fructo-oligosaccharides could benefit the host with a possible synergistic effect of consuming both sugars, in terms of Bifidobacterium and Lactobacilli growth, providing the more distal regions of the colon with a prebiotic substrate. A significant increase in the number of Bifidobacterium from feeding the GOS/FOS mixture studied here was reported in preterm, term, and weaning infants [23]. To our knowledge, the present report is the first to evaluate the effect of the GOS/FOS mixture on adults. Although Bifidobacterium was not evaluated in the present report, the obtained results showed that osteopenic rats fed the GOS/FOS mixture presented an increase in lactic acid bacteria growth, evidenced by the increase in Lactobacilli. In addition, the fermentation of the GOS/FOS mixture also showed changes in enzymatic activities, such as an increase in β-glucosidase activity and a decrease in the activity of β-glucuronidase, tryptophanase, and urease in fresh feces. SCFA was not determined; however, the enzymatic changes and the decrease in luminal pH indirectly confirm SCFA production [13, 24, 25]. Among SCFA, butyrate is used by the microbiota and serves as the primary energy source of colonocytes regulating gut cell growth and differentiation [26]. Although butyrate was not evaluated in the present report, the increase in cecum weight suggests a trophic effect of butyrate on cecum cells. Both effects, the lower luminal pH that maintains ionization of mineral salts and the proliferation of gut cells that increases the surface area of absorption, improve the active and/or the passive absorption of minerals that are essential to maintain bone health [27, 28].
CaAbs and bioavailability depends on age and on the luminal concentration of Ca. CaAbs has been found to decrease with age, reaching the lowest values in adult life. The adult osteopenic rats studied here were of similar age. However, they were fed diets that differed in Ca content, which could have affected lumen concentration of this mineral [29, 30]. It is well documented that when diet calcium content decreases, there is an adaptive increase in the fractional absorption of Ca. However, when the diet supplies a low concentration of Ca, the increment is insufficient to offset the loss in absorption and net CaAbs decreases [30]. Irrespective of diet prebiotic content, comparison of results corresponding to groups fed the NCa and LCa diets in the present study lends support to aforementioned findings, as shown by the observed increase in CaAbs % and the decrease in net CaAbs.
Studies using experimental models of bone loss prevention are controversial regarding the effectiveness of adding different prebiotics (oligofructose, polydextrose, or Synergy) to diets containing recommended amounts of Ca. Some authors reported that CaAbs was not affected [31, 32], whereas others observed an increase in CaAbs [30, 33]. The discrepancy among studies may partly be due to differences in the type of prebiotic, animal age, and the length of the study. To our knowledge, there are no previous experimental or clinical studies evaluating the effect of the assayed prebiotic mixture on mineral absorption and bone retention in an experimental model of postmenopausal osteopenia. In the present report, Ca, Pi, and MgAbs increased with prebiotic consumption irrespective of diet Ca content. The mechanisms responsible for the increase in CaAbs, as well as in Pi and MgAbs, are similar to those observed in our previous experimental studies in growing rats: an increase in lactobacilli colonies, a considerable decrease in cecal pH content, and an increase in cecal wall weight [34]. The higher Abs of Ca, Pi, and Mg from feeding the diet containing the prebiotic mixture supplied an extra amount of bony minerals associated with improving bone health.
For 5–10 years during and after menopause, women lose bone at a rate of 2–3% per year. During this period, bone loss is mostly due to estrogen deficiency, which decreases intestinal CaAbs and renal Ca re-absorption and increases PTH secretion and bone resorption [35]. There are a number of studies in the literature on the effect of prebiotics on CaAbs and bone loss prevention in OVX rats; conversely, we found no studies on the effect of prebiotics on CaAbs and bone resorption in rats with low bone mass. According to the results of the present study, the prebiotic mixture enhanced CaAbs percentage, irrespective of diet Ca content (LCa ~ 12%; NCa ~ 18%). The extra amount of Ca may have contributed to prevent a further increase in bone remodeling that could have led to further bone loss. Moreover, recent studies have demonstrated that the beneficial gut microbiota also modulates the immune system, which in turn regulates osteoclastogenesis and bone mass [36]. Osteoclastic bone resorption can be evaluated biochemically by measuring the levels of CTX, one of the most sensitive and specific markers of bone resorption. As expected, all the osteopenic OVX rats studied here showed higher CTX levels than SHAM animals; however, the GF-0.5% and GF-0.3% groups showed a decrease in CTX levels as compared to the O-0.5% and O-0.3% groups, respectively, evidencing a decrease in bone resorption. Such a decrease, together with the observed increases in femur Ca and Pi content, tibia trabecular number, bone volume, and GPC.Th, would explain the higher bone mass and density at the two areas rich in trabecular bone in the two GF groups, irrespective of diet Ca content. In addition, the absence of changes in total skeleton BMD could be explained by the composition of bone; in fact, 80% of the entire skeleton is cortical bone, which is metabolically less active than trabecular bone, found in the proximal tibia and spine.
