Abstract
Saikosaponin-A (SA), a class of native compound with numerous biological activities, may exert protective effect against postmenopausal bone loss. However, it remains unknown whether SA regulates the osteogenic differentiation of bone marrow stromal cells (BMSCs) in the treatment and prevention of osteoporosis. In this study, BMSCs were treated with various concentrations of SA to stimulate osteogenic differentiation over a 14-day period. Additionally, a canonical ovariectomized (OVX) mouse model was used to evaluate the effect of 3-month SA treatment in preventing postmenopausal osteoporosis. In vitro, we found that SA promotes alkaline phosphatase activity/staining and Alizarin red assay, stimulated the expression of osteogenic markers, i.e., runt-related transcription factor 2 (Runx2), osterix, osteopontin, and osteocalcin (OCN) in BMSCs. In vivo, the trabecular number, trabecular thickness, and trabecular bone mineral density of the distal femoral metaphysis were significantly increased in OVX mice treated intraperitoneally with SA for 3 months compared with OVX mice that not treated with SA. Moreover, the expression of Runx2 and OCN in OVX + SA mice was significantly increased than that in OVX mice. Finally, we found that SA activated the WNT/β-catenin pathway and the expression of several downstream genes including T-cell factor-1 and lymphoid enhancer factor-1. Inhibition of WNT/β-catenin pathway by Dickkopf-related protein 1 blocked the positive role of SA on osteogenesis. Therefore, SA promoted the osteogenic differentiation of BMSCs through WNT/β-catenin signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is the most common metabolic bone disease, and it affects many people such as the elderly, postmenopausal women, long-term bed-ridden patients, astronauts, and persons living sedentary lifestyles [1, 2]. Decreased bone mass and micro-architectural deterioration of bone tissues give rise to high risk of fractures of hip, vertebrae, pelvis, and wrist, causing enormous costs and substantial morbidity and mortality [3, 4]. In American, the direct fracture-caused costs are expected to raise from 17 billion dollar in 2005 to over 22 billion dollar by 2020 [5, 6]. Therefore, osteoporosis is a serious health and society problem.
Mechanistically, osteoporosis is a disorder of unbalanced bone remodeling with the increased bone resorption relative to bone formation, leading to the decreased bone mineral density (BMD) and the disruption of bone microarchitecture [6]. Bone resorption is completed by the polarization and the subsequent attachment to bone surface of osteoclasts. Meanwhile, bone formation occurs following osteoblasts phase that is to be developed into osteocytes [7]. Thus, correcting the imbalance between bone resorption and formation is an effective therapy for osteoporosis.
Currently, the clinical treatment for osteoporosis mainly include five kinds of agents, such as estrogens, bisphosphonates, calcitonin, selective estrogen receptor modulators, and monoclonal antibody [8]. Due to the potential complications associated with these agents, i.e., breast cancer, uterine bleeding, cardiovascular events, osteonecrosis, diarrhea, venous thromboembolism, and serious infections of skin, their use is restricted for treating osteoporosis [2, 9]. Besides these, single-drug target and high price of these agents also restrict their developing and applying in clinic [6, 9]. Therefore, it is urgent to exploit efficient agents for the treatment of osteoporosis.
Radix bupleuri, one of the most important crude drugs in the prescriptions of traditional Chinese medicine, made from dried roots of Bupleurum chinese DC. or Bupleurum scorzonerifolium Willd, has been utilized to treat influenza, fever, chronic hepatitis, malaria, menstrual disorders, and bone loss diseases [10–13]. Saikosaponin-A (SA), the major active constituent in radix bupleuri, has been shown to exert various pharmacological effects, including anti-inflammatory, antibacterial, immunoregulating, antiviral, antioxidative, and antiosteoporosis effects [11, 14–18]. Recently, SA has been shown to serve as a beneficial agent for prevention and treatment of osteoporosis and cancer-induced bone loss by suppressing osteoclastogenesis [10, 11]. Osteoblast differentiation and osteoclastogenesis are two important phases of bone remodeling [6, 19, 20]; however, whether SA affects the osteogenic differentiation of BMSCs in vitro and bone formation in vivo remains unknown.
