Abstract
Probiotics have been consumed by humans for thousands of years because they are beneficial for long-term storage of foods and promote the health of their host. Ingested probiotics reside in the gastrointestinal tract where they have many effects including modifying the microbiota composition, intestinal barrier function, and the immune system which result in systemic benefits to the host, including bone health. Probiotics benefit bone growth, density, and structure under conditions of dysbiosis, intestinal permeability, and inflammation (recognized mediators of bone loss and osteoporosis). It is likely that multiple mechanisms are involved in mediating probiotic signals from the gut to the bone. Studies indicate a role for the microbiota (composition and activity), intestinal barrier function, and immune cells in the signaling process. These mechanisms are not mutually exclusive, but rather, may synergize to provide benefits to the skeletal system of the host and serve as a starting point for investigation. Given that probiotics hold great promise for supporting bone health and are generally regarded as safe, future studies identifying mechanisms are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
History of Probiotics
Probiotics have been consumed by humans dating back to before 7000 BCE [1]. Probiotics are contained in many fermented products made to undergo long-term storage, such as beer, bread, wine, kefir, kumis, and cheese [2,3,4]. For example, probiotic lactobacillus strains are found in the brine of fermented olives, pickled juices, kefir, and yogurt [5]. Probiotics are also found in non-fermented foods such as meat and fruits as well as in the gastrointestinal tract of animals such as pigs, rats, and poultry [5]. Probiotic intake has long been associated with a longer, healthier life [1, 2, 6]. In the late 1800s, it was hypothesized that lactobacillus strains produce factors that benefit gastrointestinal metabolism, the intestinal epithelium, and health [1,2,3, 6, 7]. It was also thought that ingestion of beneficial bacteria could help treat disease through the restoration of the microbiota composition. The concept that beneficial microbes mediate health continues to be supported today. In fact, probiotics are defined by the Food and Agricultural Organization/World Health Organization (FAO/WHO) as “live microorganisms that when administered in adequate amounts will confer a health benefit on the host.” This definition is broad without distinction between organic/inorganic, single versus multiple mediators, effects on bacterial growth, or increased resistance to infection. At the same time the definition imposes some core requirements [6, 8].
The nomenclature for bacteria, and thus probiotics, is based on a taxonomic/genetic hierarchy where bacteria are divided into phylum, class, order, family, genus, species, and subspecies and/or strain. With more than 23 bacteria phyla, it is easy to see the abundance of specific probiotics and the complexity of their names. Evidence indicate that probiotic effects can be strain-specific. In addition, not all bacteria within a species act the same and/or can be regarded as a probiotic. Lactic acid-producing bacteria, which include lactobacilli strains, are one of the most well-known groups of bacteria/probiotics with health benefits that have been used for centuries. They are gram-positive, obtained from fermented milk products and are usually found in carbohydrate-rich environments and normal intestinal flora of many animals [3]. The genus Lactobacillus belongs to the phylum Firmicutes, class Bacilli, order Lactobacillales, family Lactobacillaceae. The most commonly isolated species are Lactobacillus acidophilus, L. salivarius, L. casei, L. plantarum, L. fermentum, L. reuteri, L. rhamnosus, L. gasseri, and L. brevis from human intestine. Several of these, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, and Lactobacillus reuteri, have been extensively studied and well documented. Lactobacillus acidophilus can colonize the human colon, has antimicrobial effects, and is used to treat intestinal infections [9]. Lactobacillus rhamnosus GG or Lactobacillus GG (LGG) tolerates low pH environment, adheres to the gastrointestinal tract, and is effective in treating diarrhea [2, 10].
Role of Probiotics in Bone Health
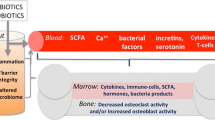
Bone health depends on the balance of bone resorption by osteoclasts and bone formation by osteoblasts. Hormones, immune cells, and the gastrointestinal system can regulate these processes [11,12,13]. While the intestine is known for its key role in calcium, phosphorous, and magnesium absorption (contributors to bone mineralization) [14], the intestine also produces endocrine factors that signal to bone cells, such as incretins [15] and serotonin [16]. Recent studies, including some from our lab, indicate that the intestinal microbiota and physiology can also regulate bone health [17,18,19,20,21,22]. Thus, probiotics which modify the microbiota composition and/or function and promote intestinal health can also benefit bone health. This is supported by numerous studies in a variety of animal models (Table 1). Several recent clinical studies confirm the benefits of probiotics to human skeletal health [23,24,25,26,27,28,29]. The mechanisms accounting for probiotic benefits on bone are not fully known, but likely involve (1) changing the intestinal microbiome, (2) modifying intestinal barrier function, and (3) impacting the immune system/immune cells (Figs. 1, 2). We discuss these possible mechanisms below.