Phosphorus is an essential macronutrient for numerous biologic processes, including bone mineralization and energy metabolism, and also provides the structural framework for DNA and RNA, and phospholipids in membranes. Approximately 85% of the body’s P is found in the bones, and the remainder is present in extracellular fluid (ECF), soft tissues, and erythrocytes [37]. Although phosphate ions are more rapidly absorbed into the circulation than ionic Ca, the present study showed that, irrespective of diet Ca content, PiAbs increased with consumption of the prebiotic mixture in a similar percentage as CaAbs. This finding was expected given that Pi and Ca metabolism are highly interconnected. Indeed, Pi regulates calcitriol and PTH synthesis, and both hormones are involved in the modulation of bone turnover and mineralization processes. Pi homeostasis is regulated mainly through the intestine and the kidney. An increase in PiAbs rapidly enhances urinary Pi excretion under the control of PTH and fibroblast growth factor 23 (FGF23). A limitation to the present study is that urinary Pi was not evaluated, and determining urinary Pi excretion might have contributed to clarifying the effect of the higher PiAbs on renal Pi excretion. Balance studies were not included in the present report because they only evidence the effect of prebiotic intake on P–Ca metabolism during a short period. Instead, evaluation of long-term consumption of the GOS/FOS mixture is more adequate to assess the impact of feeding the prebiotic mixture on bone turnover and bone mass.
Irrespective of diet Ca content, consumption of the GOS/FOS mixture was equally effective in improving Ca and Mg absorption. The simultaneous increase in Ca and Mg absorption could be associated with the characteristics of the prebiotic mixture, which contains both short-chain and long-chain non-digestible sugars. Whereas short-chain sugar increases gut bacterial fermentation in the proximal colon where Ca is absorbed, long-chain sugar favors bacterial fermentation in the distal parts of the colon where Mg absorption occurs. About 60% of total Mg is stored in the bone, either on the surface of hydroxyapatite or in the hydration shell around the crystal. Mg exerts direct effects on bone quality and skeletal fragility, influencing the architectural disposition of bone material and other factors unrelated to mineralization, such as crystal arrangement and size [38]. The higher MgAbs observed in the present study could have contributed to the improvement in parameters associated with bone quality [32]. In this regard, our data showed that both structural bone strength and resistance to fracture in the mid-length region of the tibia were increased in the two GF groups, regardless of diet Ca content, suggesting a positive effect of Mg on the quality of the material and/or on diaphysis architecture. Mg also improves bone strength via indirect effects. Mg indirectly influences bone remodeling by affecting osteoblast and osteoclast differentiation and Ca homeostasis. In this regard, Mg regulates the PTH/vitamin D axis through the activation of adenylate cyclase and 25-hydroxycholecalciferol-1-hydroxylase enzymes. In addition, unlike Ca, body Mg retention is not entirely related to bone. About 40% is intracellular and is vital for numerous physiological functions such as hormonal regulation [39]. In addition, Mg stabilizes enzymes in many ATP-generating reactions, antagonizes Ca in muscle contraction, modulates insulin signal transduction and cell proliferation, and is important for cell adhesion and membrane transport [40]. It must be taken into account that the higher Mg absorption was not concomitant with an increase in Mg retention. Mg metabolism is not as tightly regulated as Ca metabolism. Hence, it is likely that the increased Mg absorption observed in the GF-0.3% and GF-0.5% groups was balanced by an increase in urinary Mg excretion, preventing an increase in Mg in bone. Another limitation of the present report is that Mg excretion was not evaluated. Analyzing urinary Mg output might have clarified the effect of the normal Ca diet on renal Mg re-absorption. As mentioned above, the design of the present report did not include balance studies given that bone retention, density, and strength are more adequate to observe the impact of long-term consumption of the prebiotic mixture.