Bone marrow stromal cells (BMSCs) are multipotent stromal cells capable of self-renewal and capable of multilineage mesenchymal differentiation [21, 22]. It can differentiate down multiple mesenchymal lineages, including osteogenic, chondrogenic, adipogenic, myogenic, and neurogenic lineages [23]. During the aging process, BMSCs differentiation into osteoblast decreases, whereas BMSCs differentiation into adipocytes increases, thereby resulting in decreased osteogenesis and bone loss [24]. When BMSCs underdo osteogenic differentiation, a range of proteins including osteocalcin (OCN), alkaline phosphatase (ALP), osteopontin (OPN), runt-related transcription factor 2 (Runx2), osterix (OSX), osteopontin (OPN), and fibronectin will be synthesized to enhance the adhesion and maturation of the osteoblasts [24–26]. To date, BMSCs are considered as the most suitable cell source for studying osteogenic differentiation.
In this article, we used a combination of in vitro and in vivo approaches to test the hypothesis that SA stimulates the osteogenic differentiation of BMSCs and clarified the detailed molecular mechanisms of SA in BMSCs. The results demonstrate that SA promotes BMSCs to differentiate into osteoblasts by activation of WNT/β-catenin pathway.
Methods and Materials
SA Preparation
SA was obtained from Sigma(St. Louis, MO, USA) at a purity of more than 98%. SA was dissolved in 0.9% physiological saline as a stock solution, stored at −20 °C, and diluted with medium before each experiment. Working solutions were prepared before each experiment.
Animals and Experimental Procedures
8-week-old female C57BL/6 mice (n = 30) weighting 19–21 g were obtained from Southern Medical University (Guangzhou, People’s Republic of China).The mice were housed at a temperature of 21 °C under a 12-h light/dark cycle. The mice were randomly divided into three groups: Sham, ovariectomized (OVX), and OVX + SA. OVX + SA group mice were treated with SA (4 mg/kg, every other day) by intraperitoneal injection for 3 months after ovariectomy. Mice in Sham and OVX group received equal amount of normal saline compared to OVX + SA group.
Isolation of BMSCs
The isolation and culture of BMSCs from the tibias and femurs of 8-week-old C57BL/6 mice were performed as described previously [2]. Briefly, the mice were killed by cervical dislocation and sterilized using 75% ethanol for 5 min. After dissecting the metaphyseal ends of the bones under sterile conditions, the bone marrow stromal cells were flushed out with Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) using a 5-ml syringe and centrifuged at 1000 rpm for 5 min. The cells were resuspended and expanded in growth medium (α-minimum medium, Gibco) containing 10% fatal bovine serum (Gibco) and 1% penicillin streptomycin reagent (Gibco) at a concentration of 5 × 107 cells per 10 cm dish (Gibco) and incubated at 37 °C underlying conditions of 5% CO2 until 80% confluence was reached. The medium was changed every 3 days. Primary cells prior to the second passage were used in all experiments.
Osteogenic Differentiation Protocol
BMSCs were cultured in growth medium in six-well cell culture plates at a density of 1 × 105 cells/well and incubated for 24 h. Subsequently, the cells were cultured in the osteogenic induction medium (OIM), which consists of growth medium supplemented with 50 μM ascorbic acid, 0.1 μM dexamethasone, and 10 mM β-glycero-phosphate (Sigma). These cells were cultured continuously in OIM for 2 weeks with the medium replaced every third day. SA (10, 20, and 40 μM) was added to the differentiation medium of the test cultures, while controls were cultured in OIM without SA. To examine the involvement of WNT/β-catenin signaling in SA action, BMSCs under OIM were stimulated to differentiate by addition of 40 μM SA in the presence or absence of 0.1 μg/ml Dickkopf-related protein 1 (DKK1) (Peprotech, Rocky Hill, USA), a specific inhibitor of WNT/β-catenin pathway.
Assay of Cell Proliferation
BMSCs (1.0 × 103 cells/well) were plated and cultured in 96-well plates with various concentrations of SA (10, 20, 40 μM) in growth medium for 2 weeks. At the end of the culture time point, 10 μl of Cell Counting Kit-8 reagent (CCK-8; Keygen Biotech, Nanjing, People’s Republic of China) was added to the plates, followed by further incubation for 4 h at 37 ℃.The absorbance (optical density, OD) at 450 nm was measured and used as an index of cell proliferation and viability.
Alkaline Phophatase (ALP) Staining and ALP Activity Assay
BMSCs (1 × 105 cells/well) were cultured with SA (10, 20, and 40 μM) in OIM in six-well plates for 2 weeks. For ALP staining, cells were fixed with 4% paraformaldehyde for 20 min. Then cells were washed three times with phosphate-buffered saline (PBS) and stained with the ALP staining solution (Sigma) at 37 °C for 40 min. For ALP measurement, cells were washed three with PBS, scraped into 500 μl of 10 mM Tris–HCl buffer (pH 7.6) containing 0.1% Triton X-100, placed on the ice, and sonicated to lyse the cells. Protein concentrations in the lysates were determined using the Bradford protein assay. ALP activity in the cellular fraction was measured using a fluorometric detection kit (Nanjing jiancheng Biotechnology Co Ltd. Nanjing, People’s Republic of China).The absorbance (OD) at 450 nm was measured using a microplate reader (ELx800, BioTek, Winooski, VT, USA).