Probiotic ingestion alters the gastrointestinal microbiota composition, permeability, and local and systemic immune cells. Probiotic-induced changes in microbiota composition can beneficially influence intestinal barrier function and inflammation. Similarly, increasing intestinal barrier function can benefit the immune and possibly microbiota composition. Finally, a strong immune system is likely to benefit intestinal barrier function and microbiota composition
Probiotics Modify the Microbiome to Regulate Bone Health
The human body is the host for ~ 100 trillion microbes comprising ~ 1000 species and 28 different phyla [30]. In addition to microbes outnumbering human cells, the gut microbiota also express 100-fold more genes compared to human genome [30]. Changes in microbiota composition can have beneficial or harmful consequences on human health [31]. Therefore, the microbiome–host relationship is an important target for therapeutic purposes, and probiotics may be a key therapy based on their ability to influence the intestine.
Ingested probiotics enter and populate the GI tract. Depending upon the diet, the dose of naturally ingested probiotic bacteria can range from 108 to 1012 CFU per day [32]. While bacteria are robust and hearty, the acid environment of the stomach and bile and enzyme secretion in the intestine can decrease the number of viable probiotics in the gut by 50% or greater [33]. The abundance of ingested bacteria relative to residential bacteria is much greater in the small intestine where the ratio can be 1:1, whereas in the colon the ingested probiotics are greatly outnumbered by the existing resident bacteria and may comprise only 0.0001-fold of the total bacteria [32, 34]. Ingested probiotics are maintained in the gastrointestinal tract for a few days after ingestion but are rarely maintained after 1 week [32, 35, 36]. Thus, probiotics likely need to be continuously ingested to obtain maximum benefits [37].
Probiotics can modify the microbiota composition simply by the increased presence and proliferation of the probiotic in the gastrointestinal tract, as well as through their ability to compete with other bacterial strains. Probiotics can digest complex carbohydrates and generate oligosaccharides that can be further metabolized by other bacteria that subsequently proliferate and modify the microbiota composition. Probiotic bacteria also secrete antimicrobial agents that can kill certain bacterial strains in the gastrointestinal tract. For example, Lactobacilli strains produce several lactic acid, bacteriocins (antimicrobial peptides), and hydrogen peroxide [38].
Changes in the composition of the microbiota affect bone parameters under a variety of conditions [39,40,41]. A clear link has been demonstrated between microbiota composition and bone growth. For example, the microbiota of undernourished children with stunted growth differs dramatically from healthy children [42] and when transferred to germ-free mice (mice lacking a microbiome) cause poor growth [42]. More importantly, supplementation with two bacterial strains (Ruminococcus gnavus and Clostridium symbiosum) ameliorated growth abnormalities in the mice receiving the microbiota from undernourished children [42]. Similarly, treatment with the probiotic Lactobacillus plantarum restored normal growth rates in undernourished mice [43]. The L. plantarum treatment raised serum IGF-1 and IGFBP-3 to normal levels [43] consistent with reports demonstrating the microbiota regulates the IGF-1–IGF-1R axis [44]. This is a general phenomenon since germ-free drosophila repopulated with lactobacilli strains regain a normal IGF axis and grow at normal rates [45, 46]. In humans, the probiotic Bifidobacterium lactis benefited the growth of infants born to mothers with human immunodeficiency virus [47]. Taken together, these studies demonstrate that manipulation of the microbiota with probiotics can benefit bone growth during development.