Conclusion
Under the present experimental conditions and irrespective of diet Ca content, the results of this study allow concluding that the GOS/FOS® mixture studied here may help to maintain bone heath by reducing bone resorption, and increasing bone mineralization, density, and structure, due to a simultaneous increase in Ca, Pi, and Mg absorption. Although further studies are needed to reliably determine the effectiveness of this dietary intervention on bone health, the results shown here are promising, and if validated, the GOS/FOS mixture may be used in the future in conjunction with traditional pharmacological treatments, to prevent greater increases in menopause- and aging-related bone loss.
Abbreviations
- OVX:
-
Ovariectomized
- SHAM:
-
Simulated operation
- EGP:
-
Epiphyseal growth plate
- Pi:
-
Inorganic phosphorus
- CaI:
-
Calcium intake
- NDFO:
-
Non-digestible fructo-oligosaccharides
- GOS:
-
Galacto-oligosaccharides
- FOS:
-
Long-chain fructo-oligosaccharides
- AIN:
-
American Institute of Nutrition
- NCa:
-
Normal Ca content diet
- LCa:
-
Low Ca content diet
- GF groups:
-
GOS/FOS groups
- O groups:
-
Ovariectomized groups
- BW:
-
Body weight
- CO2 :
-
Oxygen dioxide
- LS:
-
Lactobacillus
- CFU:
-
Colony forming units
- I:
-
Food intake
- F:
-
Feces
- Abs:
-
Apparent mineral absorption
- Mg:
-
Magnesium
- HCl:
-
Hydrochloric acid
- HNO3 :
-
Nitric acid
- CTX:
-
C-terminal telopeptide of collagen type I
- BAP:
-
Bone alkaline phosphatase
- tsBMC:
-
Total skeleton bone mineral content
- tsBMD:
-
Total skeleton bone mineral density
- DXA:
-
Dual energy X-ray absorptiometry
- CV:
-
Coefficients of variation
- ROI:
-
Region of interest
- EDTA:
-
Ethylene-diamine-tetra-acetic acid
- BV/TV:
-
Bone volume fraction
- GPC.Th:
-
Total width of epiphyseal cartilage
References
de Barboza GD, Guizzardi S, de Talamoni NT (2015) Molecular aspects of intestinal calcium absorption. World J Gastroenterol WJG 21(23):7142
Gallagher JC, Goldgar D, Moy A (1987) Total bone calcium in normal women: effect of age and menopause status. J Bone Miner Res 2(6):491–496
Wronski TJ, Dann LM, Horner SL (1989) Time course of vertebral osteopenia in ovariectomized rats. Bone 10(4):295–301
Bryk G et al (2015) Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur J Nutr 54(6):913–923
Bryk G et al (2016) Effect of a mixture of GOS/FOS® on calcium absorption and retention during recovery from protein malnutrition: experimental model in growing rats. Eur J Nutr 55(8):2445–2458
Breuil V, Euller-Ziegler L (2004) Nutrition et vieillissement osseux: L’ostéoporose. Nutr Clin Métabol 18(4):212–218
Reid IR, Bristow SM, Bolland MJ (2015) Calcium supplements: benefits and risks. J Intern Med 278(4):354–368
Kopecky SL et al (2016) Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 165(12):867–868
Bristow SM et al (2015) Acute effects of calcium citrate with or without a meal, calcium-fortified juice and a dairy product meal on serum calcium and phosphate: a randomised cross-over trial. Br J Nutr 113(10):1585–1594
Manson JE, Bassuk SS (2014) Calcium supplements: do they help or harm? Menopause 21(1):106–108
Reid IR et al (2006) Randomized controlled trial of calcium in healthy older women. Am J Med 119(9):777–785
Coudray C et al (2003) Effects of inulin-type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur J Nutr 42(2):91–98
Roberfroid M et al (2010) Prebiotic effects: metabolic and health benefits. Br J Nutr 104(S2):S1–S63
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Oxford University Press, Oxford
Sapp RE, Davidson SD (1991) Microwave digestion of multi-component foods for sodium analysis by atomic absorption spectrometry. J Food Sci 56(5):1412–1414
Farley JR et al (1994) Quantification of skeletal alkaline phosphatase in osteoporotic serum by wheat germ agglutinin precipitation, heat inactivation, and a two-site immunoradiometric assay. Clin Chem 40(9):1749–1756
Zeni S et al (2000) Differences in bone turnover and skeletal response to thyroid hormone treatment between estrogen-depleted and repleted rats. Calcif Tissue Int 67(2):173–177