Alizarin Red Assay
After osteogenic induction, mineral deposition was assessed by staining with Alizarin red solution (Sigma) on day 14. Cells were fixed with 4% paraformaldehyde for 20 min at room temperature and then washed with distilled water. A 1% solution of Alizarin red was added and incubated for 30 min at room temperature, followed by rinsing with PBS. The stain was desorbed with 10% cetylpyridinium chloride (Sigma) for 1 h. The solution was collected and 200 μl was plated on 96-well plates. The absorbance (OD) at 590 nm was measured.
Quantitative PCR (q-PCR)
After treatment with SA (10, 20, 40 μM) for 2 weeks in OIM, total RNA was extracted using Trizol reagent (Invitrogen Corp, Carlsbad, CA, USA) according to the manufacturer’s instruction [27].The Ct (cycle threshold) values were normalized to the expression levels of GAPDH. The primers used in this study are as follows: GAPDH, 5′-CAGGGCTGCCTTCTC TTGTG-3′ (Forward), 5′-GATGGTGATGGGTTTCCCGT-3′ (Reverse); Runx2, 5′-AATT AACGCCAGTCGGAGCA-3′ (Forward), 5′-CACTTCTCGGTCTGACGACG-3′ (Reverse); OSX, 5′-GCCTACTTACCCGTCTGACTTT-3′ (Forward), 5′-GCCCACTATTGCCAACTG C-3′ (Reverse); OCN, 5′-TCTATGACCTGCAGAGGGCT-3′ (Forward), 5′-ATAGCTCGTC ACAAGCAGGG-3′(Reverse); OPN, 5′-CTGTTGCCCAGCTTCTGAGCA-3′ (Forward), 5′-TGTGGCTCTGATGTTCCAGGCT-3′ (Reverse).
Western Blot (WB) Assay
After treatment with SA (10, 20, 40 μM) for 2 weeks in OIM, cells were collected by centrifugation and washed twice with PBS. The cell pellets were resuspended in RIPA buffer (Upstate Biotechnolog, Lake Placid, NY, USA) containing 1 µg/ml each of leupeptin, aprotinin, and pepstatin, and incubated for 30 min at 4 °C. Cell debris was removed by microcentrifugation. The proteins in cell extracts were resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred by electrophoresis to a nitrocellulose membrance (Millipore, Billerica, MA, USA).The membranes were then incubated with primary antibodies, i.e., β-actin (Cell Signaling Technology Inc, Danvers, MA, USA), Runx2 (Abcam, Cambridge, MA, USA), OCN (Abcam), β-catenin (Cell Signaling Technology), T-cell factor-1 (TCF-1) (Sigma), and lymphoid enhancer factor-1 (LEF-1) (Sigma) overnight at 4 °C. Following washing in Tris-buffered saline containing Tween-20 (TBST), a FITC-conjugated goat anti-rabbit (Cell Signaling Technology) secondary antibody was incubated for 1 h at room temperature to detect bound antibodies. Signals were revealed using an enhanced chemiluminescence kit (Cell Signaling Technology). Runx2, OCN, and β-catenin protein expressions were first corrected over β-actin expression and then as fold change from no treatment.
Micro-CT Assay
Animals were euthanized after the mice had been treated with SA for 3 months, and the distal femurs were collected. All samples were scanned for bone formation with a high-resolution micro-CT (μCT 80, Scanco Medical, Brüttisellen, Zurich, Switzerland) with the following scan parameters: 89 kVp X-ray energy setting, 150 μA with a mean 20 μm slice thickness. The reconstructed femoral coronary and distal femur trabecular images were selected for quantification of bone mass. Our analyses included various bone parameters: trabecular number, trabecular thickness, and trabecular bone mineral density (BMD).
Histological Assay
Following the micro-CT scan, the samples were fixed with 4% paraformaldehyde for 24 h at room temperature, decalcified using 10% ethylenediaminetetraacetic acid for 2 weeks, and embedded in paraffin. Serial sections of 5 μm thickness were cut and mounted on polylysine-coated slides. Hematoxylin and eosin (H&E) were performed following the product manual. For immunohistochemistry, sections were incubated with primary antibodies which recognized Runx2 (Abcam) and OCN (Abcam) overnight at 4 °C. Bound primary antibodies were detected using FITC-conjugated goat anti-rabbit (Cell Signaling Technology) secondary antibody, following 1 h incubation. The sections were subsequently incubated with 3,3′-diaminobenzidine (Sigma). Finally, all sections were dehydrated, mounted with coverslips, and photographed on an Olympus light microscope.