Microbiota shifts are also associated with changes in bone density. A negative association exists between bone density and intestinal dysbiosis (microbiota imbalance favoring pathogenic over beneficial bacterial strains) observed in inflammatory bowel disease [48]. There are also positive associations, whereby shifts in microbiota composition can enhance bone density. Our lab has shown that treatment of ovariectomized mice with probiotic Lactobacillus reuteri causes a significant change in the microbiota composition and prevents estrogen deficiency-induced bone loss [19]. Shifts in microbiota composition induced by prebiotic use also support a role for the microbiota composition in regulating bone health parameters in both mice and humans [49]. In addition, many papers that indicate the probiotic ingestion can modify bone density, though links with specific microbiota components were not determined [17, 21, 22, 50]. In the past year, studies also demonstrate the role of the microbiota in periodontal disease. Diabetes was shown to shift oral microbiota composition that was shown, by transfer to germ-free mice, to be pathogenic and promote periodontal disease and tooth loss [51]. Studies also suggest that probiotics, such as Lactobacillus rhamnosus GG, Bifidobacterium, or Lactobacillus gasseri, may reduce periodontal disease and bone loss, though more work is needed to determine the specific mechanisms [52,53,54].
Probiotics Modify Intestinal Barrier Function to Regulate Bone Health
The intestinal epithelium is essential for providing a selective barrier that prevents translocation of harmful substances and pathogens and their products into the blood stream. It is composed of several layers. Importantly, intestinal epithelial cells (IECs) joined together by tight junction proteins provide the key barrier support which allows only selective transport. Additionally, a mucus layer produced by goblet cells covers the surface of the epithelial cells and prevents gut bacteria and pathogen access to host epithelial cells. The intestine also secretes immunoglobulins, defensins, and other antimicrobial products that contribute to maintaining a healthy environment.
Disruption of the epithelial barrier can lead to pathogen translocation into the bloodstream causing systemic inflammation and trigger GI diseases such as inflammatory bowel disease (IBD), celiac disease, and colon cancer [55,56,57]. Studies from our lab and others demonstrate that GI barrier dysregulation also promotes bone loss [50, 58,59,60,61]. Therefore, dysbiosis may promote bone loss by increasing intestinal permeability and serum endotoxin levels [62,63,64]. The loss of estrogen is known to promote both increased intestinal permeability as well as bone loss [65]. Consistent with this, colonic paracellular permeability decreases during the oestrus phase (high levels of estrogen) of the rat when compared to the dioestrus phase (low levels of estrogen) [65]. Our lab demonstrated increased intestinal permeability as early as 1 week following ovariectomy and estrogen loss in mice [58]. Ex vivo using chambers analyses demonstrated that the ileum had the most dynamic changes and that estrogen deficiency induces region-specific effects on intestinal permeability [58].
Similarly, while probiotic treatment can benefit dysbiosis, probiotics may mediate bone health by enhancing intestinal barrier function [66,67,68,69,70]. In a study causing enteropathogenic E. coli (EPEC)-induced dysbiosis, administration of probiotic E. coli Nissle 1917 increased specific claudin expression and prevented increases in intestinal permeability seen after infection with EPEC [71]. Additional studies also indicate that probiotics can rescue barrier function [72,73,74,75]. Consistent with a link between barrier function and bone health, probiotic treatment of estrogen-deficient mice not only prevents intestinal permeability but also prevents bone loss [50]. Probiotic Lactobacillus rhamnosus GG (LGG) as well as the probiotic supplement VSL#3 reduced gut permeability, intestinal inflammation, and completely protects against bone loss induced by estrogen deficiency [50]. Together, these data highlight the role that of the gut epithelial barrier and microbiota in bone loss induced by estrogen deficiency.
Role of the Immune System in Mediating Probiotic Effects on Bone
The intestinal immune system, one of the largest in the body, is uniquely positioned to encounter not only dietary components but also gut microbiota and pathogens. The gut immune system must recognize and differentially respond to pathogens, dietary components, and commensal and beneficial microbes [76, 77]. In addition to immune cells, paneth and goblet cells (specialized epithelial cells) play an important role in this process. Intestinal immune system dysregulation is linked to a number of diseases, including inflammatory bowel disease (IBD) and celiac disease, associated with pathologic expression of cytokines which are also important players in the regulation of bone homeostasis [78, 79]. Thus, it is not surprising that many of these intestinal conditions lead to bone loss [80, 81]. This prompted our lab and others to more closely examine the relationship of the gut and the bone in the context of changes in intestinal inflammation. In early studies, we found that induction of colitis leads to changes in the immune cell composition and cytokine expression within the bone marrow [82]. These changes in the bone marrow can signal to bone cells (stem cells, osteoblasts, and osteoclasts) to significantly alter bone homeostasis. This is indeed the case in animal models of IBD, where dysregulation of cytokines (TNFα, IL1α, IL1β, IL-6) correlates with bone loss [83,84,85,86,87]. Consistent with animal models, patients with IBD have elevated levels of cytokines [83, 84] and exhibit bone loss. Thus, the role of immune system in modulating the gut–bone axis is becoming increasingly clear.