Mastaglia SR et al (2006) Vitamin D insufficiency reduces the protective effect of bisphosphonate on ovariectomy-induced bone loss in rats. Bone 39(4):837–844
Parfitt AM et al (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2(6):595–610
Bosello O, Zamboni M (2000) Visceral obesity and metabolic syndrome. Obes Rev 1(1):47–56
Macfarlane S, Macfarlane GT, Cummings JH (2006) Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 24(5):701–714
Djouzi Z, Andrieux C (1997) Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br J Nutr 78(2):313–324
Boehm G et al (2003) Prebiotic concept for infant nutrition. Acta Paediatr Oslo Nor 91(441):64–67
Scholz-Ahrens KE et al (2007) Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr 137(3):838S–846S
Roberfroid MB (2002) Functional foods: concepts and application to inulin and oligofructose. Br J Nutr 87(S2):S139–S143
Le Blay G et al (1999) Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J Nutr 129(12):2231–2235
Scholz-Ahrens KE et al (2001) Effects of prebiotics on mineral metabolism. Am J Clin Nutr 73(2):459s–464 s
Scholz-Ahrens KE, Schrezenmeir J (2007) Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr 137(11):;2513S–2523S
Pansu D, Bellaton C, Bronner F (1983) Developmental changes in the mechanisms of duodenal calcium transport in the rat. Am J Physiol 244(1):G20–G26
Weisstaub AR et al (2013) Polydextrose enhances calcium absorption and bone retention in ovariectomized rats. Int J Food Sci doi. https://doi.org/10.1155/2013/450794
Taguchi A (1994) Effect of fructooligosaccharides on bone and mineral absorption in the rat model with ovariectomized osteiporosis. Sci Rep 33:37–43
Scholz-Ahrens KE, Açil Y, Schrezenmeir J (2002) Effect of oligofructose or dietary calcium on repeated calcium and phosphorus balances, bone mineralization and trabecular structure in ovariectomized rats. Br J Nutr 88(4):365–377
Zafar TA et al (2004) Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr 134(2):399–402
Mroczynska M, Libudzisz Z (2010) Beta-glucuronidase and beta-glucosidase activity of Lactobacillus and Enterococcus isolated from human feces. Pol J Microbiol 59(4):265–269
Zhu K, Prince RL (2012) Calcium and bone. Clin Biochem 45(12):936–942
Sjögren K et al (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27(6):1357–1367
Raina R et al (2012) Phosphorus metabolism. J Nephrol Ther S. https://doi.org/10.4172/2161-0959.S3-008
Burr DB, Robling AG, Turner CH (2002) Effects of biomechanical stress on bones in animals. Bone 30(5):781–786
Alfrey AC, Miller NL (1973) Bone magnesium pools in uremia. J Clin Invest 52(12):3019–3027
Jahnen-Dechent W, Ketteler M (2012) Magnesium basics. Clin Kidney J 5(Suppl 1):i3–i14
Acknowledgements
The authors thank technicians Ricardo Orzuza and Julia Somoza for their technical assistance.
Funding
This study was supported by the University of Buenos Aires and CONICET. This study was funded by UBACyT 20020090200037 and PIP funding programs.
Author information
Authors and Affiliations
Contributions
This study is part of the thesis of MS who participated in all stages of the experiment, data entry, and analysis of results. GB performed the biochemical determinations, and MZC participated in the anthropometric and food evaluations. MER and MLPMP participated in the design of the study and in animal and diet control, and SNZ was the director of the investigation.
Corresponding author
Ethics declarations
Conflict of interest
Mariana Seijo, Gabriel Bryk, Magalí Zeni Coronel, Marina Bonanno, María Esther Rio, María Luz Pita Martín de Portela and Susana Noemí Zeni have no conflict of interest to declare.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Seijo, M., Bryk, G., Zeni Coronel, M. et al. Effect of Adding a Galacto-Oligosaccharides/Fructo-Oligosaccharides (GOS/FOS®) Mixture to a Normal and Low Calcium Diet, on Calcium Absorption and Bone Health in Ovariectomy-Induced Osteopenic Rats. Calcif Tissue Int 104, 301–312 (2019). https://doi.org/10.1007/s00223-018-0490-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0490-5