Statistical Analysis
All experiments were performed at least six times and in triplicate. Data were analyzed with GraphPad Prism software version 5.0. One-way analysis of variance was used to determine statistical differences. Homogeneity of variance tests was used to evaluate figure homogeneity. If the variances were equal, the least-significant difference test was used. If the variances were unequal, Dunnett’s test was employed. The final results were expressed as mean ± standard error.
Results
Effect of SA on Cell Proliferation
To investigate the effect of SA on cell proliferation, we tested the viability of BMSCs using CCK8 assay. We found that SA (10, 20, 40 μM) did not significantly affect cell growth after treatment for 2 weeks (Fig. 1a).
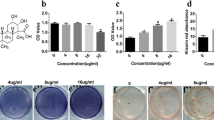
SA promotes the osteogenic differentiation of BMSCs in vitro. a CCK-8 assay was performed on BMSCs treatment with various concentrations of SA. Columns represent mean ± standard error from six well per group. *p < 0.05. ALP activity (b) and ALP staining (d) were tested on BMSCs after 2 weeks treatment with 10, 20, and 40 μM SA. Alizarin red absorbance (c) and Alizarin red staining (e) were performed after 2 weeks on BMSCs after 2 weeks treatment with 10, 20, and 40 μM SA. Columns represent mean ± standard error from six well per group. *p < 0.05, **p < 0.01, ***p < 0.001 versus with the group without SA. + p < 0.05 versus with the group with 20 μM SA; # p < 0.05 versus with the group with 10 μM SA
SA Stimulates Osteogenesis In Vitro
In order to evaluate the effects of SA on osteogenic differentiation of BMSCs, ALP activity/staining and Alizarin red staining were tested. Additionally, we examine the mRNA expression of Runx2, OSX, OPN and OCN, and the protein expression of Runx2 and OCN. Compared with the control group, ALP activity/staining (Fig. 1b, d) and Alizarin red staining (Fig. 1c, e) significantly enhanced with the increasing doses of SA. Additionally, when BMSCs were treated with SA for 2 weeks in OIM, a significant enhancement in cellular Runx2 (Fig. 2a), OSX (Fig. 2b), OPN (Fig. 2c), and OCN (Fig. 2d) mRNA expression was detected by q-PCR. Meanwhile, we used WB to confirm the effect of SA on the protein expression of Runx2 and OCN. As showed in Fig. 2e–g, SA exhibited a good promoting effect on the expression of Runx2 and OCN. Therefore, our data demonstrate that SA stimulates the osteogenic differentiation of BMSCs in vitro.
SA promotes the expression of Runx2, OSX, OPN, and OCN. BMSCs were cultured in OIM and exposed to SA (10, 20, 40 μM) for 2 weeks. Total cytosolic RNA was used for q-PCR analysis of Runx2 (a), OSX (b), OPN (c), and OCN (d). Data are expressed as fold change versus the control group, taken as calibrator for comparative quantitative analysis of GAPDH mRNA levels. Cell lysates were prepared, subjected to western blot, and analyzed using antibodies specific for Runx2 (e, f) and OCN (e, g) as described in the methods section. Columns represent mean ± standard error from three independent experiments, each performed in triplicate, *p < 0.05, **p < 0.01, ***p < 0.001 versus with the group without SA. + p < 0.05 versus with the group with 20 μM SA; # p < 0.05 versus with the group with 10 μM SA
SA Stimulates Osteogenesis In Vivo
To investigate the effect of SA on trabecular bone micro-architecture, SA was administered intraperitoneally to mice by following an ovariectomy. 3 months after treatment, the mice were sacrificed. Micro-CT results from long bones of OVX + SA mice revealed a clear trend in which trabecular number (Fig. 3a–f, g) was enhanced relative to that of the OVX group. Moreover, trabecular thickness (Fig. 3a–f, h) and BMD (Fig. 3a–f, i) in OVX + SA bones were significantly higher than in OVX mice. However, these parameters were not significantly different between the OVX and sham groups.