The intestinal microbiota is an important modulator of the gut immune system. Dysbiosis has been shown to increase osteoclastogenic activity including increased IL-17 producing cells and reduced bone mass [51]. Similarly, oral administration of “bad” bacteria to mice causes dysregulated intestinal inflammation and corresponding bone loss [86]. Thus, changes in intestinal microbiota can influence immune responses and bone health. Using germ-free mice, Sjogren et al. showed that germ-free mice have higher bone mass compared to conventional mice [88]. This was associated with reduced numbers of osteoclasts precursors as well as decreased frequency of CD4+ T cells in the bone marrow. However, the bone density findings in germ-free mice have been variable showing more, less, or no change [43, 89,90,91]; the differing compositions of the microbiota used to populate the conventionalized mice mostly likely contribute to the differing responses.
Previous studies indicate that probiotics can modulate immune function [92, 93]. Because probiotics are orally ingested, they can readily interact with the gut immune components which consequently influence local and distant organ functions. Identification of the dysregulated inflammatory pathways in dysbiosis-induced bone loss prompted us (and others) to examine the role of immune system in mediating the probiotic benefits on bone health. Consistent with its anti-TNFα activity, L. reuteri treatment decreases intestinal TNFα expression in male mice and correspondingly increases bone density [94]. Although healthy female mice did not respond to L. reuteri treatment in terms of bone density, induction of mild inflammatory state is sufficient to increase bone density following probiotic administration [95]. These results suggest that L. reuteri effects on bone are somehow linked to the inflammatory state of the organism. The precise immunological mechanisms by which L. reuteri affects bone density is still under active investigation. Interestingly however, L. reuteri reversed ovariectomy-induced bone loss and this was associated with corresponding changes in bone marrow CD4+ T cells [19]. This was associated with anti-osteoclastogenic activity of L. reuteri. This activity is suggested to be in L. reuteri-conditioned media which inhibit the differentiation of monocytic macrophages to osteoclasts [19]. Earlier studies indicate that, histamine secreted by L. reuteri, is involved in suppressing TNFα production from human monocytic cells [96]. Thus, the L. reuteri effects on bone may involve anti-inflammatory effects on the gut and potentially direct effects on bone cells. Whether these factors influence gut immune or bone immune components in vivo is still under active investigation.
Similar to our findings with L. reuteri, other groups have shown that LGG and VSL#3 administration reduces TNFα, IL-17, and RANKL expression in the gut and bone marrow of ovariectomized mice [89]. Ohlsson et al. [97] demonstrated that suppression of bone marrow T-regulatory cells by ovariectomy is partially reversed by probiotic supplementation. Consistent with regulation of anti-inflammatory T cells, bone TNFα and other pro-inflammatory cytokines were reduced by probiotic supplementation in the ovariectomized mice, and enhanced TGFβ1 expression (associated with increased T-regs) was observed. Wang et al. showed that the probiotic L. casei alters macrophage phenotype. Specifically, wear debris generated from hip implantation activates macrophages leading to a pro-inflammatory state and osteolysis [98]; however, L. casei treatment inhibited osteolysis and the pro-inflammatory state of the macrophages. Such anti-inflammatory effects of probiotics in periodontal models have also been demonstrated [54]. Other studies have indicated that probiotics can beneficially affect hematopoiesis in the bone marrow further suggesting that orally administered probiotics can significantly affect immune cells in the bone marrow that can consequently affect bone health [99]. Taken together, while these studies suggest that probiotics can significantly influence immune system and bone, further definitive studies are necessary to identify specific mechanisms.
Conclusions
The above studies indicate that probiotics hold great promise for supporting bone health. It is likely that multiple mechanisms are involved in linking probiotic signals from the gut to the bone. Here, we discussed studies that indicate a role for the microbiota (composition and activity), intestinal barrier function, and immune cells in the signaling process. These mechanisms are not mutually exclusive, but rather, may synergize to provide benefits to the skeletal system of the host and serve as a starting point for investigation. Given that probiotics are generally regarded as safe, future studies identifying mechanisms are warranted.
References
Gasbarrini G, Bonvicini F, Gramenzi A (2016) Probiotics history. J Clin Gastroenterol 50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015:S116–S119
Gogineni V (2013) Probiotics: history and evolution. J Anc Dis Prev Remeidies 1:1–7
Azizpour K et al (2009) History and basic of probiotics. Res J Biol Sci 4:409–426
Ozen M, Dinleyici EC (2015) The history of probiotics: the untold story. Benef Microbes 6(2):159–165
Fontana L et al (2013) Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr 109(Suppl 2):S35–S50
Anukam KC, Reid G (2007) Organisms associated with bacterial vaginosis in Nigerian women as determined by PCR-DGGE and 16S rRNA gene sequence. Afr Health Sci 7(2):68–72
Calatayud GA, Suarez JE (2017) A new contribution to the history of probiotics. Benef Microbes 8(2):323–325
Hill C et al (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514
McFarland LV (2015) From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Dis 60(Suppl 2):S85–S90
Gorbach SL (2000) Probiotics and gastrointestinal health. Am J Gastroenterol 95(1 Suppl):S2–S4
Cenci S et al (2000) Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 106(10):1229–1237
Hock JM et al (1988) Human parathyroid hormone-(1–34) increases bone mass in ovariectomized and orchidectomized rats. Endocrinology 122(6):2899–2904
Yamada C (2011) [Role of incretins in the regulation of bone metabolism]. Nihon Rinsho 69(5):842–847
Christakos S et al (2017) Intestinal regulation of calcium: vitamin D and bone physiology. Adv Exp Med Biol 1033:3–12
Ramsey W, Isales CM (2017) Intestinal incretins and the regulation of bone physiology. Adv Exp Med Biol 1033:13–33
Lavoie B, Lian JB, Mawe GM (2017) Regulation of bone metabolism by serotonin. Adv Exp Med Biol 1033:35–46
Ohlsson C et al (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 9(3):e92368
Parvaneh K et al. (2015) Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015:897639
Britton RA et al. (2014) Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 229:1822–1830
Collins FL et al (2016) Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS ONE 11(4):e0153180
Zhang J et al (2015) Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology 156(9):3169–3182
McCabe LR et al (2013) Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 228(8):1793–1798
Sommer F, Bäckhed F (2013) The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11:227–238
Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan Y-M, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI (2016) Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351:6275
Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F (2016) Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351(6275):854–857
Steenhout PG, Rochat F, Hager C (2009) The effect of Bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab 55:334–340
Lei M, Hua L-M, Wang D-W (2016) The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benef Microbes 7:631–637
Tu M-Y, Chen H-L, Tung Y-T, Kao C-C, Hu F-C, Chen C-M (2015) Short-term effects of Kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS ONE 10:e0144231
Lambert et al (2017) Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr 106:909–920
Fukuda S, Ohno H (2014) Gut microbiome and metabolic diseases. Semin Immunopathol 36(1):103–114
Ley RE et al (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023
Derrien M, van Hylckama Vlieg JE (2015) Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol 23(6):354–366
Oozeer R et al (2006) Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl Environ Microbiol 72(8):5615–5617
Loh G, Blaut M (2012) Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes 3(6):544–555
Firmesse O et al (2008) Lactobacillus rhamnosus R11 consumed in a food supplement survived human digestive transit without modifying microbiota equilibrium as assessed by real-time polymerase chain reaction. J Mol Microbiol Biotechnol 14(1–3):90–99
Fujimoto J et al (2008) Identification and quantification of Lactobacillus casei strain Shirota in human feces with strain-specific primers derived from randomly amplified polymorphic DNA. Int J Food Microbiol 126(1–2):210–215
Bezkorovainy A (2001) Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr 73(2 Suppl):399S–405S
Lebeer S, Vanderleyden J, De Keersmaecker SC (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72(4):728–764 (Table of Contents)
McCabe L, Britton RA, Parameswaran N (2015) Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep 13(6):363–371
Quach D, Britton RA (2017) Gut microbiota and bone health. Adv Exp Med Biol 1033:47–58
Pacifici R (2017) Bone remodeling and the microbiome. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a031203
Blanton LV et al (2016) Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351(6275):aad3311
Schwarzer M et al (2016) Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351(6275):854–857