SA improves bone micro-architecture. a–f Micro-CT scan of the distal femur shows the trabecular number (g), trabecular thickness (h), and trabecular BMD (i) in three groups. Each group contained ten mice. The values showed are the mean ± standard error from independent experiments, **p < 0.01, ***p < 0.001 versus with OVX group
H&E staining of femoral bone sections demonstrated that the number of trabeculae in OVX mice was significantly decreased when compared with the OVX + SA group (Fig. 4a).
SA increases osteogenesis in vivo. OVX mice were orally administered with SA for 3 months. a Femur sections were measured by H&E staining. b, d Runx2 expression was performed by immunohistochemistry. c, e OCN expression was tested by immunohistochemistry. The values showed are the mean ± standard error from independent experiments, **p < 0.01, ***p < 0.001 versus with OVX group. Scale bar 100 μm for (a), 5 μm for (b), and 10 μm for (c)
Expression of Runx2 and OCN in vivo were performed by immunohistochemistry. A decrease in Runx2 labeling was observed in the OVX group compared with the sham group, whereas in the OVX + SA group Runx2 expression was higher than that of the OVX group after 3-months administration (Fig. 4b, d). Similar to the expression of Runx2, a significant reduction in OCN expression was detected in the OVX group compared with the sham group, while the OCN expression of the OVX + SA group was higher than that of the OVX group after 3-months treatment (Fig. 4c, e).
Taken together, our data indicate that SA stimulates the expression of osteogenic markers in vivo and provides a protective effect against ovariectomy-induced bone loss.
Effect of SA on WNT/β-Catenin Signaling
In order to evaluate the potential mechanism of SA on osteogenic differentiation in BMSCs, we performed WB analysis to examine the expression of related proteins pretreated with an increasing dose of SA in the presence or absence of DKK1 for 2 weeks. The untreated bands were used as control group. We found the expression of β-catenin (Fig. 5a, b) elevated gradually in a dose-dependent manner. Moreover, SA treatment of BMSCs increased the protein level of β-catenin and that co-treatment with SA and DKK1 significantly inhibited increases in β-catenin (Fig. 5c, d), Runx2 (Fig. 5c, e), and OCN (Fig. 5c, f) protein expression. We also found that SA significantly increased the expression of WNT/β-catenin downstream signaling molecules, such as TCF-1 (Fig. 5g, h) and LEF-1 (Fig. 5g, i). Therefore, these results support the view that SA stimulated osteogenic differentiation of BMSCs by activating WNT/β-catenin signaling.
Effect of SA on WNT/β-catenin signaling. a, b BMSCs were treated with increasing dose SA in OIM for 2 weeks. The β-catenin protein level was detected by western blot. Columns represent mean ± standard error from three independent experiments, each performed in triplicate, *p < 0.05, **p < 0.01, ***p < 0.001 versus with the group without SA. + p < 0.05 versus with the group with 20 μM SA; # p < 0.05 versus with the group with 10 μM SA. BMSCs were cultured in OIM or OIM containing 40 μM SA with or without 0.1 μg/ml DKK1 for 2 weeks. The expression of β-catenin (c, d), Runx2 (c, e), and OCN (c, f) were detected by western blot. Columns represent mean ± standard error from three independent experiments, each performed in triplicate, + p < 0.001 versus group without SA; # p < 0.01 versus with 40 μM SA. BMSCs were cultured in OIM or OIM containing 40 μM SA for 2 weeks. The expression of TCF-1 (g, h) and LEF-1 (g, i) were detected by western blot. Columns represent mean ± standard error from three independent experiments, each performed in triplicate, ***p < 0.001
Discussion
Traditional Chinese medicines are an alternative choice to prevent and treat postmenopausal osteoporosis, which are prepared from plants and have fewer side-effects [28]. Saikosaponin present in medicinal plants, including Bupleurum chinese DC. and Bupleurum scorzonerifolium Willd, is used in traditional Chinese medicine for the treatment of numerous diseases, including osteoporosis. Recently, many natural compounds including saponins with the therapeutic effect on bone formation and skeleton construction have been reported [29–31]. Some studies have shown that several varieties of saponins such as ginsenoside [32], dioscin [6], asperosaponin VI [33], ophiopogonin [34], and achyranthes bidentata saponins [29] are protective agents against bone loss. So far, no study has yet investigated the effect of SA, a saponin compound, on osteogenic differentiation of BMSCs in vitro and in vivo. Therefore, we assessed the effects of SA on osteogenic differentiation of BMSCs in both in vitro and in vivo models.
In the present study, we present evidence, for the first time, that SA could promote BMSCs differentiation into osteoblasts in vitro and in vivo, potentially countering the bone loss caused by estrogen deficit. Notably, to elucidate the potential mechanism of SA on promoting bone formation, we found that WNT/β-catenin signaling was involved in this process.
Results obtained from our assay system indicated that SA did not exhibit cytotoxic effect on BMSCs viability at concentrations of 10, 20, and 40 μM. Our data were similar to the results of that SA had no effect on the proliferation of RAW264.7 cell and bone marrow macrophage [10].
The development of osteogenesis undergoes three stages: the osteoprogenitor, the preosteoblast, and the mature osteoblast stage [35]. ALP, a cell membrane-associated enzyme, appeared early during osteoblast differentiation, was the most widely recognized marker of osteogenic differentiation [36]. ALP activity correlates with matrix formation in osteoblasts prior to the initiation of mineralization [37]. Previous studies have found that Runx2 and OSX are the primary regulators of osteogenic differentiation and play a critical role in bone metabolism [2, 38]. OPN and OCN were involved in controlling of the mineralization process, appeared at late stage of osteogenic differentiation and characterized by mature cells of the osteoblastic lineage [37, 38]. In our study, ALP, Runx2, and OSX are used as the indicator of early osteogenic differentiation of BMSCs, while OPN and OCN are considered as the key transcriptional factor regulating late stage of osteogenesis. SA upregulated ALP activity under osteogenic condition in a dose-dependent manner, indicating the protection of earlier stages of osteogenesis by SA. The protection of the late stage of osteogenesis is evidently observed by the Alizarin red assay treated with SA on day 14. The protection of osteogenesis at both early and late stages under osteogenic differentiation condition is further supported by our results that SA increases mRNA expression level of four master osteogenic differentiation marker genes, Runx2, OSX, OPN, and OCN and stimulates protein expression of Runx2 and OCN. Taken together, we discovered that SA promoted osteogenic differentiation of BMSCs not only at the early stage, but also at the maturation stage.
In order to confirm our in vitro data, we used the OVX mouse model, the most popular animal model of postmenopausal osteoporosis, in which the acceleration of cancellous bone loss and the decrease of cortical bone are closely correlated to estrogen deficiency [39]. As expected, our micro-CT data showed that bone loss induced by OVX could be effectively rescued by SA treatment. Moreover, the expression of Runx2 and OCN were significantly upregulated, suggesting that SA stimulates osteogenic differentiation in vivo. Although our findings indicate a positive effect of SA on osteogenesis in vivo, we did not examine osteoclastogenesis in vivo. In further experiments, TRAP-positive osteoclasts should be observed in vivo, so as to know whether SA could exhibit potent abilities treat osteoporosis through enhancing osteogenesis and inhibiting osteoclastogenesis.
WNT/β-catenin signaling pathway has caught attention as a potential target for osteoporosis treatment, for it was considered to play a critical role in bone development [40]. Growing evidence suggests that activation of this pathway increases osteoblast differentiation and subsequent bone formation, while suppressing osteoclastogenesis [37]. The WNT/β-catenin pathway is not only induced by extracellular WNT proteins but also occurs in response to other chemical and mechanical stimuli [37]. Although it is well known that WNT signaling is considered as a key regulator of bone biology, the relationship between SA and WNT/β-catenin activation has not been broadly identified. DKK1, a specific inhibitor of WNT/β-catenin signaling pathway, can inhibit the stable accumulation of β-catenin by being combined with LRP5/6 competitively so that the conduction of WNT/β-catenin pathway is blocked [41]. Therefore, in order to explore whether SA increases osteogenic differentiation through WNT/β-catenin pathway activation, DKK1 was added into the OIM with the treatment of SA to block the WNT/β-catenin pathway. We found that co-culture of cells with DKK1 significantly blocked the positive effect of SA on the protein expression of osteogenic differentiation markers. Our data further demonstrate that SA stimulates the WNT/β-catenin pathway in BMSCs. For one thing, SA obviously increased β-catenin protein expression. For another, SA improved WNT/β-catenin downstream genes TCF-1 and LEF-1 protein expression in BMSCs. However, we have not examined how DKK1 suppresses the effect of SA on osteogenesis of BMSCs in this study. We thought that SA treatment gives rise to higher concentration of WNT proteins in the media, which in turn results in the elevated β-catenin level in response to SA treatment. Therefore, further studies are needed to assay the regulation of SA on WNT proteins such as Wnt3 and Wnt3a.
In conclusion, our results demonstrate for the first time that SA promotes osteogenesis, which is dependent on increasing ALP, Runx2, OSX, OPN, and OCN expression, thereby stimulating the lineage differentiation of BMSCs toward osteoblasts in vitro and in vivo. Furthermore, WNT/β-catenin signaling might be the specific signaling pathway mechanism in this process. Therefore, our findings shed light on the mechanisms of how SA potentiates BMSCs differentiation into osteoblasts, and indicating that SA may be a suitable candidate for the treatment of patients with postmenopausal osteoporosis.