Yan J et al (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 113(47):E7554–E7563
Storelli G et al (2011) Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14(3):403–414
Hyun S (2013) Body size regulation and insulin-like growth factor signaling. Cell Mol Life Sci 70(13):2351–2365
Steenhout PG, Rochat F, Hager C (2009) The effect of Bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab 55(4):334–340
Sylvester FA (2017) Inflammatory bowel disease: effects on bone and mechanisms. Adv Exp Med Biol 1033:133–150
Whisner CM, Weaver CM (2017) Prebiotics and bone. Adv Exp Med Biol 1033:201–224
Li JY et al (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126(6):2049–2063
Xiao E et al (2017) Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 22(1):120–128 e4
Gatej SM et al (2017) Probiotic Lactobacillus rhamnosus GG prevents alveolar bone loss in a mouse model of experimental periodontitis. J Clin Periodontol 45:204–212
Ricoldi MST et al (2017) Effects of the probiotic Bifidobacterium animalis subsp. lactis on the non-surgical treatment of periodontitis. A histomorphometric, microtomographic and immunohistochemical study in rats. PLoS ONE 12(6):e0179946
Kobayashi R et al (2017) Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci Rep 7(1):545
France MM, Turner JR (2017) The mucosal barrier at a glance. J Cell Sci 130(2):307–314
Konig J et al (2016) Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7(10):e196
Nagao-Kitamoto H et al (2016) Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res 14(2):127–138
Collins F et al. (2017) Temporal and regional intestinal change in permeability, tight junction and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. https://doi.org/10.14814/phy2.13263
Irwin R et al (2016) Intestinal inflammation without weight loss decreases bone density and growth. Am J Physiol Regul Integr Comp Physiol 311(6):R1149–R1157
Irwin R et al (2013) Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis 19(8):1586–1597
Harris L et al (2009) Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol 296(5):G1020–G1029
Tremellen K, Pearce K (2012) Dysbiosis of gut microbiota (DOGMA)—a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses 79(1):104–112
Maruyama K, Sano G, Matsuo K (2006) Murine osteoblasts respond to LPS and IFN-gamma similarly to macrophages. J Bone Miner Metab 24(6):454–460
Moriyama H, Ukai T, Hara Y (2002) Interferon-gamma production changes in parallel with bacterial lipopolysaccharide induced bone resorption in mice: an immunohistometrical study. Calcif Tissue Int 71(1):53–58
Braniste V et al (2009) Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol 587(Pt 13):3317–3328
Rosenfeldt V et al (2004) Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr 145(5):612–616
Stratiki Z et al (2007) The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 83(9):575–579
Madsen K et al (2001) Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121(3):580–591
Zareie M et al (2006) Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55(11):1553–1560
Bron PA et al (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117(1):93–107
Zyrek AA et al (2007) Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 9(3):804–816
Anderson RC et al (2010) Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol Lett 309(2):184–192
Resta-Lenert S, Barrett KE (2006) Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130(3):731–746
Qin H et al (2009) L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol 9:63
Moorthy G, Murali MR, Devaraj SN (2009) Lactobacilli facilitate maintenance of intestinal membrane integrity during Shigella dysenteriae 1 infection in rats. Nutrition 25(3):350–358
Mowat AM, Agace WW (2014) Regional specialization within the intestinal immune system. Nat Rev Immunol 14(10):667–685
Kundu P et al (2017) Our gut microbiome: the evolving inner self. Cell 171(7):1481–1493
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14:329–342
Strober W, Fuss IJ (2011) Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1756–1767
Bjarnason I et al (1997) Reduced bone density in patients with inflammatory bowel disease. Gut 40:228–233
Zanchetta MB, Longobardi V, Bai JC (2016) Bone and celiac disease. Curr Osteoporos Rep 14:43–48
Trottier MD et al (2012) Enhanced production of early lineages of monocytic and granulocytic cells in mice with colitis. Proc Natl Acad Sci USA 109(41):16594–16599
Ali T et al (2009) Osteoporosis in inflammatory bowel disease. Am J Med 122:599–604
Ciucci T et al (2015) Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 64:1072–1081
Harris L et al (2009) Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol 296:G1020–G1029
Irwin R et al (2013) Colitis induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis 19:1586