References
Meng J, Ma X, Wang N, Jia M, Bi L, Wang Y, Li M, Zhang H, Xue X, Hou Z, Zhou Y, Yu Z, He G, Luo X (2016) Activation of GLP-1 receptor promotes bone marrow stromal cell osteogenic differentiation through β-catenin. Stem Cell Rep 6:633
Li SF, Tang JJ, Chen J, Zhang P, Wang T, Chen TY, Yan B, Huang B, Wang L, Huang MJ, Zhang ZM, Jin DD (2015) Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des Dev Ther 9:5169–5183
Wang D, Ma W, Wang F, Dong J, Wang D, Sun B, Wang B (2015) Stimulation of Wnt/β-catenin signaling to improve bone development by naringin via interacting with AMPK and Akt. Cell Physiol Biochem 36:1563–1576
Li M, Wu W, Tan L, Mu D, Zhu D, Wang J, Zhao B (2015) Low-magnitude mechanical vibration regulates expression of osteogenic proteins in ovariectomized rats. Biochem Biophys Res Commun 465:344–348
Curtis JR, Cai Q, Wade SW, Stolshek BS, Adams JL, Balasubramanian A, Viswanathan HN, Kallich JD (2013) Osteoporosis medication adherence: physician perceptions vs. patients’ utilization. Bone 55:1–6
Tao X, Qi Y, Xu L, Yin L, Han X, Xu Y, Wang C, Sun H, Peng J (2016) Dioscin reduces ovariectomy-induced bone loss by enhancing osteoblastogenesis and inhibiting osteoclastogenesis. Pharmacol Res 108:90–101
Lewiecki EM (2011) New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol 7:631–638
Mulder JE, Kolatkar NS, LeBoff MS (2006) Drug insight: existing and emerging therapies for osteoporosis. Nat Clin Pract Endocrinol Metab 2:670–680
Chen JS, Sambrook PN (2012) Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol 8:81–91
Zhou C, Liu W, He W, Wang H, Chen Q, Song H (2015) Saikosaponin a inhibits RANKL-induced osteoclastogenesis by suppressing NF-kappaB and MAPK pathways. Int Immunopharmacol 25:49–54
Shin JE, Kim HJ, Kim KR, Lee SK, Park J, Kim H, Park KK, Chung WY (2015) Type I saikosaponins a and d inhibit osteoclastogenesis in bone marrow-derived macrophages and osteolytic activity of metastatic breast cancer cells. Evid Based Complement Alternat Med 2015:582437
Ye M, Bi YF, Ding L, Zhu WW, Gao W (2016) Saikosaponin a functions as anti-epileptic effect in pentylenetetrazol induced rats through inhibiting mTOR signaling pathway. Biomed Pharmacother 81:281–287
Chen E, Chen J, Cao SL, Zhang QZ, Jiang XG (2010) Preparation of nasal temperature-sensitive in situ gel of Radix Bupleuri and evaluation of the febrile response mechanism. Drug Dev Ind Pharm 36:490–496
Bermejo P, Abad MJ, Diaz AM, Fernandez L, De Santos J, Sanchez S, Villaescusa L, Carrasco L, Irurzun A (2002) Antiviral activity of seven iridoids, three saikosaponins and one phenylpropanoid glycoside extracted from Bupleurum rigidum and Scrophularia scorodonia. Planta Med 68:106–110
Sun Y, Cai TT, Zhou XB, Xu Q (2009) Saikosaponin a inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int Immunopharmacol 9:978–983
Ma Y, Bao Y, Wang S, Li T, Chang X, Yang G, Meng X (2016) Anti-inflammation effects and potential mechanism of saikosaponins by regulating nicotinate and nicotinamide metabolism and arachidonic acid metabolism. Inflammation 39:1453–1461
Fu Y, Hu X, Cao Y, Zhang Z, Zhang N (2015) Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in Human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radic Biol Med 89:777–785
Yu YH, Xie W, Bao Y, Li HM, Hu SJ, Xing JL (2012) Saikosaponin a mediates the anticonvulsant properties in the HNC models of AE and SE by inhibiting NMDA receptor current and persistent sodium current. PLoS One 7:e50694
Liu YQ, Hong ZL, Zhan LB, Chu HY, Zhang XZ, Li GH (2016) Wedelolactone enhances osteoblastogenesis by regulating Wnt/beta-catenin signaling pathway but suppresses osteoclastogenesis by NF-kappaB/c-fos/NFATc1 pathway. Sci Rep 6:32260
Du L, Nong MN, Zhao JM, Peng XM, Zong SH, Zeng GF (2016) Polygonatum sibiricum polysaccharide inhibits osteoporosis by promoting osteoblast formation and blocking osteoclastogenesis through Wnt/β-catenin signalling pathway. Sci Rep 6:32261
Su X, Liao L, Shuai Y, Jing H, Liu S, Zhou H, Liu Y, Jin Y (2015) MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis 6:e1851