Metzger CE et al (2017) Inflammatory bowel disease in a rodent model alters osteocyte protein levels controlling bone turnover. J Bone Miner Res 32:802–813
Sjogren K et al (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–1367
Li J-Y et al (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126:2049–2063
Yan J et al (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 113:E7554–E7563
Quach D et al. (2018) Microbiota reconstitution does not cause bone loss in germ-free mice. mSphere. https://doi.org/10.1128/mSphereDirect.00545-17
Klaenhammer TR et al (2012) The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol 12(10):728–734
Frei R, Akdis M, O’Mahony L (2015) Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol 31(2):153–158
McCabe LR et al (2013) Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 228:1793–1798
Collins FL et al (2016) Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS ONE 11:e0153180
Thomas CM et al (2012) Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 7:e31951
Ohlsson C et al (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 9:e92368
Wang Z et al (2017) Probiotics protect mice from CoCrMo particles-induced osteolysis. Int J Nanomed 12:5387–5397
Salva S et al (2012) Dietary supplementation with probiotics improves hematopoiesis in malnourished mice. PLoS ONE 7(2):e31171
Chiang SS, Pan TM (2011) Antiosteoporotic effects of lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem 59:7734–7742. https://doi.org/10.1021/jf2013716
Maekawa T, Hajishengallis G (2014) Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J Periodontal Res 49:785–791. https://doi.org/10.1111/jre.12164
Messora MR, Oliveira LFF, Foureaux RC et al (2013) Probiotic therapy reduces periodontal tissue destruction and improves the intestinal morphology in rats with ligature-induced periodontitis. J Periodontol 84:1818–1826. https://doi.org/10.1902/jop.2013.120644
Oliveira LFF, Salvador SL, Silva PHF et al (2016) Benefits of Bifidobacterium animalis subsp. lactis probiotic in experimental periodontitis. J Periodontol. https://doi.org/10.1902/jop.2016.160217
Ghanem KZ, Badawy IH, ABDEL-SALAM AM (2004) Influence of yoghurt and probiotic yoghurt on the absorption of calcium, magnesium, iron and bone mineralization in rats. Milchwissenschaft 59:472–475
Tomofuji T, Ekuni D, Azuma T et al (2012) Supplementation of broccoli or Bifidobacterium longum-fermented broccoli suppresses serum lipid peroxidation and osteoclast differentiation on alveolar bone surface in rats fed a high-cholesterol diet. Nutr Res 32:301–307. https://doi.org/10.1016/j.nutres.2012.03.006
Rodrigues FC, Castro ASB, Rodrigues VC et al (2012) Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food 15:664–670. https://doi.org/10.1089/jmf.2011.0296
Kruger MC, Fear A, Chua W-H et al (2009) The effect of Lactobacillus rhamnosus HN001 on mineral absorption and bone health in growing male and ovariectomised female rats. Dairy Sci Technol 89:219–231. https://doi.org/10.1051/dst/2009012
Kim JG, Lee E, Kim SH et al (2009) Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int Dairy J 19:690–695. https://doi.org/10.1016/j.idairyj.2009.06.009
Narva M, Collin M, Lamberg-Allardt C et al (2004) Effects of long-term intervention with Lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab 48:228–234. https://doi.org/10.1159/000080455
Amdekar S, Kumar A, Sharma P et al (2012) Lactobacillus protected bone damage and maintained the antioxidant status of liver and kidney homogenates in female wistar rats. Mol Cell Biochem 368:155–165. https://doi.org/10.1007/s11010-012-1354-3
Rovenský J, Švík K, Maťha V et al (2004) The effects of Enterococcus faecium and selenium on methotrexate treatment in rat adjuvant-induced arthritis. Clin Dev Immunol 11:267–273. https://doi.org/10.1080/17402520400001660
Mutuş R, Kocabagli N, Alp M et al (2006) The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci 85:1621–1625
Plavnik I, Scott ML (1980) Effects of additional vitamins, minerals, or brewer’s yeast upon leg weaknesses in broiler chickens. Poult Sci 59:459–467
Nahashon SN, Nakaue HS, Mirosh LW (1994) Production variables and nutrient retention in single comb White Leghorn laying pullets fed diets supplemented with direct-fed microbials. Poult Sci 73:1699–1711
Acknowledgements
The authors would like to acknowledge funding from the National Institute of Health, Grants RO1 DK101050 and RO1 AT007695.
Funding
This review was supported by NIH grants (RO1 DK101050 and RO1 AT007695) to LRM and NP.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
LRM and NP have no conflict of interest.
Rights and permissions
About this article
Cite this article
McCabe, L.R., Parameswaran, N. Advances in Probiotic Regulation of Bone and Mineral Metabolism. Calcif Tissue Int 102, 480–488 (2018). https://doi.org/10.1007/s00223-018-0403-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0403-7