Jackson WM, Nesti LJ, Tuan RS (2012) Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 1:44–50
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749
Ma ZP, Liao JC, Zhao C, Cai DZ (2015) Effects of the 1, 4-dihydropyridine L-type calcium channel blocker benidipine on bone marrow stromal cells. Cell Tissue Res 361:467–476
Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, Liu R, Olivos D, Saunders M, Lam KS, Nolta J, Olvera D, Ritchie RO, Lane NE (2013) Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells 31:2003–2014
Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18:456–462
Jiang J, Li J, Jia X (2014) The antiosteoporotic activity of central-icaritin (CIT) on bone metabolism of ovariectomized rats. Molecules 19:18690–18704
Sun W, Wang YQ, Yan Q, Lu R, Shi B (2014) Effects of Er-Zhi-Wan on microarchitecture and regulation of Wnt/beta-catenin signaling pathway in alveolar bone of ovariectomized rats. J Huazhong Univ Sci Technolog Med Sci 34:114–119
He G, Guo W, Lou Z, Zhang H (2014) Achyranthes bidentata saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK MAPK signaling pathway. Cell Biochem Biophys 70:467–473
Wang Y, Huang X, Tang Y, Lin H, Zhou N (2016) Effects of panax notoginseng saponins on the osteogenic differentiation of rabbit bone mesenchymal stem cells through TGF-β1 signaling pathway. BMC Complement Altern Med 16:319
Schilling T, Ebert R, Raaijmakers N, Schutze N, Jakob F (2014) Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J Steroid Biochem Mol Biol 139:252–261
Siddiqi MH, Siddiqi MZ, Ahn S, Kim YJ, Yang DC (2014) Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. J Pharm Pharmacol 66:1763–1773
Niu Y, Li Y, Huang H, Kong X, Zhang R, Liu L, Sun Y, Wang T, Mei Q (2011) Asperosaponin VI, a saponin component from Dipsacus asper wall, induces osteoblast differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway. Phytother Res 25:1700–1706
Huang Q, Gao B, Wang L, Zhang HY, Li XJ, Shi J, Wang Z, Zhang JK, Yang L, Luo ZJ, Liu J (2015) Ophiopogonin D: a new herbal agent against osteoporosis. Bone 74:18–28
Wang L, Zhang YG, Wang XM, Ma LF, Zhang YM (2015) Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem Biol Interact 242:255–261
Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J (2010) Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res 28:1267–1275
Ying X, Chen X, Feng Y, Xu HZ, Chen H, Yu K, Cheng S, Peng L (2014) Myricetin enhances osteogenic differentiation through the activation of canonical Wnt/β-catenin signaling in human bone marrow stromal cells. Eur J Pharmacol 738:22–30
Tao K, Xiao D, Weng J, Xiong A, Kang B, Zeng H (2016) Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicol Lett 240:68–80
Xiao JJ, Zhao WJ, Zhang XT, Zhao WL, Wang XX, Yin SH, Jiang F, Zhao YX, Chen FN, Li SL (2015) Bergapten promotes bone marrow stromal cell differentiation into osteoblasts in vitro and in vivo. Mol Cell Biochem 409:113–122
Marie PJ, Kassem M (2011) Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol 165:1–10
Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML (2005) Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol 25:4946–4955
Acknowledgements
We thank Cong Luo (Southern Medical University, PR China) for excellent technical support with micro-CT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Weiqi Huang, Xiaoling Zheng, Xiaodong Yang, and Shicai Fan state that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This study was ethically approved by the Institutional Animal Care and Use Committee of Southern Medical University and performed in accordance with the criteria defined by the rules of the committee.
Rights and permissions
About this article
Cite this article
Huang, W., Zheng, X., Yang, X. et al. Stimulation of Osteogenic Differentiation by Saikosaponin-A in Bone Marrow Stromal Cells Via WNT/β-Catenin Pathway. Calcif Tissue Int 100, 392–401 (2017). https://doi.org/10.1007/s00223-017-0242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0242-y